Keywords
Pumpkin, Cellic C-Tec2, cellulase and hemicellulase, colloidal stability, functional foods, total carotene, antioxidants
Pumpkin, Cellic C-Tec2, cellulase and hemicellulase, colloidal stability, functional foods, total carotene, antioxidants
Pumpkin is a rich source of vitamin A precursor carotenoids such as β-carotene (4.28– 94.14 mg/100 g db) (Atencio et al., 2022; Kulczynski & Gramza-Michałowska, 2019; Priori et al., 2017), found mainly in the pulp and peel (Durante et al., 2014; Pazinato et al., 2018; Pazinato & Cardozo, 2019). The physical composition of the fresh pumpkin fruit ranges from 71.85%–86.06% in pulp, 8.20%–13.86% in peel, and, 2.7%–5.89% in seeds (Jacobo et al., 2011). The peel is normally discarded as waste, but significant amounts of β-carotene (52.7 mg/100 g db) (Lima et al., 2021) and phenolic compounds (22.92±1.06 mg of eq gallic acid GAE/g db) (Bahramsoltani et al., 2017) are reported. Its seed contains a high percentage of the total lipid content as monounsaturated (25.42±0.51%) and polyunsaturated (51.66±0.68%) fatty acids of the total lipid content (Amin et al., 2019); and a high proportion of the total bioactive compounds as total tocopherol (16.3–29 mg/100 g of oil) (Chellini et al., 2022); and as protein (21.4%–27.4%) (Amin et al., 2019; Mi et al., 2012). Therefore, pumpkin fruits and their fractions, such as seeds and peels, constitute a good source of nutrients for the development of novel functional foods.
Enzymatic maceration refers to the process of reducing a substance in the presence of enzymes following its reaction with the aqueous phase. It has been used extensively in the food industry, encompassing the clarification of juices, the production of nectar and fruit and vegetable purees, the extraction of oil from oilseeds, and other functional ingredients (Álvarez, 2018). Enzymatic hydrolysis can reduce the interaction between antioxidants and other substances such as proteins, pectin, and polysaccharides, allowing the release of tocopherol and phenolic content and increasing the functional value of food (Li et al., 2016). The addition of a multi-enzymatic complex that mainly includes cellulase, hemicellulase and β-glucosidase enzymes as pretreatments in substrates rich in heteropolysaccharides together with a combined action using homogenization by shear, allows minimizing the impacts of these compounds on the characteristics of the final product, such as color, texture, turbidity, viscosity, and an increase in phenolic content (Álvarez, 2018; Danalache et al., 2018).
Homogenization is the physical process of subdividing large particles into many smaller particles by mechanical effects, and it is a previous operation in many food operations, including spray-drying processing. The physicochemical properties of the colloidal system, rheological behavior, type of suspension, multiscale particle sizes, content of bioactive compounds, and many interacting constituents, together with the operating parameters of the homogenization equipment and operation principle, are important conditions for obtaining a high-quality and physiochemically stable product (Janiszewska et al., 2015; Schramm, 2005a; Shishir & Chen, 2017). Along with pulp in pumpkin suspensions, the presence of insoluble compounds from seed and peel, such as cellulose (41%–49%), hemicellulose (24%–29%) and lignin (9%–26%) (Nansikombi et al., 2019; Nor lia et al., 2022), generates a thermodynamically unstable colloidal system that ends in phase separation quickly or gradually, depending on the particle-particle and particle-phase interactions that occur from the predominance of attractive forces (Van der Waals forces) against repulsive (electrostatic), steric, hydration, and hydrophobic forces, among others (Salehi, 2020). Therefore, technological processes such as homogenization by shear are required to reduce the size of the particles by mechanical enabling, which decreases the attractive forces and improves the physicochemical stability of the colloidal suspension, as well as the extraction of soluble compounds such as lignin, proteins, and polysaccharides from the cell wall (Hua et al., 2017). Besides, this type of mechanical processing produces the release of total soluble solids, bioactive components, and dietary fibers, improving texture, rheology, and functional thermodynamic and compositional stability (Kamble et al., 2022). Hence, the combined action of mechanical forces (shearing) and pretreatment of vegetable material using enzymes could produce significantly positive results on the colloidal system (Hua et al., 2017; Lan et al., 2023).
