Keywords
Ebastine, cocrystal, benzamide, solvent evaporation, slurry , liquid assist grinding
Ebastine, cocrystal, benzamide, solvent evaporation, slurry , liquid assist grinding
Approximately 60-70% of medicinal compounds have been classified as BCS Class II (low solubility/high permeability) or IV (low solubility/poor permeability) over the years.1
The solubility and rate of dissolution play an essential role in gastrointestinal absorption in oral drug delivery systems.2
To improve the solubility of pharmaceuticals, researchers have examined a number of techniques, including particle size reduction, solid dispersion, complexation, salt creation, self-emulsifying drug delivery systems, the inclusion of cosolvents, and cocrystal formation. Each technique has its own advantages and disadvantages.
Cocrystallization changes a compound’s molecular structure and, therefore, its physical characteristics. This alteration can be applied in an industrial setting to reduce the need for extra additives and enhance the physicochemical properties of medications, including solubility, dissolution rate, flowability, and stability.3
Like salification, cocrystallization occurs when a hydrogen bond donor group interacts with an acceptor group. The key distinction between cocrystallization and salts is that cocrystals do not result in a proton transfer between the two fragments.4
By calculating the difference between the pKa values, it can predict whether the active pharmaceutical ingredient (API) and coformer will be able to form a cocrystal. The formation of a cocrystal is predicted when the difference in pKa between the API and co-former is negative, as there is no proton transfer. On the other hand, salt formation is predicted when the difference in pKa is more than 3, as there is full proton transfer.5
The functional groups of the API and co-former engage with one another in a cocrystal by non-covalent interactions such as hydrogen bonds, van der Waals bonds, and π interactions.1
The “synthon” technique, which builds a supermolecule inside the cocrystal by using certain molecular fragments to generate “supramolecular synthons,” is the most common basis for choosing co-formers.6 According to the synthon method, certain functional groups on the drug and the co-former will be crucial in producing cocrystals. Co-formers should have complementary functional groups to those on the drug for successful cocrystallization.7
Ebastine (EB) is a selective nonsedating H1 antihistamine. It is a white crystalline powder with a molecular weight of 469.66 g/mol and a chemical structure shown in Figure 1A and B. It is poorly soluble in water and belongs to BCS class II. EB has a partition coefficient (Log P) of 6.8 and a melting point of 86°C.8,9
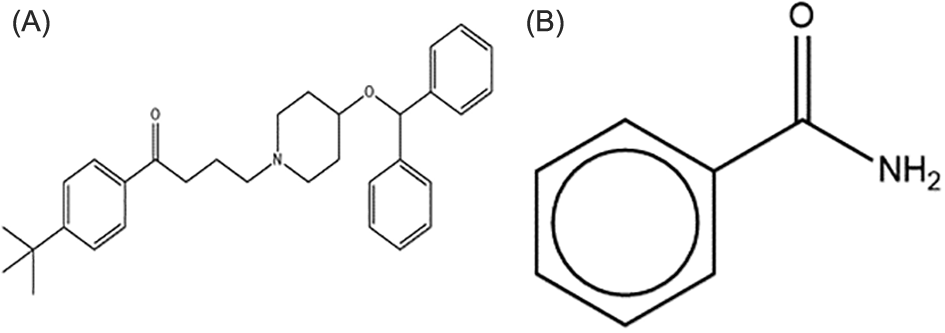
This figure is an original figure produced by the author(s) for this article.
Many trials were made to enhance the solubility of EB, including solid dispersion, spherical crystal agglomerates, and formulating microemulsion.8,10,11
This research aimed to enhance the solubility of EB by the formation of cocrystals using benzamide (BENZ) as a conformer Figure 1B.12
This study done in department of Pharmaceutics College of Pharmacy, University of Baghdad, Baghdad, Iraq in 2022.
Ebastine (EB) was purchased from Hyper-Chem LTD CO, Chin, and benzamide (BENZ) from Avonchem UK. All other chemicals used were of analytical grade.
