Keywords
cotinine, blood cotinine levels, malondialdehyde, glutathione, smokers, safe work
cotinine, blood cotinine levels, malondialdehyde, glutathione, smokers, safe work
Smoking is a serious public health problem. Smoking has been implicated as a significant risk factor in the cause and progression of several diseases. Active smoking is a significant cause of preventable disability and premature death in humans (Groppetti et al., 2023). Data from the World Health Organization (WHO) showed that in 2020, 22.3% of the global population used tobacco, 36.7% of all men, and 7.8% of the world’s women. More than 80% of the world’s 1.3 billion tobacco users live in low- and middle-income countries, where the burden of tobacco-related illness and death is greatest (WHO, 2022). Tobacco use contributes to poverty by redirecting household expenditure away from basic needs such as food and shelter to tobacco. The negative effects of cigarette smoking on physical health are well known. There is no safe exposure to smoking. Smoking can cause various types of cancer, heart disease, stroke, lung diseases, diabetes, and chronic obstructive pulmonary disease (COPD) (CDC, 2020).
Nicotine is one of the most important ingredients in tobacco. The nicotine concentration is approximately 5% per 100 grams of tobacco weight. Nicotine has a strong and stimulant effect on the human body. Nicotine has addictive and psychoactive effects that lead to pleasure, anxiety reduction, tolerance, and physical attachment (Prihatningtias et al., 2022). After nicotine enters the body (blood flow), these substances will go to the brain and circulate throughout the body (McEwan et al., 2022). Nicotine has a plasma half-life of approximately 30 min (González et al., 1996) and is quickly converted to its major metabolite, cotinine. Cotinine is a metabolite of nicotine with concentrations much higher than those in the blood. Also, cotinine has a longer half-life than nicotine, ranging from 10 to 30 hours (González et al., 1996).
Various harmful chemicals contained in cigarettes have a negative impact on health, especially cotinine, which is often used as a biomarker of nicotine levels in the body. Research conducted by Suryadinata and Wirjatmadi (2020) states that when cigarette smoke enters the airways, this condition may decrease the expression of superoxide dismutase and glutathione, which represents a decrease in the body’s antioxidant activity, and may increase the expression of malondialdehyde. Increased malondialdehyde expression suggests that excess free radicals limit the ability of antioxidants to inhibit oxidation, while oxidative stress is also positively correlated with lipid peroxidation as a mechanism of tissue damage (Suryadinata & Wirjatmadi, 2020).
Several studies found that smoking exposure can cause oxidative stress. Research by Muthumalage et al. (2018) shows exposure to cigarettes smoking can cause oxidative stress and damage lung tissue. Research by Shields et al. (2022) showed that exposure to cigarette smoking can increase reactive oxygen species (ROS), which is a free radical body. A free radical is a molecule that has lost an electron from its lone pair, allowing it to react with other molecules around it. Imbalance between free radicals with deep antioxidants body can cause oxidative stress such as cell and tissue damage. One of the markers of free radicals is malondialdehyde and glutathione. Research by Solak et al. (2005) and Bangsa, Retnoningrum and Bhima (2019) shows increased levels of malondialdehyde and decreased levels of glutathione in smokers compared to non-smoker cigarette users. Therefore, the objective of this article was to conduct a meta-analysis to evaluate the relationship between blood cotinine levels, glutathione levels, and malondialdehyde levels in smoker.
The research method used was meta-analysis and was developed according to the criteria proposed by the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (Tualeka et al., 2023). To find relevant articles, we conducted a comprehensive literature search on electronic databases, including PubMed (RRID:SCR_004846), Science Direct, DOAJ - Directory of Open Access Journals (RRID:SCR_004521), ResearchGate (RRID:SCR_006505) and Google Scholar (RRID:SCR_008878) databases for cross-sectional and case-control studies to identify all relevant studies on the relationship between blood cotinine levels, glutathione levels, and malondialdehyde levels in smokers from March 03, 2023 to April 30, 2023. The following keywords were used: “Cotinine” OR “Blood cotinine” AND “Glutathione” AND “Malondialdehyde” AND “Smokers”. Our research was limited to studies involving humans reported in English. We also reviewed reference lists based on the recognized literature to find additional eligible studies.