The combined action of homogenization processing by shearing and enzymatic treatment has not been studied and reported in heterogeneous colloidal systems of integral pumpkin, where this assessment could be especially important for obtaining food products, such as dehydrated materials in powder form. This research aimed to evaluate the physicochemical stability of a colloidal system based on the pulp, seed, and peel of pumpkin (C. maxima), through the integration of the technological processes: enzymatic maceration and homogenization by shear, with potential applications in diverse food processing applications, including powder materials by spray drying.
Pumpkins (C. maxima) were harvested at physiological maturity in the municipality of Dabeiba, Antioquia, Colombia; then, they were stored (25°C and 65±2% RH). They were washed, disinfected with commercial sodium hypochlorite (Makro, Aro) to 100 ppm, and peeled and chopped manually, reserving 25% of the total peel and all the seeds. The fruit was subjected to blanching at 90°C for 5 min (Thermostat bath, Memmert), and the pulp, peel, and seed were manually separated.
Enzymatic maceration
To reduce the firmness of the seeds and peels, enzymatic hydrolysis was performed using a complex of cellulase, hemicellulose, and β-glucosidase, Cellic CTec2 (Novozymes Cellic C-Tec2, Batch VCNI0018). The enzymatic activity was determined at 105 FPU/ml by the method proposed by (Adney and Baker, 1996), where a unit of filter paper activity (FPU) is defined as the amount of enzyme that forms 1 μmol of glucose (reducing sugars as glucose) per minute. The enzymatic treatments were subjected to optimum hydrolysis temperatures of 45°C and shaking at 280 rpm (Heidolph Promax 1020, Germany), where 100 g of seed and peel substrate was liquefied (3 V 4655/Oster, Hilliard, Ohio, USA) for 5 min with water in a 1:1 ratio at a pH of 6.2. The enzyme dosage (X1) and hydrolysis time (X2) were varied. At the end of the hydrolysis time, it was inactivated at 95°C for 60 s (Thermostatic bath, Memmert) and the substrate was stored at -8°C for 12 h.
Homogenization by shear
Initially, 1700 g of previously scalded pumpkin pulp was chopped and processed in a colloidal mill (JML-50 Longqiang, China) with 100 g of water for 5 min with a cooling bath at 8°C; then, together with the hydrolyzed seed and peel substrate, homogenization was performed by under high shear (Silverson, L5 series mixer, MA, USA), at a constant rotation of 10,000 rpm. The homogenization time (X3) was varied during the process, and the integral suspension was maintained at a constant temperature (20°C) using a water-cooling bath. The feed to the shear mixer was kept at 6% total solid for each batch of 2000 g of suspension (90% milled pulp and 10% of the pumpkin seed and peel substrate).
Statistical design
A response surface methodology was used with a centered central composite design (α=1), considering as independent variables: enzyme dose (X1=21, 31, 41 FPU/g db of the substrate), hydrolysis time (X2=2, 3.5, 5 h) and homogenization time (X3=5, 7.5, 10 min) with 16 experiments (Table 1) and dependent variables such as the apparent viscosity (μ), zeta potential (ζ), particle size (D[3;2], D[4;3]), R index, the total carotenoid content, and antioxidant capacity by DPPH• and ABTS•+. The multiple regression method (confidence level of 95%) was used for the prediction of the linear and quadratic coefficients and the interaction of the independent variables in the response surface models, according to Equation 1.
