Keywords
COVID-19, cytology, neurological disorders, leukocytes, glucose, protein, SARS-CoV-2, Peru
COVID-19, cytology, neurological disorders, leukocytes, glucose, protein, SARS-CoV-2, Peru
The COVID-19 pandemic caused by SARS-CoV-2 has affected millions of people around the world, resulting in an unprecedented health and economic crisis.1 The virus mainly affects the respiratory system; however, there is increasing evidence to suggest that it can also affect the nervous system, resulting in symptoms and neurological comprlications.2 The interaction between SARS-CoV-2 and the nervous system is complex and not yet fully understood.3
Neurological symptoms associated with COVID-19 range from mild symptoms, such as headache and anosmia, to severe symptoms, such as encephalitis and stroke.4 Additionally, COVID-19 can exacerbate pre-existing neurological conditions, such as Parkinson’s disease and multiple sclerosis.5,6 The mechanisms underlying the neurological effects of COVID-19 are thought to involve direct viral invasion of the nervous system, immune-mediated damage, and hypercoagulability. However, the precise mechanisms and long-term effects of COVID-19 on the nervous system remain unclear.7
Recognizing and managing neurological cases related to COVID-19 presents a significant challenge that requires a comprehensive understanding of the underlying pathophysiological mechanisms.4 COVID-19 has been associated with a wide range of neurological syndromes, including encephalopathies, cerebrovascular events, and neuropathies such as Guillain-Barré Syndrome, which affects the entire neuraxis, including the cerebral vasculature.8 In patients with neurological manifestations related to COVID-19, brain MRI abnormalities such as leptomeningeal enhancement and increased inflammatory markers in the cerebrospinal fluid are frequently observed. Furthermore, acute disseminated encephalomyelitis with hemorrhagic changes is highly prevalent in severe cases of COVID-19.9
Most cytochemical studies of cerebrospinal fluid (CSF) in patients infected with SARS-CoV-2 have shown abnormal results, including elevated leukocyte and protein counts.10 Given the importance of timely management of neurological complications in critically ill patients, it is crucial to assess the impact of cerebrospinal fluid analysis. Wu et al.11 have emphasized that requesting a CSF analysis in a timely manner can significantly improve patient outcomes, but further studies are needed to fully characterize the range of neurological conditions associated with COVID-19 and to identify CSF profiles and biomarkers that may help in the diagnosis of this disease.10
The objective of this study was to determine the physical, cytological, and chemical alterations of the CSF cytochemical examination of COVID-19 patients with neurological symptoms in Peru.
This study has followed the guidelines of the Helsinki Guide12 and has been approved by the Ethics Committee of the Edgardo Rebagliati Martins Hospital (N°881-GRPR-ESSALUD-2021). Based on a retrospective approach, the reference records were used to obtain only the data corresponding to the results, excluding any other information from the clinical record (for example, the names of the patients), to preserve effective confidentiality and protection of the participants involved.
This cross-sectional observational study was conducted during the first outbreak of COVID-19. The study was developed in the Clinical Laboratory of the Emergency Center of Metropolitan Lima (CELIM) of the Edgardo Rebagliati Martins Hospital in Lima, Peru. This hospital belongs to the Social Security (EsSalud) and annually receives 350 thousand patients. During the pandemic, it has become a reference hospital for the care of patients with COVID-19 with isolation centers and 24-hour care. This study followed the guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guide.13
The study population consisted of 94 CSF samples obtained by lumbar puncture from inpatient patients. The inclusion criteria were samples of patients with COVID-19 diagnosed by polymerase chain reaction (PCR) from patients of both sexes and over 18 years of age with neurological symptoms and who had a complete cytochemical analysis (physical, cytological, and chemical). Samples from pregnant patients, with cancer or HIV were excluded. Only 30 samples met the criteria, which is why they were included in the study (Figure 1).
The age and sex of the participants were obtained by analyzing the medical records, the SAGER guidelines were considered to report information about sex and gender.14 The CSF cytochemical analysis variable included physical (color and appearance), cytological (cellular characteristics), and chemical (glucose and protein) characteristics. Taking into account the reference values in CSF, for glucose from 40 to 70 mg/dl and for proteins from 15 to 45 mg/dl. The other variable was neurological disorders defined according to clinical neurological guidelines and DSM-V.15,16 CSF samples were sent to the laboratory for cytochemical examination following the recommendations of the Clinical and Laboratory Standards Institute (CLSI) H-56A and C49-A guidelines.17,18 The clinical records were obtained from the digital database in MS-Access (Microsoft, Redmond, US) of the emergency laboratory – CELIM.
The data analysis has been done in SPSS v24.0 (Armonk, USA). Data analysis was initially descriptive to estimate the frequencies of the continuous variables and the mean and the standard deviation of the categorical variables. We used paired T-test and ANOVA one-way with Bonferroni post-hoc test to determine the differences in the values of biochemical markers of CSF considering the p-value <0.05 and the confidence interval of 95% as the significant. The database containing the original data as it was collected, without having been processed or analyzed is available under Underlying data (Database.sav).19
The age range of the 30 patients with COVID-19 was 22 to 77 years (mean 52.2±14.2 years) and 17 (57%) were male. The most frequent neurological disorders were encephalopathy and brain tumor in 13 (43%) and 7 (23%) patients, respectively. Table 1 describes the demographic and clinical characteristics of the patients with COVID-19, whose cerebrospinal fluid was analyzed.
