Keywords
Biomarkers, Ulcerative colitis, Disease activity, Remicade, Remsima,
Biomarkers, Ulcerative colitis, Disease activity, Remicade, Remsima,
Ulcerative colitis (UC) is a chronic inflammatory bowel disease that necessitates effective treatment strategies to alleviate symptoms and improve patients’ quality of life.1 Over the years, biological therapies such as Remicade® (infliximab originator) and Remsima® (infliximab biosimilar) have emerged as valuable options in managing UC.2 One contributing factor to the elevated cost of biologics is the implementation of patent protection, which serves to restrict market competition. However, with the expiration of certain patents in recent years, alternative manufacturers have entered the market, offering biosimilar molecules at more competitive prices. These biosimilars are typically priced at a discount of approximately 45% compared to the original biologic product. In light of the increasing demands placed on healthcare systems to achieve cost effectiveness, biosimilars have emerged as a pivotal asset.3 Biosimilars are medicinal products that possess an amino acid chain that is comparable and exhibit a biochemical activity that is extremely analogous when compared to the original drug. Nevertheless, due to the intricate molecular composition of biologics and biosimilars, slight variations in their molecular structure may result from changes in base materials and manufacturing circumstances, these differences have the potential to impact the effectiveness and safety of the drug theoretically.4
Biosimilar infliximab, referred to as CT-P13 (Remsima® and Inflectra®) and SB2 (Flixabi®), is a monoclonal antibody of significant size that has been granted permission to be marketed in various countries for the treatment of multiple conditions such as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, inflammatory bowel diseases.5 The evidence submitted to regulatory authorities consisted of in vitro assay activity data, comparative pharmacokinetic data from individuals with ankylosing spondylitis, and comparable pharmacodynamic data from individuals with active rheumatoid arthritis treated with the original product. The Canadian regulatory authority, Health Canada, initially differed from its American and European counterparts in its reluctance to accept evidence from patients with rheumatoid arthritis and ankylosing spondylitis as endorsed for the indication of Crohn’s disease and ulcerative colitis. This decision was based on concerns regarding the reliability of in vitro testing activity data and the absence of safety data.6 Despite the forthcoming provision of additional evidence to support the utilization of biosimilars in the treatment of inflammatory bowel disease indications such as Crohn’s disease (CD), fistulizing CD, and ulcerative colitis (UC), there remains a significant gap in knowledge regarding the safety profile and the potential development of neutralizing antibodies when patients are switched between the originator product and the biosimilar, particularly in cases that involve multiple changing.6
The measurement of infliximab trough levels provides valuable insights into the drug’s concentration in a patient’s bloodstream, serving as an indicator of the therapeutic drug monitoring.7,8 By comparing the trough levels between the two treatment groups, and assess the drug’s pharmacokinetics and determine if there are differences in the drug exposure.8
Anti-infliximab antibodies, known as immunogenicity, play a pivotal role in response to biological therapies.9 The presence of these antibodies can impact drug efficacy by neutralizing the therapeutic effects of infliximab.10 Comparing the development of anti-infliximab antibodies between patients receiving Remicade® and Remsima® allows to evaluate the immunogenic potential of these treatments and their impact on treatment outcomes.11
Serum calprotectin is a non-invasive biomarker used to assess intestinal inflammation.12 It reflects the activity of UC and can indicate response to treatment.13 Comparing the changes in serum calprotectin levels between Remicade® and Remsima®-treated groups provides valuable insights into the drugs’ abilities to reduce inflammation and achieve disease remission.14
Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are commonly used markers of inflammation and are indicative of disease activity in UC patients.15–19 By comparing the changes in ESR and CRP levels in patients treated with Remicade® and Remsima®, to evaluate the drugs’ effectiveness in reducing systemic inflammation.20
Tumor necrosis factor alpha (TNFa) and oncostatin M are pro-inflammatory cytokines involved in the pathogenesis of UC.21–24 Monitoring their serum levels allows to assess the drugs’ ability to modulate the inflammatory response. Comparing the changes in TNFa and oncostatin M levels between the treatment groups provides valuable information on the drugs’ mechanisms of action and their impact on disease activity.25
By thoroughly analysing these key parameters, we aimed to provide a comprehensive comparison between the efficacy of Remicade® and Remsima® in the treatment of UC.
The study adhered to the protocols outlined by the Ethics Committee of the College of Pharmacy at Mustansiriyah University, as indicated by the assigned research number 31, approval number 19, and reference number 72, on 3 July 2022. Written informed consent has been obtained from each participant. No incentives were provided, and the participation was entirely voluntary.