The process in this article involved identifying specific criteria for including or excluding studies in a systematic review or research study. These criteria were based on the components of Problem, Intervention, Comparison, Outcome, and Study (PICOS). The studies were grouped for the syntheses based on the following criteria:
1. Problem: The relationship between cotinine levels in the blood with glutathione, and malondialdehyde levels of smokers.
2. Intervention: Smoking.
3. Comparison: Non-smokers or control group.
4. Outcome: Cotinine levels, glutathione levels, and malondialdehyde levels.
5. Study: Systematic reviews and meta-analyses that investigated the relationship between cotinine levels in the blood with glutathione, and malondialdehyde levels of smokers.
Articles published in 2003-2023 were carefully examined, and there were no country restrictions in our study. Studies qualified for inclusion met the following criteria: 1) Articles were original research; 2) articles discussing the relationship between blood cotinine levels, glutathione levels, and malondialdehyde levels; 2) open access articles; 3) participants were classified as smokers and non-smokers; and 4) blood cotinine levels, glutathione levels, and malondialdehyde levels were reported as the outcomes. Accordingly, studies were excluded based on the following criteria: 1) Articles with qualitative research type; 2) reported association without any retrievable data; 3) variable measurement parameters were saliva, urine, and hair; 4) duplicate data reported; and 5) animal studies.
This article used a two-stage screening process to decide whether a study met the review’s inclusion criteria. The first stage involved screening the titles and abstracts of all records retrieved from the electronic databases. The second stage involved screening the full-text reports of the potentially relevant studies identified in the first stage. One author independently screened each record and each report retrieved.
The inclusion criteria for the review were studies that investigated the relationship between cotinine levels in the blood with glutathione, and malondialdehyde levels of smokers. The exclusion criteria were studies that did not meet the inclusion criteria, studies that were not published in English, and studies that were not conducted on human subjects. Mendeley Data (RRID:SCR_002750) was used to manage the references and to screen the studies. The studies that met the inclusion criteria were grouped for the syntheses based on the PICOS design, as described above.
The following data were extracted from each eligible study using Microsoft Excel (RRID:SCR_016137) included in the review: last name of first author, year of data publication (2003-2023), country of the population, sample size, cotinine levels, glutathione levels, and malondialdehyde levels. We extracted data from the six articles meeting eligibility criteria for this research.
This article sought data on the following outcomes: i) Cotinine levels in blood; ii) glutathione levels in blood; and iii) malondialdehyde levels in blood. This article sought data on the following variables:
• Participant characteristics: the studies included in the review investigated the relationship between cotinine levels in the blood with glutathione, and malondialdehyde levels of smokers. The participants were smokers and non-smokers, and some studies validated smoking status based on serum cotinine levels.
• Intervention characteristics: the intervention was smoking, and some studies investigated the effect of smoking reduction or cessation on the plasma levels of glutathione.
This article does not provide information about the methods used to assess the risk of bias in the included studies. Based on the Cochrane (2011) handbook, the risk of bias assessment can only be performed when there are at least 10 studies included in the meta-analysis, whereas in this article there are only six studies included in the meta-analysis.
Mean differences (MDs) with a 95% confidence interval were used to assess the relationship between blood cotinine levels, glutathione levels, and malondialdehyde levels in smokers. Pooled MDs were calculated for this research.