1. pKa rule
Benzamide (Pka 14.5) was used as a conformer to prepare cocrystal with EB (Pka 8.19) since according to the “pKa rule,”
ΔpKa = pKa (acceptor (EB)) − pKa (donor (BENZ))13,14
The ΔpKa = -6.31, supposing the formation of cocrystal15
Knowing that
Where:
∆G = Gibbs free energy
HA = hydrogen donor
B = hydrogen acceptor
R = ideal gase costant
∆pK = difference in pka between drug and co-former
Thus, positive ΔpKa resembles negative and therefore prefer proton transfer from donor to acceptor means salt formation, while negative ΔpKa resembles positive and prefer cocrystal formation.16 For EB-BENZ the
3. Computational cocrystal design
The pharmaceutical cocrystal formulation process involves coformer selection, computational analysis, and characterization of cocrystals.17 The conformational flexibility of molecules and the location of their functional groups work out significantly in shaping the degree of cocrystallization. Although some of the coformers comply with the ∆pka rule and Gibbs free energy, they cannot form cocrystals with the required API, so computational design (like avocadro software) is an important step in the prediction of cocrystal formation.18,19
Computational design for cocrystal screening favors co-formers which can be engaged with API depending on whether or not they are suitable supramolecular heterosynthon.20
Depending on the above rules, BENZ was used as a co-former for preparing EB cocrystals. Since it may form H-bond with EB according to the computational cocrystal design, as shown in Figure 2.
This figure is an original figure produced by the author(s) for this article.
Three different methods, including solvent evaporation, slurry, and liquid asset grinding with different molar ratios (Table 1), were used for the preparation of EB cocrystals.
Solvent evaporation method (SE)
Three formulas (EB-BENZ1 - EB-BENZ3) were prepared by this method using 1:1, 1:4, and 1:8 (EB: BENZ) molar ratio, respectively, in which the drug and the conformer were dissolved in 20 ml of methanol with stirring (Magnetic Stirrers - Hei-Mix S from- Heidolph Instruments GmbH & Co. KG Walpersdorfer Str. 12 - Germany) for one hour at 1000 rpm.21
Slurry method
Three formulas (Eb-Benz4 - Eb-Benz6) were prepared using 1:1, 1:4, and 1:8 (EB: BENZ) molar ratios, respectively. The drug and the conformer were dissolved in 5 ml of methanol in a closed container with stirring for one hour at 1000 rpm; then, the cover was removed and left aside over night for slow evaporation of the solvent.22
Liquid asset grinding (LAG)
Three formulas (EB-BENZ7 - EB-BENZ9) were prepared by using 1:1, 1:4, and 1:8 (EB: BENZ) molar ratio, respectively, by grinding with mortar and pestle for 45 min with the addition of a drop of methanol every ten min during grinding.23–25
Using a spatula, the powders were geometrically combined in a glass mortar to prepare the physical mixture needed for the chosen cocrystal formula after prepration of the optimum formula.
Determination of percentage yield
The percentage yield of the prepared cocrystal to determine the pecent of produced cocrystal in compared with starting material was calculated by using the following equation1,26
Determination of drug content
To determin the amount of bastine in cocrystale. EB-BENZ cocrystals equivalent to10 mg EB were dissolved in 10 ml methanol with stirring for 30min, then after suitable dilution, the drug content was estimated by determining the absorbance of the resultant solution at 253 nm.11 The following equation calculated the percentage of drug content in the cocrystal
Solubility study
The solublity of each cocrystal formula and compaire with solublity of pure drug. The solubility of EB-BENZ cocrystals was determined by adding excess amounts of co-crystals in the test tube containing 10 ml distilled water placed in a water bath shaker (WNB3. From Memmert GmbH + Co. KG, - Schwabach, Germany) at 50 rpm and 25°C for 48 hr. The sample was then filtered using a Whatman filter paper, and after suitable dilution, it was analyzed by UV spectroscopy (Varian Cary 100 Bio UV-Visible Spectrophotometer, Agilent Technologies Co. Santa Clara, California. United States) at 257 nm.27 This study was done in triplicate.
In vitro dissolution study
The USP type II apparatus (paddle dissolution vessel) (Copley dissolution 8000, UK) was used to perform the dissolution testing for EB-BENZ cocrystal formulations with the highest solubility. Cocrystals equivalents to 10 mg EB were dispersed into the 1000 ml dissolution medium of 0.1 N HCl (pH 1.2).
The temperature was set at 37± 0.5°C, with the rotation speed at 100 rpm for 60 min. The amount of the released EB was measured spectrophotometrically at 257 nm.28 The results obtained from the dissolution studies were statistically validated using the similarity factor (f2).
The similarity factor fits the result between 0 and 100. f2 higher than 50 indicates the similarity of the dissolution profile, while that less than 50 indicates nonsimilar profiles.
The selection of the best formula depended on the solubility study and the dissolution profile of EB from cocrystals.