| Randomization | Experiments | [X1] | [X2] | [X3] |
|---|---|---|---|---|
| FPU/g db | (h) | (min) | ||
| 8 | 1 | 31 | 3.5 | 7.5 |
| 13 | 2 | 42 | 2 | 10 |
| 9 | 3 | 31 | 3.5 | 7.5 |
| 2 | 4 | 21 | 2 | 10 |
| 11 | 5 | 31 | 5 | 7.5 |
| 4 | 6 | 21 | 5 | 5 |
| 12 | 7 | 42 | 2 | 5 |
| 14 | 8 | 42 | 3.5 | 7.5 |
| 7 | 9 | 31 | 3.5 | 5 |
| 1 | 10 | 21 | 2 | 5 |
| 10 | 11 | 31 | 3.5 | 10 |
| 16 | 12 | 42 | 5 | 10 |
| 6 | 13 | 31 | 2 | 7.5 |
| 3 | 14 | 21 | 3.5 | 7.5 |
| 5 | 15 | 21 | 5 | 10 |
| 15 | 16 | 42 | 5 | 5 |
Where , , is the constants obtained by multiple regression.
For the multi-response optimization (desirability approach), the weight and impact were established for each of the response variables that affect the stability of the colloidal system previously defined. For the optimal condition, the experimental data were compared with the adjusted values predicted by the statistical models based on the relative mean error (REM) between the experimental and predicted value.
Viscosity (μ)
The apparent viscosity (μ) was determined in a Brookfield DV-III Ultra (rotational mode), coupled to a Brookfield model TC-502 thermostatic bath, maintaining the suspension temperature of 25°C. Rheological determination (flow curve) was performed using a RV3 spindle, speed from 0.01 to 100 rpm, and the viscosity value was reported at a rotation of 100 rpm. Results were expressed as mPa.s (Castaño et al., 2022).
Zeta potential (ζ)
The zeta potential (ζ) of the integral suspensions was determined from the electrophoretic mobility using a Zetasizer Nano ZS90 (Malvern Instruments Ltd., Worcester, UK), a DTS 1060 capillary cell, and dilution in distilled water (1:100) (Liu et al., 2022).
Particle size
The particle size was determined in a Mastersizer 3000 analyzer (Malver Instrument Ltd., Worcestershire, UK), calculating the D[3;2] (equivalent surface diameter) and D[4;3] (equivalent volume diameter), where the samples were dispersed in 500 mL of distilled water until a darkening value of 10±1% was obtained (Cardona et al., 2021).
Spectral absorption (R index)
The stability index by spectral absorption was calculated from the absorbance ratio at two wavelengths (800 and 400 nm) (A800/A400) as described by Kaufman and Garti (1981).
Granular morphology by binocular microscope
The granular morphology of the suspension was determined by a binocular microscope (Leica, DM1000 LED, Japan), a 10 μL aliquot was poured onto a glass slide, and the microphotographs were acquired under fields of clear and polarized light, with a magnification of 10× and 40×, using a digital camera (Leica, ICC50W, Japan) (Atencio et al., 2022).
Total carotenoid
For the quantification of total carotenoid in the pumpkin suspension, 1.5 g were weighed and mixed with 20 mL of the acetone:ethanol (Merck) solution in a 9:1 ratio and incubated at 4°C for 30 min. It was shaken in a Fisher Scientific vortex mixer and centrifuged at 4000 rpm for 10 min (Zapata et al., 2017). Carotenoids, were quantified by spectrometry, using acetone as a blank at an absorbance of 450 nm, and β-carotene was used as a standard Sigma-Aldrich St. Louis, MO, USA; results were expressed as mg β-carotene/100 g db, according to modification of the methodology by Biswas et al., (2011).