To determine which studies were eligible for each synthesis, eligible studies must have used blood samples to measure cotinine levels. The studies must have investigated the relationship between cotinine levels and glutathione or malondialdehyde levels in smokers. The results must have been presented as mean differences. This study presented the results in a table. The table presents the mean and standard deviation of cotinine levels (ng/ml), glutathione levels (μM/ml), and malondialdehyde levels (nmol/ml) in smokers and non-smokers, and sample size
To perform the meta-analysis, a specific model was selected to aggregate the results from the included studies. The specific model uses a random effects model. The random-effect model was used when the I2 statistic was 50% or more indicating substantial heterogeneity among the included studies. To identify the presence and degree of statistical heterogeneity among studies statistical tests such as the Cochran’s Q test and I2 statistics were used to assess the degree of variability between study results. Additionally, the software used to carry out this study was RevMan (RRID:SCR_003581), specifically (RevMan Cochrane version 5.4, London, UK) to calculate and reported the pooled effect sizes as mean differences between the groups being compared.
Pooled MDs were determined by Z test. A p-value less than 0.05 indicates statistically significant data. The Cochrane Q test was used to assess heterogeneity of these statistical data. Statistical heterogeneity was assessed using the I2 statistic: if the value is I2 less than 50%, then this meta-analysis uses a fixed effect model; if value I2 is 50% or more, so this meta-analysis uses a random effect. An estimate of potential publication bias was performed using a funnel plot. An asymmetric plot suggests possible publication bias. In this study, the distribution of data is heterogeneous so it uses the random-effect model. This study was not tested for publication bias because the number of data in the meta-analysis was less than 10 articles.
A flow diagram summarizing the process of study selection is shown in Figure 1. A total of 400 potentially relevant articles were identified using our search strategy from databases. Overall, 331 studies remained after ruling out duplicates. After screening the titles and abstracts, we excluded 40 articles that did not satisfy the inclusion and exclusion criteria. After scanning the whole main body of the reserving 21 records, another 14 articles were excluded. Five other studies (Ambad et al., 2021; Nsonwu-Anyanwu et al., 2019; Pawar, 2019; Rasheed & Al-Rubayee, 2012; Safyudin & Subandrate, 2016) were also excluded because of insufficient publicly available data. Six studies were not included in the analysis because they used different parameters i.e., saliva (Balasubramaniam & Arumugham, 2022; Begum et al., 2018; Neshat et al., 2020) or urine (Hackett et al., 2003; Oh et al., 2022; Zandy et al., 2020).
Finally, six qualified articles met the eligibility criteria for this meta-analysis. Table 1 presents the baseline characteristics of the six studies included in our analysis.
| First Author/Year | Country | Sample size | Cotinine levels (ng/ml) | Glutathione levels (μM/ml) | Malondialdehyde levels (nmol/ml) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Smokers | Non-smokers | Smokers | Non-smokers | Smokers | Non-smokers | Smokers | Non-smokers | ||
| (Chelchowska et al., 2011) | Poland | 60 | 80 | 413.6 ± 181.1 | 25.0 ± 4.08 | - | - | 3.6 ± 0.7 | 3.1 ± 0.7 |
| (Kim et al., 2003) | Korea | 19 | 19 | 295.6 ± 57.5 | 14.3 ± 3.0 | 0.026 ± 0.001 | 0.029 ± 0.001 | 3.4 ± 0.2 | 2.1 ± 0.1 |
| (Nsonwu-Anyanwu et al., 2019) | Nigeria | 50 | 40 | 77.87 ± 51.55 | 0.83 ± 1.12 | 12.66 ± 3.15 | 13.87 ± 3.36 | 32.38 ± 20.7 | 11.7 ± 6.97 |
| (Khan et al., 2019) | Iraq | 26 | 25 | 83.76 ± 26.36 | 0.44 ± 0.12 | - | - | 34.9 ± 4.83 | 35.57 ± 2.09 |
| (Mahrous, 2019) | Egypt | 40 | 20 | 13.9 ± 5.26 | 1.47 ± 1.06 | - | - | 96.69 ± 25.22 | 28.24 ± 24.82 |
| (Colsoul et al., 2023) | Belgium | 138 | 83 | 17.1 ± 12.1 | 55.0 ± 55.0 | 122.06 ± 98.76 | 131.66 ± 98.75 | 11.3 ± 7.4 | 6.5 ± 4.9 |
Table 2 shows a summary of the individual studies.