Scanning electron microscopy
Using a scanning electron microscope (VEGA3 TESCAN Co.,Warrendale, PA USA), at 500× magnitude the surface morphology of the produced cocrystals.29,30
Fourier transform infrared spectroscopy
The Fourier transform infrared spectroscopy (FTIR) spectra of EB and BENZ selected formula, and its physical mixture was determined using an FTIR spectrometer (FTIR-8300 Shimadzu, Japan). The samples were scanned between 4000–400 cm-1.1,31
Raman spectroscopy
A Raman spectrometer (BRUKER - Raman apparatus (Germany) was used with a spectral range of 3500–50 cm–1. This test was done to detect the interaction between the drug and the conformer quantitatively and qualitatively.32,33
Differential scanning calorimetric
The thermodynamic characteristics of EB, BENZ, the selected formula, and its physical mixture were measured using a DSC-60 plus apparatus (Shimadzu, Japan).30
Powder X-ray diffraction
A powder X-ray diffraction (PXRD) study was performed to evaluate changes in the crystalline nature of the drug.and to detect the formation of a new crystalline form.34,35 By using an X-ray diffractometer (XRD-6000 Shimadzu, Japan) Under these conditions, tests were conducted: filter K, target metals Cu, 45 kV voltage, and 30 mA current. Samples were scanned across a 2 range of 10-90°C with a 0.04° step size.
The results were analyzed by one-way (ANOVA) test using SPSS Statistic version 26.
Cocrystals prepared by different methods produce high PY, good drug content and enhanced solubility by 347 fold in distilled water with enhanced dissolution. The FTIR and Raman spectroscopy showed the possibility of hydrogen bond formation between the drug and the coformer, while the PXRD and DSC results confirmed the formation of new crystal lattice.
Percentage of yield
A high percentage yield was obtained from all the cocrystal formulas that ranged between 88-97%, as shown in Table 2, indicating that all methods were efficient.52
Drug content
The percentage drug content of all formulas was in the range of 95%-102% Table 2. indicated that there was a minor loss of drug throughout the cocrystallization process.
Solubility study
The results of solubility are shown in Table 2. It was found that there was a significant increase p < 0.05 in the solubility of EB by preparing it as cocrystals which were increased as the ratio of drug: conformer increased.36
This result can be attributed to the properties of cocrystals which are believed to feature a mechanism that promotes solubility by changing the lattice and solvation energies and by increasing the solvent affinity due to the presence of coformer.37,38
On the other hand, it was found that the solubility of EB was not significantly enhanced p > 0.05 by using the same ratio in preparing the cocrystals by the different methods, indicating that the coformer rather than the method influenced the Solubility of EB.
Dissolution study
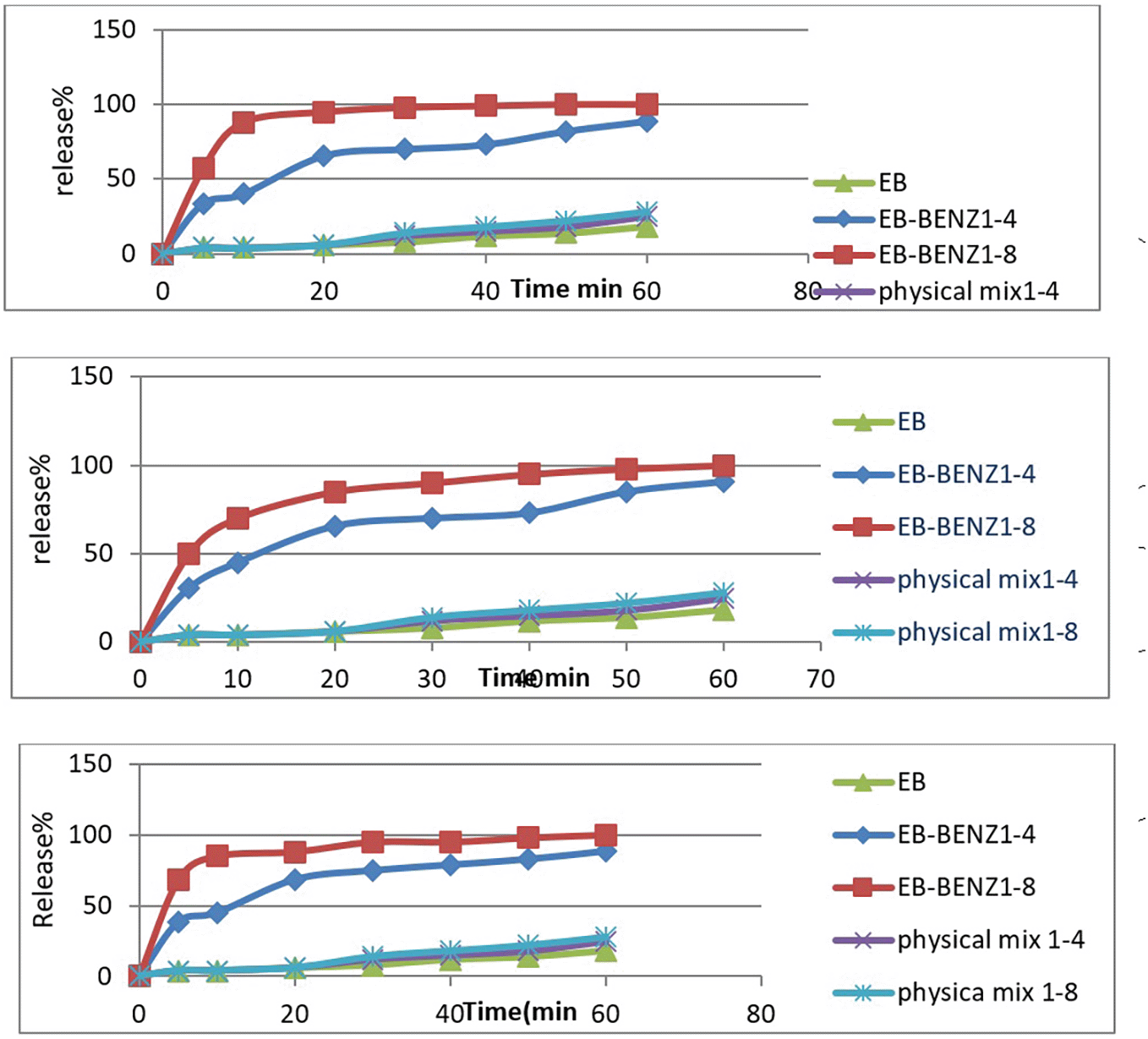