The research employed a cross-sectional observational design to examine the treatment outcomes of Remicade and Remsima in patients diagnosed with ulcerative colitis. The present investigation adhered to the STROBE guidelines for reporting cross-sectional observational studies.26
The present study was conducted at Baghdad Teaching Hospital, located in Baghdad, Iraq, lasting from July 2022 to February 2023.
The G*Power software version 3.1.9.7, with the Research Resource Identifier (RRID) SCR_013726, was utilized to estimate the necessary sample size for the study. The study employed a one-tailed alpha level of 0.05, a confidence interval of 95%, a power of 95%, and an effect size of 0.50. Hence, the minimum sample size required was determined to be 34 (f). The study involved the enrolment of 43 individuals.
The study included individuals between the ages of 21 and 57 years who had been previously diagnosed with inflammatory bowel disease (IBD), specifically ulcerative colitis (UC). These patients received treatment protocols prescribed by physicians at the Gastrointestinal Tract (GIT) Centre in Baghdad Teaching Hospital, located in Baghdad, Iraq. The patients received biological treatment with infliximab at a dosage of 5mg/kg for a duration exceeding one year. The infliximab administered could either be the originator brand (Remicade®) or the biosimilar brand (Remsima®).
The study excluded individuals who had the following coexisting conditions: rheumatoid arthritis, systemic lupus erythematosus, diabetes mellitus; cardiovascular, hepatic, or renal diseases; organ transplant recipients; individuals who smoke tobacco; and those taking medications that could potentially affect infliximab levels or accurate measurements. Patients who were previously subjected to any anti-TNF agent before initiating infliximab were excluded from the study.
This study utilizes random selection, a rigorous and systematic approach, to ensure that each participant from the population of infliximab users (both originator and biosimilar) has an equal opportunity to be included in the sample. The aforementioned concept forms the foundation of probability sampling and holds significant importance in probability methodologies and the ability to generalize findings. The utilization of random selection effectively mitigates the presence of sampling selection bias.
Demographic data such as age, gender, weight, height, working status, disease duration, smoking status, income, marital status, family history, and presence of extra-intestinal manifestations were collected via direct patient interviews using a patient data chart specially designed for this study. The body mass index (BMI) was computed by dividing the weight in kilograms by the square of the height in meters.
From each patient, a 10 ml venous blood sample was obtained and divided into 2 samples, first one was used for erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) and the second one was allowed to clot. Later the clot was removed by centrifuging at 2,000-3,000 rpm for 20 minutes. The resultant supernatant was kept in deep freeze (-80°C) till the time of analysis of biomarkers.
Quantitative analysis of free infliximab in serum samples
The measurement of infliximab trough levels was conducted using Shikari® (Q-INFLIXI) ELISA kits manufactured by Matriks Biotek® Turkey, as indicated in Table 1. The analysis employed a solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. The standards and samples, particularly serum or plasma, were incubated in a microtiter plate that had been infused with the suited reacting agent intended for infliximab. After the incubation process the wells are subjected to a washing procedure. Following this, the horse radish peroxidase (HRP) conjugated probe is added. It establishes a binding association with the immobile infliximab facilitated by the reactant present on the outer layer of the wells. Following the incubation period, the wells undergo a washing procedure to eliminate anything that has not bonded to the surface. The detection of the enzymatic activity immobilized in the wells is accomplished by introducing a chromogenic substrate known as tetramethylbenzidine (TMB). In conclusion, the process is terminated by utilizing an acidic stop solution. The degree of color observed is positively associated with the quantity of infliximab detected in the sample or standard. The determination of sample results can be accomplished by utilizing the standard curve.