| Author | Title | Sample size | Result | Conclusion |
|---|---|---|---|---|
| (Chelchowska et al., 2011) | The effect of tobacco smoking during pregnancy on plasma oxidant and antioxidant status in mother and newborn | 140 healthy pregnant women Smokers (n = 60) Non-smokers (n = 80) |
| |
| (Kim et al., 2003) | Influence of Smoking on Markers of Oxidative Stress and Serum Mineral Concentrations in Teenage Girls in Korea | Female senior high school students (15–17 years old) in a rural community in Korea. Smoker (n = 19) was defined as a person who had smoked 10 or more cigarettes/day continually for at least 1.5 years Non-smoker (n = 19) was a person who had no previous smoking experience. |
| Smoking is associated with increased oxidative stress and decreased antioxidant capacity in teenage girls, which may have negative health consequences. |
| (Nsonwu-Anyanwu et al., 2019) | Cigarette Smoke and Oxidative Stress Indices in Male Active Smokers | 90 consenting male subjects aged 18–60 years old Smokers (n=50) Non-smokers (n=40) |
| Cigarette smoke is associated with increased oxidative stress and decreased antioxidant capacity in male active smokers. |
| (Khan et al., 2019) | Systemic biomarkers of inflammation, oxidative stress and tissue injury and repair among waterpipe, cigarette and dual tobacco smokers | Smokers (n=26) Non-smokers (n=25) | Smoking is associated with elevated levels of biomarkers of inflammation, oxidative stress, immunity, tissue injury, and repair, and that dual use of waterpipe and cigarette smoking is more harmful than exclusive waterpipe or cigarette smoking. | |
| (Mahrous, 2019) | Blood biomarkers of nicotine-induced toxicity in healthy males | 90 male participants Non-smokers (n=20) Active smokers (n=40) | Nicotine and cotinine exposure is associated with increased oxidative stress and toxicity in healthy males, which may have negative health consequences. | |
| (Colsoul et al., 2023) | Biological effect of cigarette smoking in endothelial dysfunction: Study of biomarkers of endothelial function, oxidative stress, inflammation, and lipids | Smokers (n=138) Non-smokers (n=83) | Cigarette smoking is associated with increased oxidative stress and decreased antioxidant capacity, which may contribute to endothelial dysfunction and cardiovascular disease. |
Based on Table 1, six selected studies showed the relationship between blood cotinine levels, glutathione levels, and malondialdehyde levels in smokers. Covering a total of 333 participants who were smokers and 267 participants who were non-smokers were registered in our analysis. Three of six articles explain the relationship between cotinine levels in the blood and glutatione levels in smokers, and six of six articles explain the relationship between cotinine levels in the blood and malondialdehyde levels in smokers.
Meta-analysis of the relationship of blood cotinine levels and glutathione levels in smokers
The meta-analysis revealed a significant level of between-study heterogeneity for blood cotinine levels and glutathione levels in smokers among the available studies (I2 = 100%, p < 0.00001). Random-effects modeling of the combined results revealed a non-significant difference in the blood cotinine levels and glutathione levels in smokers (MD = 85.02, p 0.39, 95% CI = -108.04 to 278.09, Figure 2a). The pooled mean difference for blood cotinine levels in smokers showed an increase compared to glutathione levels in smokers. The mean difference is positive, indicating that the average blood levels of cotinine in smokers tend to be higher than glutathione levels in smokers.

a) relationship of blood cotinine levels and glutathione levels in smokers; b) relationship of blood cotinine levels and glutathione levels in non-smokers; c) relationship of blood cotinine levels and malondialdehyde levels in smokers; d) relationship of blood cotinine levels and malondialdehyde levels in non-smokers.