In the present study, all formulas were dissolved to determine the effect of the conformer ratio and the preparation method on the dissolution profile of EB. Figure 3 and Table 3 show that the release of all formulas was nonsimilar, faster than pure drug and their physical mixture.
The increased dissolution rate of the prepared cocrystals can be attributed to the increased solubility of EB. The result can be explained by Noyes and Whitney equation,
The EB-BENZ3 prepared by a solvent evaporation method using 1:8 EB: BENZ was selected as the best formula.
Morphology
SEM (Figure 4) scans revealed the change in the surface morphology of cocrystals compared to the pure EB and BENZ. Crystal habit of EB-BENZ3 showed rod-shaped irregular particles with smooth surface morphology.

SEM (a), EB (b), BENZ (c), EB-BENZ3 cocrystal.
Fourier transform infrared spectroscopy
FTIR spectroscopy is an important spectroscopic technique in determining the interaction between the drug and the coformer.
The typical IR absorption peaks of EB (Figure 5a) are 1269 cm-1 (C-N stretch), 1450 cm-1 (C=C stretch), 1678 cm-1 (C=O stretch) and 3053 cm-1 (C-H stretch) which were in accordance with documented results.42
The typical IR absorption peaks of BENZ (Figure 5b) are 3363.86 cm-1,3167.12 cm-1 (NH) stretching vibrations, the primary amide scissoring peak is seen at 1620.21 cm-1,1651.07 (C=O stretch) and 1396.46 cm-1 (C-N stretches). These results were in agreement with previous studies.43
The N–H group in BENZ is identified as a hydrogen donor group. While the oxygen (carbonyl) in EB and is considered as hydrogen acceptor, this peak was disappeared from the spectra of EB-BENZ cocrystals (Figure 5d). This result indicated the involvement of this group in hydrogen bond for cocrystal formation.44–46
Raman spectroscopy
Raman spectra are shown in Figure 6. EB has a characteristic peak at 1031 cm-1 for C-O-C stretching, 1067 cm-1 for C-N-C stretching and 1676 cm-1 for C=O stretching and 1600 cm-1 for the aromatic ring (Figure 6a). The results were in agreement with previous studies.47
Major bands of the Raman spectra of BENZ at 1000 cm-1 for in-plane C-H,1142 cm-1 NH2 rocking mode, 1600 cm-1 for C-C ring stretching mode and 1,685 cm-1Amide (Figure 6b). The results were following the documented values.33
The Raman spectral results showed that the C=O stretch for EB disappeared, while the amid band for BENZ strongly overlapped and shifted to 1650 cm-1, corresponding to proton vibrations. These changes were due to multiple hydrogen bond formation (Figure 6d)48
This confirms that the cocrystal is not simple hydrogen bonding between the individual starting materials, but multiple hydrogen bonds resulting from the interaction between one BENZ molecule with one EB molecule and between BENZ molecules that form a series around the EB molecule, which forms a completely different lattice phase.49
Differential scanning calorimetry
The DSC of EB shows a sharp endothermic peak at 87.97°C, while that of BENZ shows a sharp endothermic peak around 130.9°C, representing their melting points as shown in Figure 7a and b, respectively.