| Diagnostic kits | Supplier | Cat. No. |
|---|---|---|
| Shikari® (Q-INFLIXI) Infliximab ELISA | MATRIKS BIOTECHNOLOGY CO., LTD. | INF-FD-REMI |
| Shikari® (Q-ATI TOTAL) Anti-Infliximab ELISA (Free/Total Ab) | MATRIKS BIOTECHNOLOGY CO., LTD. | INF-QNFT-REMI |
| Human TNFa (Tumor Necrosis Factor Alpha) ELISA Kit | MyBioSource | MBS8800192 |
| Human Oncostatin M (OSM) ELISA KIT | Melsin Medical Co., Limited | EKHU-2288 |
| Human calprotectin (CAL) ELISA Kit | MyBioSource | MBS2601681 |
| HumaReader HS | HUMAN | REF16670 |
Quantitative measurement of total Antibodies to Infliximab
The method utilizes the enzyme-linked immunosorbent assay (ELISA) technique to quantitatively determine the levels of both total and liberated antibodies specific to infliximab in blood samples. The production of anti-infliximab antibodies was achieved through the utilization of the SHIKARI® (Q-ATIDUO) enzyme-linked immunosorbent assay (ELISA) kit manufactured by Matriks Biotek® in Turkey. In the initial phase of incubation, dissociating buffering was introduced to aid in the disassociation of the antibody-immune complex specific to infliximab, as depicted in Table 1. After transferring the dissociating blend onto the plate, the antibodies present in the blood samples of individuals were isolated from infliximab and bound to the drug infliximab that was immobilized on the walls of the microtiter wells. This binding was facilitated by the use of a horse radish peroxidase (HRP) conjugated probe. The detection of a reaction between tetramethylbenzidine (TMB) chromogen substrate to samples that underwent a washing process to eliminate any unbound components. Subsequently, a stop solution with corrosive properties is employed to terminate the reaction process. A positive correlation exists between the intensity of the reaction color and the number of infliximab antibodies present in the specimen.
TNFa values obtained using (Tumor Necrosis Factor Alpha) ELISA Kit (MyBioscore Inc., USA) Table 1, Sandwich enzyme immunoassay is the testing method utilized by this kit. This preparation includes a microtiter plate pre-coated with a TNFa-specific antibody. Next, the microtiter plate wells are coated with a biotin-conjugated anti-TNFa antibody, which is the suitable choice. Subsequently, the addition of Avidin-Horseradish Peroxidase (HRP) conjugate is carried out, followed by an incubation period within each well of the microplate. The change in color following the addition of the TMB substrate solution will only occur in wells that contain TNFa, a biotin-conjugated antibody, and enzyme-conjugated Avidin. The enzymatic activity of the enzyme-substrate complex is inhibited by the addition of a solution containing sulphuric acid. Subsequently, the resulting change in color is quantitatively assessed using spectrophotometry at a wavelength of 450 nm with a bandwidth of 10nm. Subsequently, the concentration of TNFa in the samples is evaluated through a comparison of the optical density of the specimens with the standard curve.
All samples and standards have been inserted into the Microelisa Stripplate in duplicate. Establish standard wells and sample wells for the purpose of testing. Dispense 50 microliters of the standard solution into each well designated for the standard. The testing sample should be supplemented with 10 μl. The testing sample well should be supplemented with 40 μl of sample diluent. In every hole, introduce 100 microliters of HRP-coupled reagent. Proceed to seal the wells with a sealing film and subject them to incubation at a temperature of 37 degrees Celsius for a duration of sixty minutes. Perform aspiration and subsequent cleansing of each well in the experimental setup on four occasions, resulting in a cumulative total of five washes. The wells should be filled with 400 μl of Wash Solution using either a spray bottle, manifold dispenser, or automatic washer. The complete removal of all liquid during each phase is imperative for achieving optimal performance. Ensure the complete removal of any residual cleanse solution by employing the method of aspiration or decantation subsequent to the ultimate cleansing process. To achieve the desired outcome, it is necessary to invert the dish and subsequently remove any excess moisture by gently pressing clean paper towels against its surface. It is recommended that each well be allocated a volume of 50l for both chromogen solution A and chromogen solution B. Gently combine the components and subject the mixture to incubation at a temperature of 37°C for a duration of 15 minutes. Protect from exposure to light. The recommended procedure involves the addition of 50 microliters of Stop Solution to each well. Alter the coloration of the wells, shifting them from a blue hue to a yellow hue. In the event that the wells exhibit a green color or if the color change is not uniformly distributed, it is advisable to gently tap the plate to promote comprehensive blending. The Optical Density (OD) at 450 nm should be measured using a microtiter plate reader within a time frame of 15 minutes.
The calprotectin evaluations were conducted utilizing the Human calprotectin (CAL) ELISA Kit manufactured by MyBioscore.Inc. which is based in the United States. The process can be simply outlined as follows: The ELISA Kit should be taken out of the fridge approximately 20 minutes prior to commencing the test, allowing it to equilibrate at ambient temperature. To achieve a less concentrated washing buffer, it is recommended to dilute it by a factor of 1:25 using double-distilled water. The lyophilized standard vial should be supplemented with 1.0 ml of Standard Diluent, followed by a 30-minute incubation period. Once the standard solution has completely dissolved, combine it and appropriately label the tube gently. To perform the experiment, it is necessary to extract a quantity of Enzyme Conjugate solution that is directly proportional to the number of wells to be tested. This extracted solution should then be diluted with Enzyme Diluent in a ratio of 1:100. It is recommended to allocate a period of 30 minutes prior to the intended usage for adequate preparation. Furthermore, it is advised against reutilizing the prepared material for subsequent testing purposes. The OD values of each sample and standard should have the values of the blank well subtracted from them.
