Keywords
PNET, Sinonasal tract, Cytodiagnosis
This article is included in the Datta Meghe Institute of Higher Education and Research collection.
Malignant sinonasal tumors are rarely encountered in the practice of oncology as they constitute only 1% of total body tumors. The occurrence of primitive neuroectodermal tumor (PNET) in the sinonasal tract is even more uncommon. The PNET belongs to Ewing sarcoma family and forms the differential diagnosis of a large group of lesions generally described as malignant round cell tumors. The unique pathogenetic chromosomal alteration in this lesion is the EWS FLI1 fusion gene. This case report describes the unusual occurrence of PNET for its cytomorphological diagnosis along with its clinical features, radioimaging findings and immunohistochemistry. Such case reports are scarce in literature and cytodiagnosis of PNET in sinonasal tracts warrants its sharing with medical fraternity as such diagnoses are stumbled along rarely in clinical practice.
PNET, Sinonasal tract, Cytodiagnosis
A primitive neuroectodermal tumor (PNET) of the sinonasal tract is one of the rare malignant tumors of the sinonasal tract.1–3 Therefore, it remains clinically unsuspected whenever the swelling within the maxilla or over the palate is noticed. The literature search on Google Scholar yielded very few cases of PNET of paranasal sinus. Most of these cases were diagnosed on histopathological examination.3–6 The diagnosis of PNET of sinonasal tract by fine needle aspiration cytology (FNAC) has been reported but very rarely.1,7
The malignant sinonasal tumors constitute about 1% of the total body tumors and around 3-5% of the head and neck cancer.1 In children and adolescents, malignant sinonasal tumors are uncommon and diagnosis is delayed. This is due to nonspecific yet confusing clinical presentation and plethora of radio-imaging diagnosis.2,3,8,9
The PNET that belongs to the Ewing sarcoma family shares common neuroectodermal origin. These tumors exhibit a primitive morphology of small round cell and are highly malignant. It also has unique pathogenetic chromosomal translocation t(11:22) (q24;q12) leading to formation of EWSR1-FLI1 fusion gene protein.10
The reported cases of PNET are rare, as are the reports of FNAC of sinonasal PNET. The reports of FNA diagnosis of PNET in sinonasal tract are still more rare.
The following report describes the clinical and radiological findings and the cytodiagnosis of PNET by FNAC, as well as its histopathological appearances. Its immunohistochemistry (IHC) is also described in present case report of 15-year-old female.
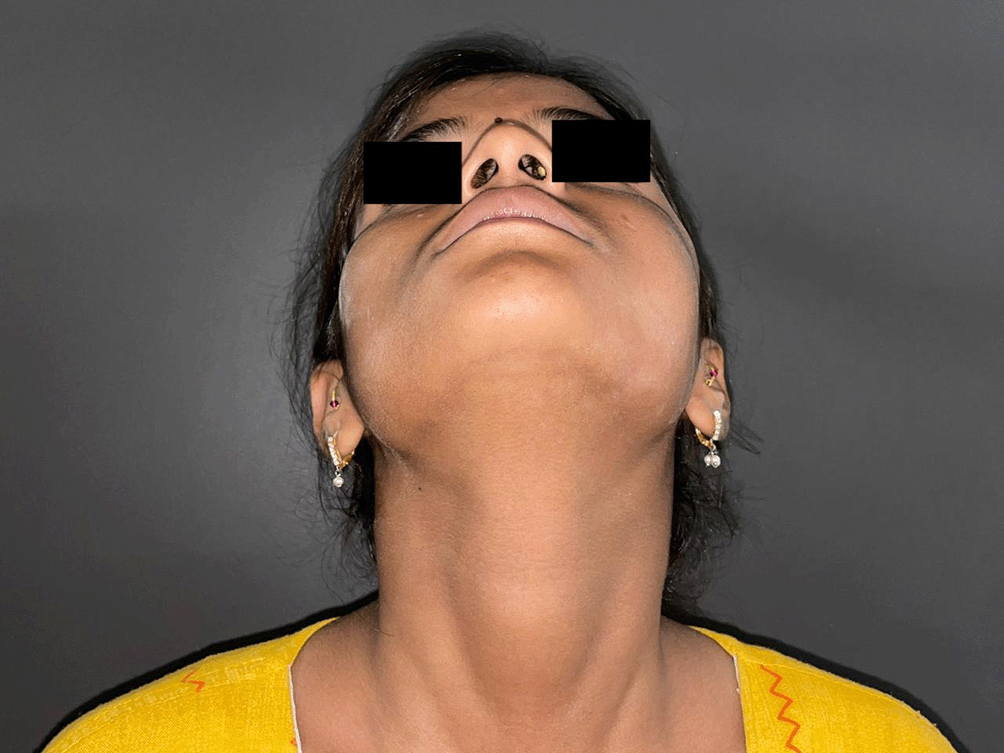
A 15-year-old girl presented to the outpatient department of oral surgery with complaints of swelling on the left side of face and bulge on the hard palate that caused in difficulty in eating and swallowing. The patient had no family history of any of their close relatives suffering from tumors of the sinonasal tract, maxilla or mandible. The patient also complained of pain of moderate intensity at the swelling and surrounding area. The swelling had gradually increased over a period of 6 months. She complained of loosening of the last of her three left molar teeth in a span of 6 months. She also complained of pain at the floor of the orbit which was producing difficulty in her vision (Figures 1, 2). She had no history of medications used to treat the illness. She also complained of watery discharge from the left nostril for past 15 days. She was provisionally diagnosed with an odontogenic tumor of the left maxilla. She was referred to the Department of Radiology for the CECT of the head in assessment of suspected tumour.