Antioxidant capacity by DPPH• and ABTS•+
For the antioxidant capacity determined by DPPH• and ABTS•+, approximately 2 g of the suspension was weighed and 10 mL of ethanol inoculated at 25°C for 24 h in the dark. Subsequently, the supernatant was filtered and the solvent rotoevaporated (Hernández et al., 2018). Extractions were performed for each treatment in triplicate. For the determination of antioxidant capacity by the Brand-Williams et al. (1995) method of DPPH• (2,2-diphenyl-1-picryl-hydrazyl) modified by Zapata et al. (2017), a 10 μL was aliquot extracted with 990 μL of DPPH• in a methanol solution for 30 min at room temperature, then a standard curve was performed using Trolox and the results were expressed as μmol Trolox Eq/100 g db. The absorbance was determined at 517 nm. It was performed for each repetition of treatment and three extractions. The antioxidant activity determined by ABTS•+ (2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid) was carried out according to the modified methodology of Londoño et al. (2017). A 10 μL volume of the extract was added to 990 μL of ABTS•+ diluted in ethanol, and the resulting solution was incubated at room temperature for 7 min in the dark. Absorbance was measured at 734 nm against a blank. Trolox standard solution was used to perform the calibration curves, and the results were expressed as μmol Trolox Eq/100 g db.
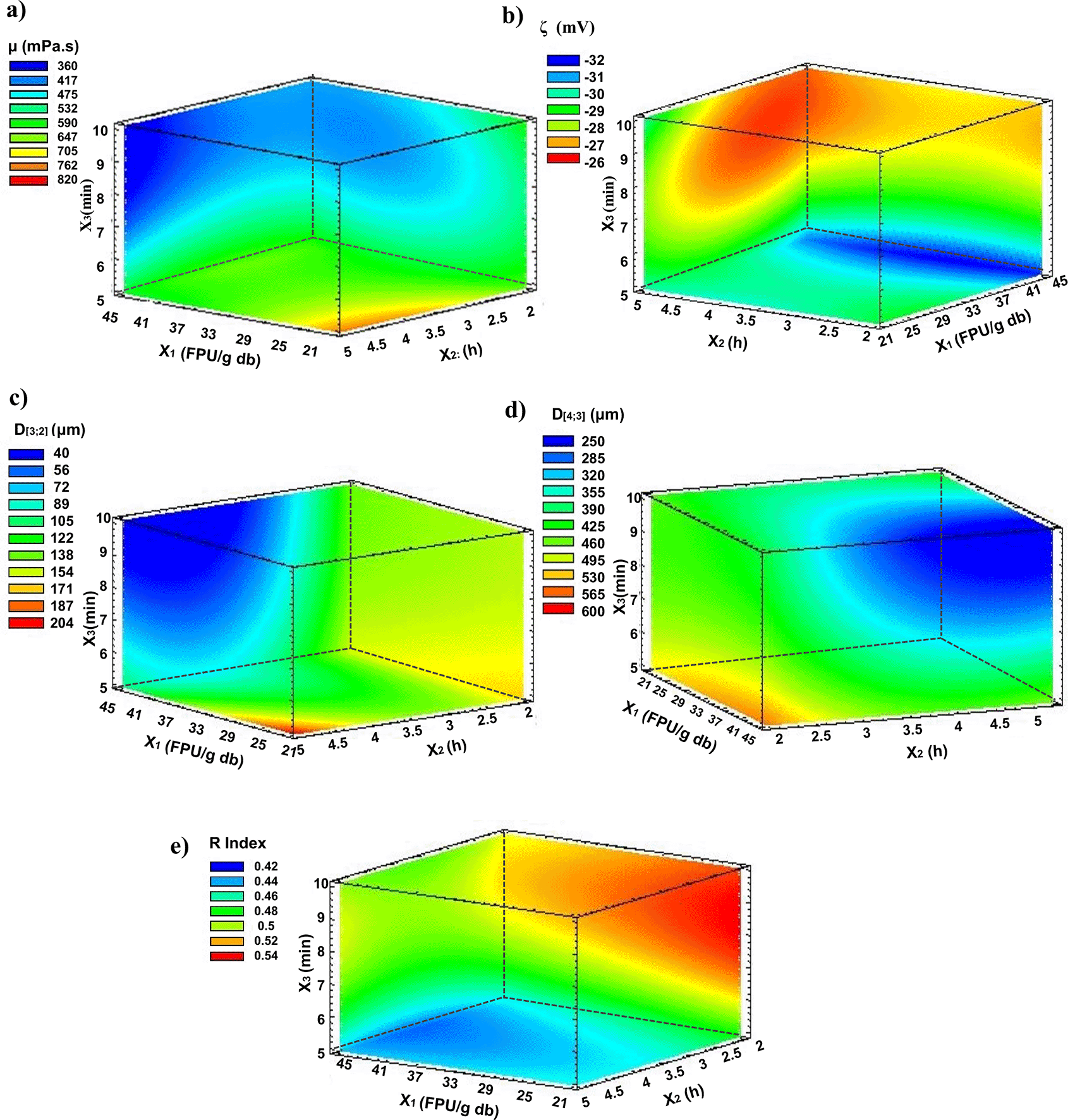
Figure 1 displays the response volume graphs of apparent viscosity (μ) (mPa.s) (Figure 1a), zeta potential (ζ) (mV) (Figure 1b), particle size (Figure 1c and 1d), and (R) index (Figure 1e) in the integral suspension of pumpkin (C. maxima). Table 2 reports the polynomial regression coefficients for the surface model of the colloidal system and statistically significant values with the R2 coefficient adjusted.
| Variable | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| μ | 997.85 | -9.36* | 230.38 | -144.57* | 0.31 | -2.59 | -1.21 | -15.33 | -4.2 | 11.36 | 87.65 |
| ζ | -39.3 | 0.11 | -2.19 | 3.16 | -0.0066 | 0.016 | 0.037 | 0.23 | 0.0033 | -0.26 | 42.04 |
| D[3,2] | 312.63 | 2.18* | -76.07 | -8.018* | -0.003 | -1.25* | 0.0068 | 19.08* | -3.93 | 0.8 | 89.77 |
| D[4,3] | 694.57 | 12.14 | -63.18 | -53.26* | -0.033 | -2.23 | -0.74 | 22.96 | -6.03 | 4.5 | 87.3 |