Meta-analysis of the relationship of blood cotinine levels and glutathione levels in non-smokers
The meta-analysis revealed a significant level of between-study heterogeneity for blood cotinine levels and glutathione levels in non-smokers among the available studies (I2 = 100%, p < 0.00001). Random-effects modeling of the combined results revealed a non-significant difference in the blood cotinine levels and glutathione levels in non-smokers (MD = -19.75, p 0.09, 95% CI = -42.89 to 3.39, Figure 2b). The pooled mean difference for blood cotinine levels in non-smokers showed a decrease compared to glutathione levels in non-smokers, however, the overall effect was not significant. The mean difference is negative, indicating that the average blood levels of cotinine in non-smokers tend to be lower than glutathione levels in non-smokers.
Meta-analysis of the relationship of blood cotinine levels and malondialdehyde levels in smokers
The meta-analysis revealed a significant level of between-study heterogeneity for blood cotinine levels and malondialdehyde levels in smokers among the available studies (I2 = 100%, p < 0.00001). Random-effects modeling of the combined results revealed a significant difference in the blood cotinine levels and malondialdehyde levels in smokers (MD = 115.37, p 0.0004, 95% CI = 51.92 to 178.82, Figure 2c). The pooled mean difference for blood cotinine levels in smokers showed an increase compared to malondialdehyde levels in smokers. The mean difference is positive, indicating that the average blood levels of cotinine in smokers tend to be higher than malondialdehyde levels in smokers.
Meta-analysis of the relationship of blood cotinine levels and malondialdehyde levels in non-smokers
The meta-analysis revealed a significant level of between-study heterogeneity for blood cotinine levels and malondialdehyde levels in non-smokers among the available studies (I2 = 100%, p < 0.00001). Random-effects modeling of the combined results revealed a non-significant difference in the blood cotinine levels and malondialdehyde levels in non-smokers (MD = 1.46, p 0.91, 95% CI = -24.50 to 27.43, Figure 2d). The pooled mean difference for blood cotinine levels in non-smokers showed an increase compared to malondialdehyde levels in non-smokers. The mean difference is positive indicating that the average blood levels of cotinine in non-smokers tend to be higher than glutathione levels in non-smokers.
Smoking-related diseases have been implicated in the various components of tobacco smoke and their specific effects on vital organs and tissues, but these have not yet been elucidated. The adverse health effects of smoking has been attributed to the smoke’s production of ROS and oxidative stress and its detrimental effects on biomolecules such as lipids, membrane proteins and nucleic acids. Levels of biomarkers of oxidative stress associated with duration and number of cigarettes smoked in smokers were estimated. Overall, this study provides evidence for the harmful effects of smoking on biomarkers of oxidative stress and antioxidant capacity in the blood of smokers.
One limitation of this review is the relatively small number of studies available on the relationship between cotinine levels, glutathione levels, and malondialdehyde levels in smokers. The limited number of studies may restrict the generalizability of the findings and the ability to draw robust conclusions. All studies included in this review were assessed as being of high quality. These studies reported only on the search results providing the specific values of the mean difference, standard deviation, and 95% CI of cotinine levels, glutathione levels, and malondialdehyde levels in the blood of smokers and non-smokers.
In this study, the blood cotinine levels in smokers and non-smokers were significantly higher than the malondialdehyde levels of smokers and non-smokers. A significant positive association was observed between blood cotinine levels and malondialdehyde levels of smokers studied. However, it was found that there was no significant positive association between blood cotinine levels and malondialdehyde levels of non-smokers studied. These results are similar to a study conducted by Mahrous (2019), which found that blood cotinine levels were higher in active smokers compared to non-smokers. Similarly, serum cotinine concentrations were higher in smokers than in non-smokers (295.6 ± 57.5 ng/mL for smokers and 14.3 ± 3.0 ng/mL for non-smokers; p < 0.001) (Kim et al., 2003). In the smoking group, concentration of malondialdehyde was positively correlated with cotinine levels (Chelchowska et al., 2011).