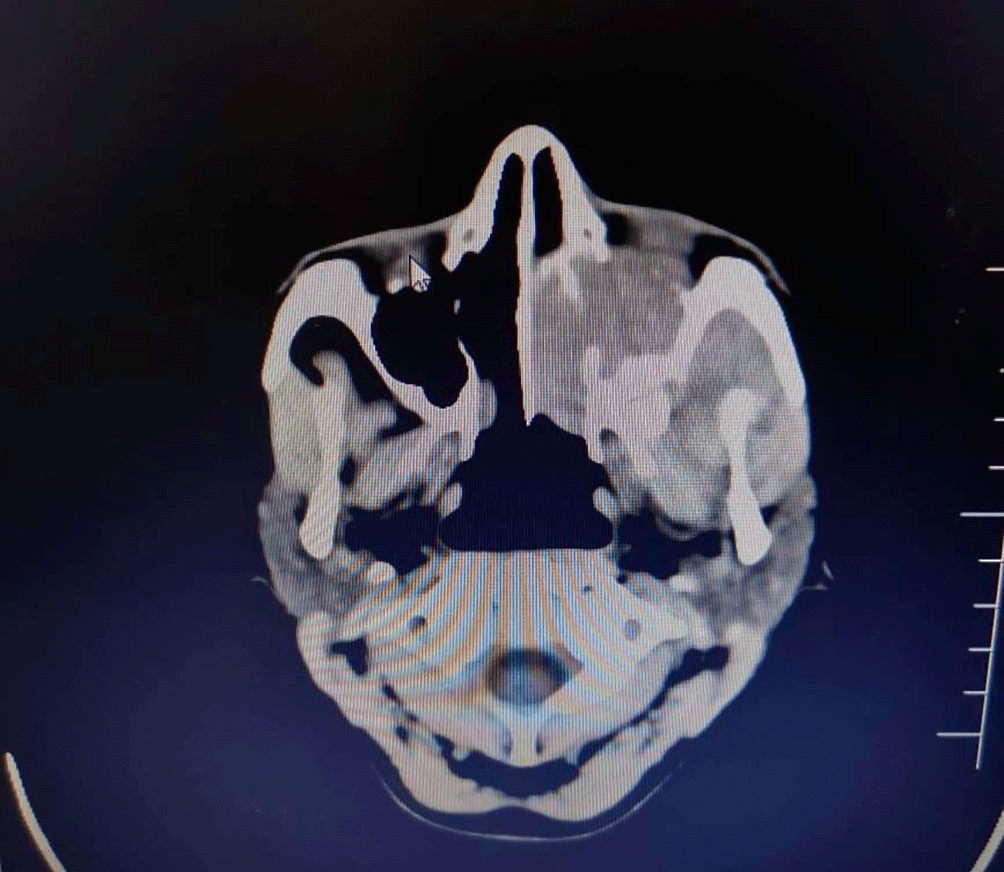
The CECT showed minimally enhancing soft tissue density lesion in left maxillary sinus with extension into masticator space and upper gingivobuccal space. The mass was also eroding alveolar ridges of the maxilla and left orbital cavity causing abetment of inferior and lateral rectus muscle. The brain parenchyma wasn’t affected and no focal lesions were located in it.
The differential diagnoses on the CT examination were of maxillary sinus mucocele or aggressive neoplastic lesion of maxilla.
The patient underwent haematological investigations and coagulation profile before conduction of FNAC procedure. Her haematological profile was normal except for high ESR and decreased value of Hb g/dl.
This report and CT prompted the surgeons to refer the patient for guided FNAC of the mass lesion in left maxilla (Figure 3).