| R Index | 0.31 | -0.0024 | -0.054 | 0.08* | 0.014 | 0 | 0.0003 | 0 | -0.0004 | -0.005 | 69.02 |
| Total carotenoids | -154.11 | 3.71* | 81.13 | 11.89 | 0.066 | -1.14* | -0.33 | -4.58 | -0.55 | 0.014 | 77.51 |
| DPPH• | 1958.32 | -63.26 | -177.03 | 81.79* | 0.9 | -1.36 | 1.25 | 29.01 | 6.36 | -12.66 | 81.53 |
| ABTS•+ | -38.41 | -249.5 | 57.53 | 1392.9 | 5.02 | 12.19 | -11.29 | -80.61 | 25.6 | -73.04 | 65.89 |
Viscosity (μ)
The μ values of the suspensions ranged from 371 to 811 mPa.s where a statistically significant effect (p<0.05) of X1 and X3 was obtained. Figure 1a shows that increasing X1 and X3 ends up diminishing the suspension’s viscosity. The time of shear forces during homogenization causes the disruption of individual cells and the rupture of small groups, such as pectin structures where polygalacturonic acid chains protrude, which are affected by the cutting time, releasing simple monomer structures, these chains are shorter and may have a reduced ability to form a three-dimensional gel network (Aghajanzadeh et al., 2017). These phenomenologies were consistent with the viscosity values obtained, denoting adequate fluidity for feeding in spray-drying processes. High viscosities in the feed can influence the properties of the powder mixtures, such as moisture, bulk density, porosity, and solubility, among others (Janiszewska et al., 2015). Also, molecular rearrangement during the homogenization process may lead to lower gel strength; a similar effect was reported by Kamble et al. (2022). Significant pectin contents have been reported in pumpkin fruits: 1.27±0.08% in the pulp (Torkova et al., 2018) and ranges from 2.28% to 2.91% in the peels (Nor lia et al., 2022), which gives the fluid a viscous character. Also, the dose of the Cellic CTec2 enzyme complex acts on polysaccharides of the cell wall, such as cellulose and hemicellulose, contained in both the pumpkin seed and the peel, facilitating disintegration during homogenization and favoring the rheology of the system (Donzella et al., 2022). Some researchers have reported values of colloidal systems based on fruit and vegetables treated with homogenization processes; Gallón Bedoya et al. (2020) of cape gooseberry, strawberry, and blackberry (496 mPa.s–604 mPa.s) and, Cardona et al. (2021) for pineapple with peels and crown with enzymatic pretreatment, using Viscozyme L (97 mPa.s–277 mPa.s).