Meanwhile, in this study the blood cotinine levels in non-smokers were significantly lower than in smokers, and glutathione levels in smokers and non-smokers. No significant positive association was observed between blood cotinine levels and glutathione levels of smokers and non-smokers studied. Serum glutathione were lower in smokers than in non-smokers (Kim et al., 2003). Exposure to cigarette smoke has been reported to cause a decrease in the glutathione concentration and in the expression or activity of several antioxidant enzymes (Nsonwu-Anyanwu et al., 2019). These results are similar to studies conducted by Allen et al. (2019) which found no significant associations between blood cotinine and blood glutathione in smokers.
When cigarette smoke enters the respiratory tract, this condition can reduce the expression of superoxide dismutase and glutathione peroxidase, which describes a decrease in the body’s antioxidant activity and can increase the expression of malondialdehyde (Figure 3). Increased malondialdehyde expression suggests that excessive free radicals limit the ability of antioxidants to inhibit oxidation, while also suggesting oxidative stress is positively correlated with lipid peroxidation as a tissue damage mechanism.
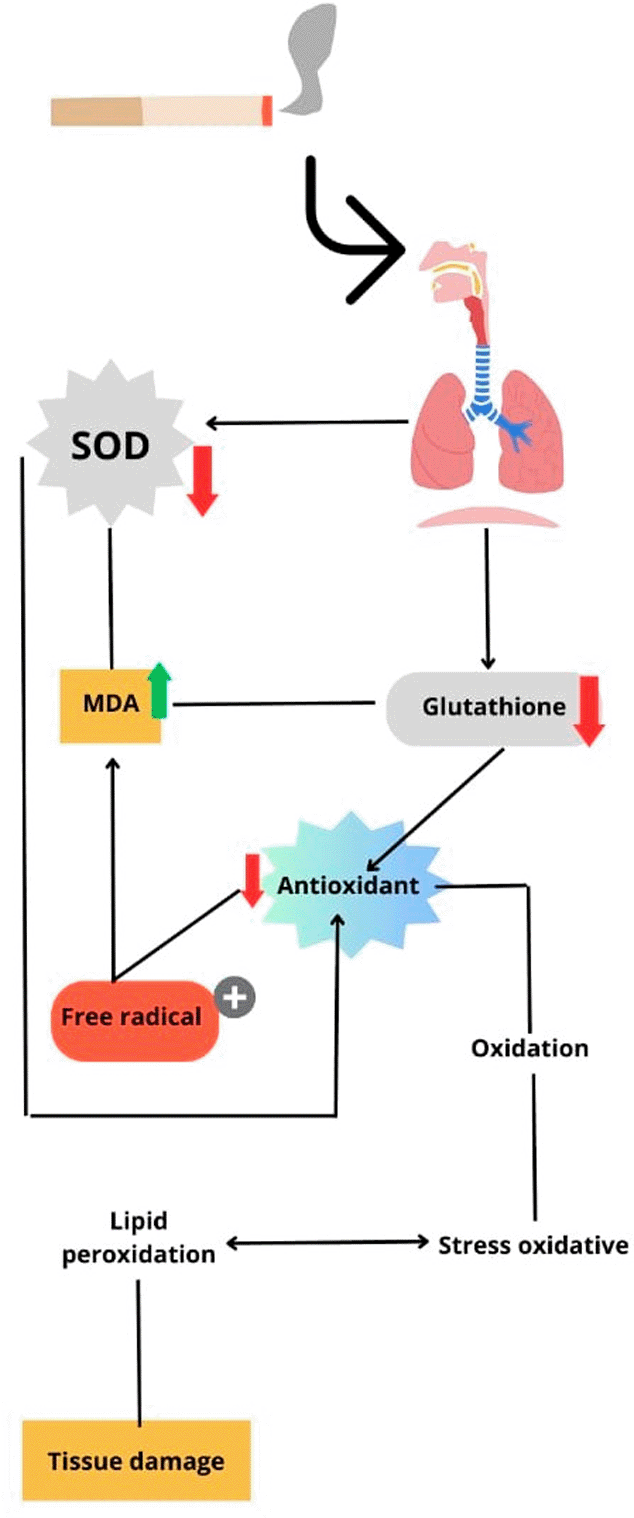
SOD, superoxide dismutase; MDA, malondialdehyde.