The FNAC was carried out percutaneously from left facial swelling under sonographic guidance after ascertaining minimal distance between skin and tumor mass.
The aspirate of swelling was blood mixed and sticky. The part of the aspirate was prepared into smears. The other part of it was processed to cell block/tissue block after formalin fixation.
The smears were wet fixed in 95% absolute alcohol and later stained by Papanicolaou stain and Hematoxylin and Eosin stain. The dry smear underwent Giemsa stain.
The stained smears from the mass lesion showed hypercellularity of small cells with cellular monomorphy. Smears dominantly showed round cell population which was placed diffusely in perivascular forms and small nodular aggregates along with few attempted pseudorosettes. Cells carried a high N:C ratio. Nuclei were hyperchromatic and were mildly pleomorphic. Chromatin was microvesicular and there were sparse inconspicuous nucleoli. The cytoplasm was absent to scant and showed vacuoles in few. Background showed smudged nuclei. There were light-stained and dark-stained nuclei within the nodular aggregates. The scattered lymphocytes and a granular blue material to was evident in the background.
The cytodiagnosis of malignant small round cell tumor with subtyping as primitive neuroectodermal tumor was made (Figures 4, 5, 6).
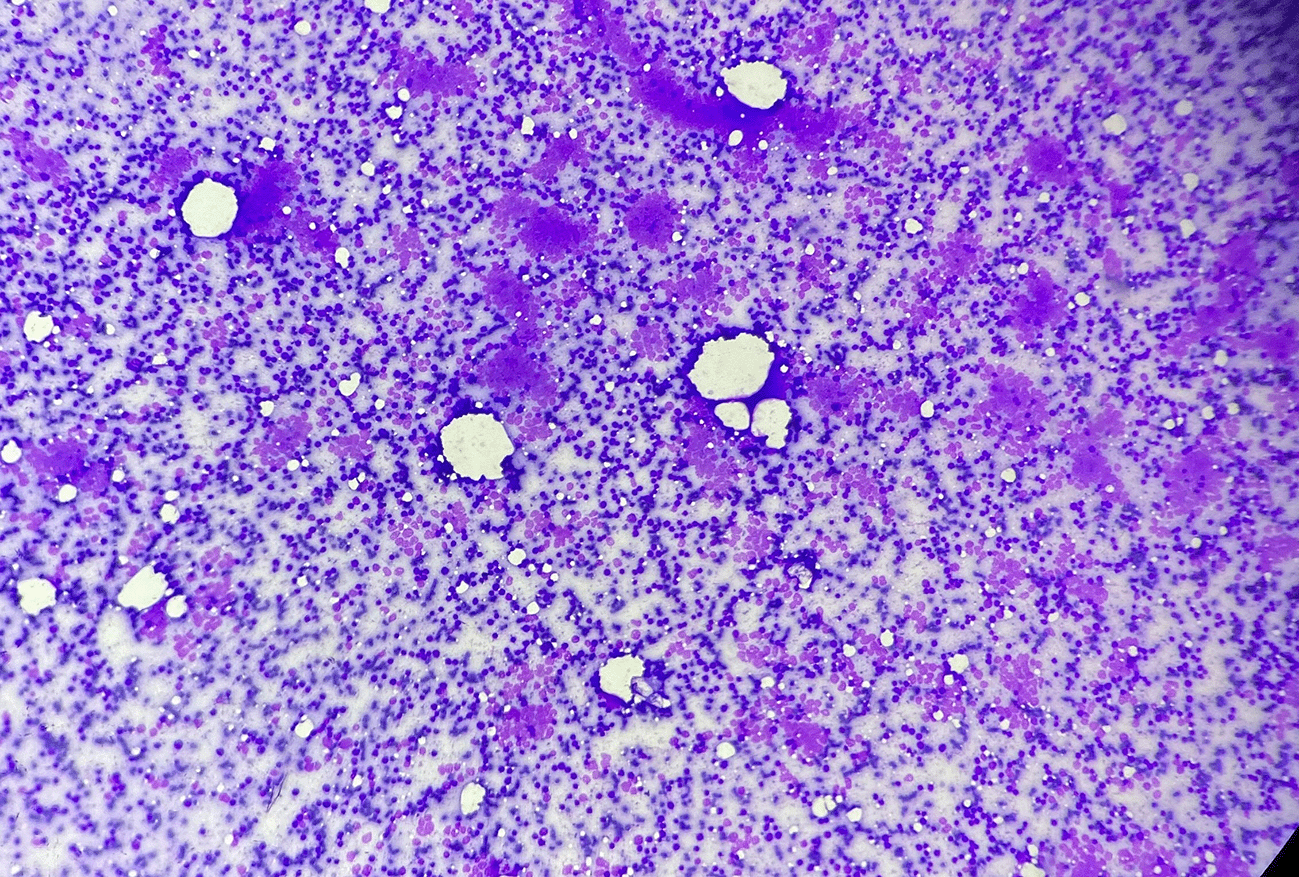
Background lacks lymphoglandular bodies (MGG, 4×).