Among the most outstanding physical characteristics, 14 (47%) liquids were colorless and 21 (70%) had a transparent appearance, corresponding to encephalopathies as the most frequent neurological complication in both cases (Table 2). Brain tumors and intracerebral hemorrhage were neurological complications that frequently presented a cloudy appearance and reddish color in the macroscopic evaluation.
Glucose concentration in COVID-19 patients with encephalopathy, brain tumor, intracerebral hemorrhage, and others was 70.9±20 mg/dl (95%CI 60 to 81.8), 67.6±38.5 mg/dl (95%CI 39.1 to 96.1), 76.8±42.3 mg/dl (95%CI 43 to 110.7), and 56.5±26.0 mg/dl (95%CI 31 to 82), respectively. Among the biochemical parameters, we found that glucose in the CSF of 9 (30%) patients with encephalopathy had values greater than 70 mg/dl, 4 (13%) patients with brain tumors had glucose concentrations between 40 and 70 mg/d. Finally, only 4 (13%) patients with intracerebral hemorrhage had glucose >70 mg/dl.
Protein concentration in COVID-19 patients with encephalopathy, brain tumor, intracerebral hemorrhage, and others was 68.1±74.1 mg/dl (95%CI 27.8 to 108.3), 80.4±96.3 mg/dl (95%CI 9 to 151.7), 221.5±326.3 mg/dl (95%CI -39.6 to 482.6), and 273±271.6 mg/dl (95%CI 6.8 to 539.2), respectively. Proteins >45 mg/dl corresponded to 6 (20%) patients with encephalopathy and 5 (17%) patients with hemorrhage and brain tumor. No differences were found in glucose concentration between neurological disorders (Figure 2); however, differences were observed in protein concentration (p=0.001).
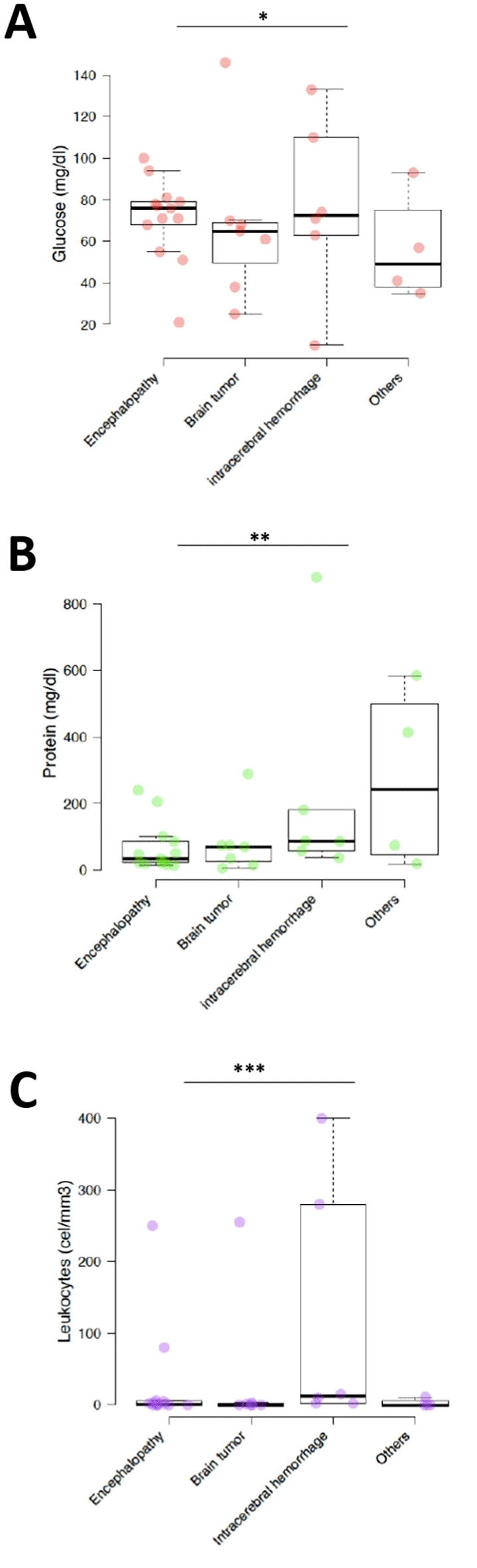
*p>0.05, **p<0.001 and ***p<0.05 (significant).
Among the cytological characteristics, the leukocyte count <5 cells/ul was found in 10 (33%) patients with encephalopathy and in 6 (20%) patients with brain tumors. Patients with encephalopathy and Intracerebral Hemorrhage had a leukocyte count >5 cells/ul which was significant (p>0.05).
This study demonstrated in a cohort of Peruvian patients with COVID-19 an increase in leukocyte count in patients with encephalopathy or intracerebral hemorrhage. In addition, the brain tumor and intracerebral hemorrhage had very marked cytochemical changes and the protein concentration varied significantly according to the neurological condition.