Chemical constituents in cigarette smoke can induce oxidative stress by generating ROS, increasing pro-oxidant load and decreasing antioxidant protection. Oxidative stress plays an important role in the pathogenesis of several diseases such as lung cancer, chronic obstructive pulmonary disease and atherosclerosis (Mohod et al., 2014). Cigarette smoke-derived ROS cause oxidative damage to cellular components, modulate cellular responses, and activate numerous signaling pathways that ultimately lead to pathological changes in cellular function (Nielsen et al., 1997; Dietrich et al., 2003; Halliwell and Poulsen, 2016). Nicotine and cotinine have been shown to disrupt the mitochondrial respiratory chain and increase the formation of superoxide anions and hydrogen peroxide (Mohod et al., 2014). This increase in pro-oxidants leads to lipid peroxidation, induction of DNA strand breaks, inactivation of certain proteins and rupture of membranes, disruption of cellular function and integrity, and is associated with many adverse health effects (Mohod et al., 2014).
Cotinine, the major metabolite of nicotine, is currently considered the best predictor of tobacco smoke exposure. Smokers can be exposed to environmental tobacco smoke through airborne smoke inhalation and percutaneous absorption. The amount of nicotine absorbed by a smoker is highly dependent on the concentration of nicotine in the smoke, the individual’s smoking pattern, and the pH of the smoke (Hwang et al., 2012). This means that the more nicotine and oxidants in cigarette smoke you are exposed to, the higher your cotinine levels.
Malondialdehyde is a product of lipid peroxidation. The higher levels observed in smokers are consistent with increased ROS formation associated with smoking. Chronic smoking may induce increased activity of the antioxidant system to neutralize the increased ROS associated with smoking in order to restore redox equilibrium between prooxidants and antioxidants (Nsonwu-Anyanwu et al., 2019). Glutathione levels in smokers and non-smokers may result from an adaptive response involving the upregulation of antioxidant glutathione defenses in response to smoking-induced oxidative stress. The adaptive glutathione response consists of coordinated reactions during glutathione synthesis, utilization, recycling, and transport (Gould et al., 2015). Exposure to cigarette smoke has been reported to decrease glutathione levels and reduce the expression or activity of several antioxidant enzymes (Bazzini et al., 2013). This depletion may be directly related to increased lipid peroxidation, which could be attributed to increased ROS generation by cigarette smoking, in addition to uptake by the antioxidant enzyme glutathione.
In conclusion, the present meta-analysis suggested that blood cotinine levels were significantly associated with malondialdehyde levels in smokers. However, this study did not find a significant association between cotinine levels in the blood of smokers and glutathione levels in the blood of smokers. Smoking can increase blood cotinine levels of smokers in comparison with that in non-smokers.
All data underlying the results are available as part of the article and no additional source data are required.
Zenodo: PRISMA checklist for ‘The relationship between cotinine levels in the blood with glutathione, and malondialdehyde levels of smokers: Meta-analysis’, https://doi.org/10.5281/zenodo.8053129 (Tualeka et al., 2023).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors would like thank to the rector of Airlangga University. The authors would like to acknowledge The relationship between cotinine levels in the blood with glutathione, and malondialdehyde levels of smokers: a systematic review and meta-analysis.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)