The thermogram of the physical mixture (Figure 7c) shows the sharp endothermic peak for each component at nearly the same position, indicating that the crystalline form of each component was preserved. The slight decrease in the intensity of these peaks may be due to dilution. EB-BENZ3 cocrystals show sharp endothermic peaks appearing at 87.5°C (shifting by 0.5°C from that of EB) and 129.21°C (shifting from BENZ main peak by 1.7°C) (Figure 7d). These slight differences in the melting point of cocrystals compared to the melting point of the starting component do not exclude the possibility of cocrystal formation. This result was the following results obtained by Saganowska P et al.50
Powder x-ray diffraction
Each crystalline form of a drug has a characteristic PXRD pattern. The diffractograms of EB, BENZ, and EB-BENZ3 and their physical mixture are presented in (Figure 8). The major diffraction peaks of EB are shown at 2θ of 16.8°, 18.5°, 23.5°, 33°, 37°, 40°, 48° and 50° with high intensities as shown in Figure 8a, while the major diffraction peaks of BENZ are shown at 2θ of 15°, 23°, 26°, 28° and 36° as shown in Figure 8b. These results were in agreement with previous studies.26,43
Moreover, the PXRD of EB-BENZ3 showed a new intense peak at 2θ of 12° (Figure 8d). This result indicated the formation of a new crystal lattice.35,51 This peak was also found in the physical mixture (Figure 8c) but with lower intensity compared to that found in the diffractogram of the selected formula, indicating the possibility of formation of cocrystals even by simple mixing.44
Cocrystal is a promising approach to modify the poor solubility and dissolution of EB using BENZ as a coformer.
It has been confirmed by FTIR and Raman spectroscopy that EB interacts with BENZ to form cocrystals by hydrogen bonding. These cocrystals exhibited different crystal lattices, as identified by DSC and PXRD studies.
Zenodo: supplementary data Designing and Evaluation of Ebastine –Benzamide Cocrystals. https://doi.org/10.5281/zenodo.7544700.52
This project contains the following underlying data:
- grinding.xlsx
- slurry.xlsx
- smilarity test for differant method.xlsx
- solvent evapo.xlsx
- all formula 2.spv (contain Solubility analysis by spss of All formula)
- benzamid formula.sav (Solubility analysis by spss of benzamide formula)
- the supplemantry (2).docx (contain the following
○ Fig (1) Ebastine chemical imaging by raman spectroscopy
○ Fig (2) benzamide chemical imaging by raman spectroscopy
○ Fig (3) Ebastine- Benzanide (1_8) molar ratiophysical mixture chemical imaging by raman spectroscopy
○ Fig (4) Ebastine- Benzanide (1_8) molar ratio cocrystal chemical imaging by raman spectroscopy
○ Fig (5) SEM of ebastine
○ Fig (5) SEM of benzamide
○ Fig (6) SEM of Ebastine- Benzanide (1-8) molar ratio cocrystal
○ Fig (7) ebastine structure by Avogadro software
○ Fig (8) BENE structure by Avogadro software
○ Fig (9) EB-BENZ cocrystal (1-8) molar ratio
○ Fig (10) release profile of cocrystal in EB-BENZ (1-4) molar ratio in a different method
○ Fig (11) release profile of cocrystal in EB-BENZ (1-8) molar ratio in a different method
○ Fig (12) optical microscope (a)EB(b) BENZ(c)EB-BENZ3(SE)(d)EB- BENZ6slurry(e) EB-BENZ9(LAG)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Eltobshi A, Sultan A, El Maghraby G: Hydrotropy and co-crystallization for synergistic enhancement of dissolution rate and in vivo anti-inflammatory efficacy of ebastine. Journal of Drug Delivery Science and Technology. 2025; 104. Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: co-crystalization for enhancement of dissolution rate
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 08 Nov 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)