This is the first comprehensive Peruvian study describing the physical, cytological, and biochemical characteristics of CSF in COVID-19 patients with neurological complications. Our study addresses a critical gap in understanding the unique characteristics of CSF in this patient population and contributes to advancing the field of neurological health.20 By characterizing the CSF in patients with COVID-19, we provide valuable information that can inform and improve care processes for neurological complications associated with COVID-19. Our study also offers significant implications for laboratory professionals, providing critical information on the biochemical and cytological features of these disorders. This knowledge is essential to recognize the key cellular and biochemical changes observed in the CSF of COVID-19 patients and ensure the quality of clinical laboratory results. By providing a detailed understanding of the biochemical and cytological profile of CSF, we hope to contribute to more effective diagnosis and management of neurological complications in patients with COVID-19.
The study found that the most frequent neurological disorders were encephalopathy (43%) and brain tumor (23%). Likewise, the age group with the highest frequency of patients was between the ages of 50 to 60 years (40%) with a mean ±SD of 52.2±14.2 years and 57% were male. Some investigations such as the studies by Neumann et al.,4 Poyiadji et al.,21 and Ghosh et al.,22 indicate an association between SARS-CoV-2 infection with encephalopathies as one of the neuropathological manifestations with greater relevance in this disease. A systematic review identified 430 patients with COVID-19 presenting with neurological symptoms, with the majority (56%) having encephalopathy.
In the present investigation, no statistically significant differences were found in glucose concentration between neurological disorders (p>0.05); however, differences in protein concentration were observed (p=0.001) and the highest levels were observed in patients with intracerebral hemorrhage and brain tumors. An investigation in patients with and without neurological disease found that elevated total protein concentrations were associated with the presence of neurological disease, mental disorders, and central nervous system infections.23 On the other hand, Espindola et al.9 observed that patients with encephalopathy had a mean glucose concentration of 64.5 mg/dl in CSF and total protein of 35.5 mg/dl, finding an elevation of total protein in 29.2% of patients; however, the analysis of these analytes did not present significant differences with other neurological manifestations.
Espindola et al.9 and Keller et al.,24 described a high incidence of acute disseminated encephalomyelitis with hemorrhagic changes in these patients, in this work, Intracerebral Hemorrhage was detected as one of the three main diagnoses, this limits the possibility that a patient without any risk factor could develop the formation and rupture of an aneurysm as described in their work Al Saiegh et al.25
When comparing the results with what Jaijakul et al.,26 in their work where they indicate that 25% of patients with central nervous system (CNS) viral infections are typically characterized by lymphocytic or neutrophilic pleocytosis of the cerebrospinal fluid (CSF), we could not determine this because, in most Of the study cases, the COVID-19 patients with neurological alterations did not have more than 5 cells/ul, so the cytomorphological differential study was not performed.
Miller et al.,10 in their work mentioned that the few cytochemical studies of the CSF in patients infected with SARS-CoV-2 are mostly abnormal, results with which this work is not related, because, unlike the present work This author found elevated leukocyte counts, in terms of proteins if we had similarity in the study, finding hyperproteinorrachia in both investigations.
The study has some limitations. First, because it is a cross-sectional study, it was not possible to establish a causality criterion between the independent variables and the dependent variable. Second, the small sample size is a limitation that may limit the generalizability of the findings; because 30 samples of cerebrospinal fluid were obtained using a non-probability sampling for convenience, rigorously classified according to the eligibility criteria, now although the sample may not be very representative, it must be taken into account that it was carried out in full swing of the COVID-19 pandemic, where studies were carried out day by day so that the scientific community began to detail a wealth of knowledge that is available to date about the virus. In addition, the study does not provide information on the long-term outcomes of the patients. Despite these limitations, the study provides important information on the neurological complications of COVID-19 and highlights the need for further research in this area of cytology applied to neurology.
These findings suggest that the characteristics of CSF may differ depending on the type of neurological complication experienced by patients with COVID-19. These differences may be helpful in diagnostic and treatment decisions for these patients. However, more research is needed to fully understand the relationship between CSF characteristics and neurological complications of COVID-19.
Early recognition, investigation, and treatment of neurological diseases related to COVID-19 are crucial to improve patient outcomes and reduce disease burden. A better understanding of the neurological effects of COVID-19 is essential to optimize patient care and develop effective preventive and therapeutic strategies.
Figshare: Cerebrospinal fluid cytochemical analysis from COVID-19 patients with neurological disorders, DOI: https://doi.org/10.6084/m9.figshare.23620983. 19
This project contains the following underlying data:
- Database.sav 19
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC BY 4.0 Public domain dedication).
Figshare: STROBE checklist for Cerebrospinal fluid cytochemical analysis from COVID-19 patients with neurological disorders: a cross-sectional study’, https://doi.org/10.6084/m9.figshare.23621217.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Movement disorders, particularly Huntington's and Parkinson's disease; COVID-19 related neurological disorders; Neuroimaging.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 18 Oct 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)