Zeta potential (ζ)
This variable is one of the most important physicochemical stability parameters to consider in the assessment of food suspensions because it is related to the repulsive forces in colloidal systems, which are directly responsible for their physicochemical stability (Schramm, 2005a). Figure 1b shows values between -23.2 and -31.6 mV and although changes in the negative electrical potential are denoted between the treatments, there was no statistically significant evidence (p>0.05). This behavior can be attributed to the anions from the dissociation of mineral salts and the electrical charge on the surface of the pectin particles present in the pumpkin pulp (Amin et al., 2019). Pectin is an acidic polymer containing a carboxyl group and a methoxyl group, and these groups can ionize in aqueous solutions and generate negative charges. As the concentration of pectin in the solution increases, the number of ionizable functional groups increases, leading to an increase in the electrical charge on the surface of the pectin particles (Aghajanzadeh et al., 2017). The surface charges present in a colloidal system produce forces of electrostatic repulsion between adjacent particles in aqueous phases; thus, when these charges are eliminated, the colloid particles agglomerate and settle. Therefore, values of |ζ|> 21 mV begin to favor the thermodynamic stability of a suspension (Patel & Pathak, 2021; Schramm, 2005a). Some authors have reported on colloidal systems with physicochemical stability values of |ζ|; Castaño et al. (2022) for strawberry suspensions with gum arabic (25–28), Liu et al. (2019) of carrot beverage (11–35), and Chávez-Salazar et al. (2019) of plantains suspensions (33–50).
Particle size D[3;2] (equivalent superficial diameter) and D[4;3] (equivalent volume diameter)
D[3;2] (equivalent superficial diameter), a statistically significant effect (p<0.05) was evidenced in the interaction between the variables X1 and X2, obtaining values from 45.1 μm to 203 μm, where at higher X1 and X2 of the enzyme complex, a reduction of the particle size is reached (Figure 1c). Also, the value of D[4;3] (equivalent volume diameter) varied from 281 μm to 586 μm (Figure 1d), having a statistically significant effect (p<0.05) on the shearing time during the homogenization (X3), where increasing the shearing processing time produces a smaller particle size. The polysaccharides present in the seed and shell substrate are previously treated with the Cellic CTec2 enzyme complex, which allows fragmentation of the fibers as well as cellulose, hemicellulose, starch, protein, and lignin, allowing the volumetric diameter of the particle size to decrease. Similar effects were found by Cardona et al. (2021). The most stable suspensions will be those that have a size distribution that is highly skewed towards the smallest sizes (Drapala et al., 2018; Schramm, 2005b).