A few pseudorosettes structures are evident, light and dark nuclei along with frequent nuclear smudging (Papanicolaou Stain, 40×).
The part of the aspirate which was processed as a biopsy in a cell block/tissue block preparation underwent paraffin block sections. The sections were stained by haematoxylin and eosin stain. The morphologic features observed were consistent with features of PNET. The special stain of IHC for PAS, S100 protein and NSE was carried out.
The patient underwent the appropriate treatment of radiation therapy and chemotherapy (vincristine, doxorubicin and cyclophosphamide). The surgery was deferred due to extensions of the tumor that may produce extensive mutilations; at time of writing, the surgery has not yet occurred. The patient remained in follow up for three months. The assessment of the tumor at the end of three months in post chemotherapy and radiotherapy treatment revealed the improvement in clinical parameters and radio images.
The incidence of PNET in paranasal sinuses extending into the maxilla and orbital bones is low. Therefore, it’s mostly reported in literature as case reports.1,11–14
In previous studies, the patients suffering of PNET of paranasal sinus and maxilla were in the paediatric and adolescent age groups.1,4,8 The diagnosis of PNET in paranasal and maxillary area is rare.1,3,5 The incidence of PNET in the paranasal sinuses and maxilla is common in the first and second decades of life as the literature mentions.1,4,8
The presently reported case was of a 15-year-old patient, which is similar to the age range reported for PNET. The present case also showed the involvement of orbit in the disease process, similar to those described by other authors.11–14
The diagnostic cytomorphology of PNET in the sinonasal tract and maxilla has been described by Singh et al.7
The cytomorphology of PNET is no different from the cytomorphology of the Ewings Sarcoma as reported in a few case reports.15–17
FNAC of the present case showed the cellular smears with dominant small cell monomorphy. The cells were dominantly round and showed the placements like solid groups with central cellular heaping, pseudorosettes and perivascular placements. The cells were also lying isolated in the background devoid of cytoplasm. The individual cells were carrying hyperchromatic nuclei with a high nuclear: cytoplasmic ratio. The chromatin of the nuclei was microvesicular in many. The mild pleomorphism too was noticed. The cytoplasm of the cell was scanty with blue rims and in many places the cytoplasm showed the vacuoles.
The nuclei showed easy fragility. The background showed the structureless nuclear-smudged material. The background appeared bluer in the Giemsa-stained preparation. Some these features were commonly cited in case reports published by authors for diagnosis of PNET.7,15
The cytodiagnosis of PNET was confirmed on histopathological examination in the present case. The paraffin section of the tumor tissue also underwent the histochemistry of PAS stain which was positive for it. The IHC on the tissue sections for CD99 was performed and it also showed the positive results. IHC analysis of PNET shows positive immunoreactivity for CD99, vimentin, S100 protein and NSE has been reported in a few studies6,11
The PNET belongs to the Ewing sarcoma family of tumors and is a group of undifferentiated small round blue cell known to be of neuroectodermal origin. The consistent molecular pathology associated with PNET is of EWS FLI1 translocation. This translocation stimulates oncogenesis that synergizes the events of tumor development and progression.10
The reports of PNET in the sinonasal region and maxillary sinus are rare.3,4,5,8,15
The described case in the present report is an uncommon clinical encounter. The occurrence of PNET of the sinonasal tract in a teenager has rarely been reported in the literature. The FNA diagnosis of PNET at this unusual place is another rarity.
The cytodiagnosis of PNET by FNAC in the sinonsal tract has not been reported very frequently. Therefore this report stands on the value of its rarity and clinical appearance. The material obtained at FNAC holds a good option for histopathological examination by cell block when it is in enough quantity. This avoids additional biopsy procedures of such vascular tumor masses.
This case offered a comprehensive spectrum of its clinicoradiological presentation, cytomorphological evaluation, histopathological examination, and IHC. If clinicoradiological information is adequate then the diagnosis of PNET can be easily achieved by cytology with exclusion of the differentials of malignant small round cell tumors.
Informed consent for publication of their clinical details and clinical images was obtained from the patient’s parent. The patient gave her assent for the publication.
All data underlying the results are available as part of the article and no additional source data are required.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)