Spectral absorption (R index)
Figure 1e shows the absorption R index, which is related to the particle size distribution and light scattering properties; low values indicate a close relationship between small particles, and the larger the range of particle sizes in the suspension (Kaufman & Garti, 1981). In Figure 1e, values from 0.43 to 0.53 were obtained. Similar results have been favorable for the stability of the fruit-based suspension reported by some authors (Cardona et al., 2021; Castaño et al., 2022; Gallón Bedoya et al., 2020). Although there is a statistically significant effect (p<0.05) of the shearing time during the homogenization (X3), this increase in the R index could be because the shear-off time allowed the presence of insoluble cell fragments such as hydrocolloids or components that were not completely dissolved and released mainly during enzymatic maceration, which varies in size from microscale to larger fragments, which confers a change in the optical properties of the suspension (Danalache et al., 2018).
Granular morphology by binocular microscope
Figure 2 shows the morphology of the suspension through optical microscopy; this technique is useful to observe the size of the oil globules and the aggregates of proteins, fibers, starches, and other compounds within the complex multiphase systems of the food (Drapala et al., 2018). In Figure 2a, 2b, and 2c, the impact of the highest doses of enzyme and the homogenization time on the reduction of the particle size is evidenced; it is also observed that the chromoplasts containing the carotenoids and no intact cells of sizes from 4 to 8 μm are observed, surrounded by drops of oils obtained from the lipid content of the seeds, as observed by Atencio et al. (2022). In Figure 2c, it is observed the affectation of the spatial conformation structures of the concentric rings and a typical birefringence (“Maltese cross”) with the hila in the center of the granules in squash starch by polarized optical microscopy. In different cultivars of pumpkin Cucurbita sp. there have been reports of values ranging from 1.05 to 6.47g/100 g fresh pulp of total starch content related to the textural properties of a pumpkin-based suspension (Yuan et al., 2022) and that can provide a greater encapsulating benefit for fat-soluble compounds during the spray-drying phase (Shi et al., 2012).

( Points out chromoplasts containing the carotenoids and drops of oils of the integral suspension of pumpkin
Brightness modification (30 more points).
Total carotenoid
Figure 3a shows the total carotenoid content. The carotenoids content changed from 72.1 to 155.0 mg β-carotene/100g db) where X1 and X3 showed a statistically significant effect (p<0.05). The increase in the dose of enzymes and hydrolysis time allowed greater extraction and aqueous availability of carotenoids (Atencio et al., 2022; Sharma & Sogi, 2022). Cellulases mainly follow the lock and key mechanism when joining cellular components, facilitating the rupture of cell walls and allowing the extraction of polysaccharides, pectin, and some bioactive compounds such as carotenoids and phenolic compounds (Das et al., 2021), which, when integrated with shear operations during homogenization, releases phytochemicals and pigments by breaking the cell wall of plant cells, mainly from the peel of pumpkin (C. maxima) (Kamble et al., 2022). For the total carotenoid in pumpkin, different authors reported the following values: pulp: 8.83 to 70.35 mg/g db (Itle & Kabelka, 2009; Kulczynski & Gramza, 2019; Zdunić et al., 2016), peel: 76.9 to 481 mg/100 g db (Lima et al., 2021; Mi et al., 2012) and seed: 0.4 to 6.6 mg/100 g db (Mi et al., 2012; Singh & Kumar, 2023). These results indicate an increase in the concentration of this bioactive by adding this type of agro-industrial coproducts to the suspension.

Antioxidant capacity by DPPH• and ABTS•+
The values of antioxidant capacity by DPPH• range from 683.6 to 1163 μmol Trolox Eq/100 g db (Figure 3b). The statistical analysis shows a significant effect of the shearing time (p<0.05), where the increase in shearing processing (X3) leads to a decrease in antioxidant activity. This response could be due to the degradation of some bioactive that are affected by the thermal energy released during homogenization (Kamble et al., 2022). Bioactive such as tocopherol have been shown to have a high antioxidant capacity by the DPPH• because this method focuses its effectiveness on hydrophobic compounds and free radical scavenging (Oroian & Escriche, 2015). Likely, the α-tocopherol content of the seed oil (1.69–6.24 mg/100 g) is related to the susceptibility of degradation during homogenization and hence the decrease in antioxidant capacity by DPPH•. A wide range of values are reported of antioxidant capacity by DPPH• for Cucurbita sp., from 237.2 to 3059.7 μmol Trolox Eq/100 g of fresh pulp, which can be attributed to the phenotypic conditions of the crop (Zhou et al., 2017).
Figure 3c shows the antioxidant activity by ABTS•+ ranging from 853 to 3242 μmol Trolox Eq/100 g db; however, this variation did not present statistically significant (p>0.05) changes with the independent variables. The antioxidant capacity obtained by this method has higher values than those obtained by DPPH•, because highly pigmented and hydrophilic antioxidants are better reflected by the ABTS•+ (Floegel et al., 2011). This cation is more reactive towards most phenolic antioxidants, vitamin C, flavonoids, hydroxycinnamates, carotenoids, and plasmatic antioxidants (Re et al., 1999). The scavenging of free radicals is very possibly closely related to the presence of β-carotene present in the pumpkin peel and pulp (Jiao et al., 2014), where carotenoids are used to protect fatty foods from photooxygenation processes and also act as a photochemical scavenger of singlet oxygen formed by some sensitizer in the reaction medium (Oroian & Escriche, 2015). The antioxidant capacity of Cucurbita sp determined by ABTS•+ has been reported to range from 1500 to 1800 μmol Trolox Eq/100 g db (Aydin & Gocmen, 2015), similar results were found in this study.
The optimization was defined under the criteria of minimizing μ, ζ, R, D[3;2], D[4; 3] and maximizing total carotenoid and antioxidant capacity by DPPH• and ABTS•+. The numerical results indicated a value of 79.9% to desirability in values of X1=42 FPU/g db, X2=4.68 h, and X3=8.54 min. Table 3 shows the values of the experimental validation and the theoretical values obtained from the quadratic models in the optimal condition determined, where the absolute values of relative mean error (RME) (<10%) validate the prediction of mathematical models. The variables of apparent viscosity (μ), zeta potential (ζ), particle size (D[3;2]) and total carotenoid content present the values obtained in triplicate with error percentages lower than 6% absolute compared to the theoretical values; while the R index, size particle (D[4,3]) and antioxidant capacity by DPPH• y ABTS•+ showed a high percentage of error due to the complexity of the adjustment for multiple responses. Optimization desirability values have been obtained for colloidal systems based on 67 % to Aegle marmelos L pulp (Kamble et al., 2022), pineapple pulp and peel with 84% (Cardona et al., 2021), cape gooseberry and strawberry of 80% (Gallón Bedoya et al., 2020) and watermelon of 84% (Aghajanzadeh et al., 2017).
A formulation of a multiphase suspension of integral pumpkin (pulp, seed, and peel) was evaluated considering the dual effects of enzymatic and shear homogenization processing. The interaction of enzyme doses and hydrolysis time allowed for greater extraction and availability of total carotenoid. The impact of the effect of enzymatic maceration together with homogenization by shearing showed synergistic effects, resulting in a significant decrease in particle size and a rheological response effective for the prevention of sedimentation rate. The suspensions presented an electrical surface charge, showing enough repulsive force between particles to prevent aggregation. The combined use of enzymatic hydrolysis allowed greater cell disruption, a reduction in homogenization time, and improved availability of bioactive in extraction processes. The results found for the suspension allowed for optimal conditions for its subsequent application in technological processes such as spray drying.
Figshare: Results effect of enzymatic maceration and homogenization of pumpkin suspension 1.1.xlsx (datasets). https://doi.org/10.6084/m9.figshare.23549859.v4 (Caballero et al., 2023a).
Figshare: Photomicrographs obtained from 10X, 40X bright field optical microscopy, and 40X polarized light optical microscopy.jpg (photographic images). https://doi.org/10.6084/m9.figshare.24162789.v3 (Caballero et al., 2023b)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Sustainable food systems
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 09 Oct 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)