The statistical analysis in the present research was performed employing GraphPad Prism version 8, which has the Research Resource Identifier (RRID) SCR_002798. The Shapiro-Wilk test was chosen as the statistical test to assess the normality of the data. The data is provided in the format of mean plus or minus standard deviation. The standard error of the mean (SEM) is a statistical metric used to assess the level of variability or uncertainty linked to the estimation of the population mean derived from a sample. The data underwent statistical analysis utilizing an unpaired Student’s t-test and a two-way analysis of variance (ANOVA). Following the identification of significant differences among the datasets through the analysis of variance (ANOVA), a posthoc test called Tukey’s multiple-comparisons test was utilized to compare the datasets. A P-value that falls below the predetermined threshold of 0.05 is deemed to be statistically significant.
A total of 43 patients diagnosed with ulcerative colitis were included in the analysis. Of these, 22 patients received Remicade, and 21 patients received Remsima, as presented in Figure 1. The demographic and disease characteristics of the two treatment groups are summarized in Table 2. There were no significant differences in age, gender distribution, disease duration, or extent of colonic involvement between the Remicade and Remsima groups (P > 0.05).
The mean infliximab trough levels in the Remicade group were 3.264 ± 1.776 μg/mL, while in the Remsima group, the mean trough levels were [3.248 ± 1.889] (microgram) ng/mL. Statistical analysis revealed no significant difference in infliximab trough levels between the two groups (P = 0.977). This suggests comparable drug exposure and pharmacokinetics in patients receiving Remicade and Remsima Figure 2.
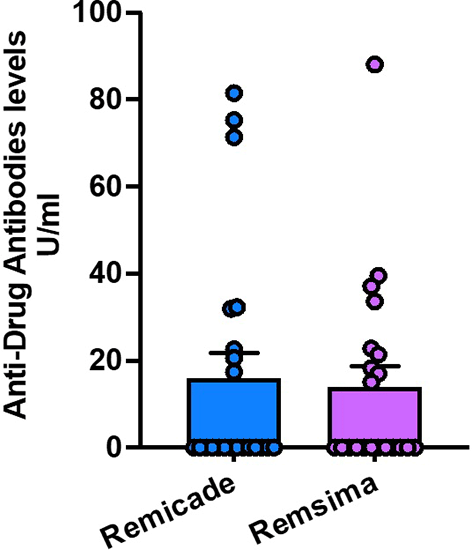
Among patients receiving Remicade, 8 patients, 36% developed anti-infliximab antibodies, while [9 patients, 42%] of patients in the Remsima group tested positive for these antibodies. The difference in the development of anti-infliximab antibodies between the two groups was not statistically significant (P = 0.7799). These findings indicate a similar immunogenic potential between Remicade and Remsima in ulcerative colitis patients Figure 3.
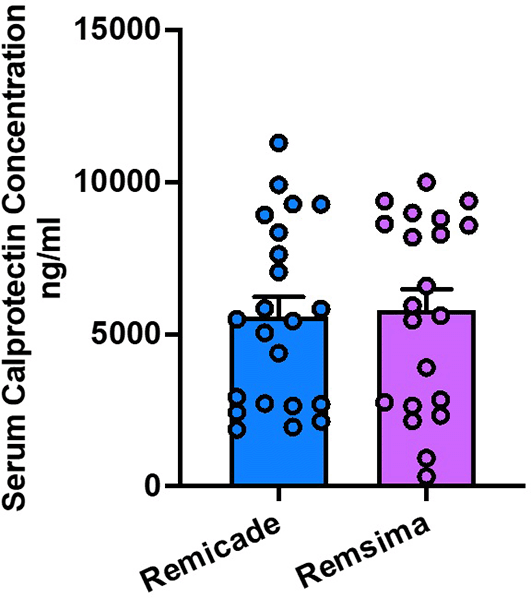
The mean serum calprotectin level measured just before the second dose of treatment was 596 ± 2982 μg/ml in the Remicade group and [5795 ± 3174] μg/ml in the Remsima group. There was no statistically significant difference in serum calprotectin levels between the two treatment groups (P = 0.8334) Figure 4.
The mean ESR value measured just before the second dose of treatment was [18.33 ± 18.02] mm/hr in the Remicade group and [16.43 ± 15.13] mm/hr in the Remsima group. There was no statistically significant difference in serum calprotectin levels between the two treatment groups (P = 0.7052) Figure 5.
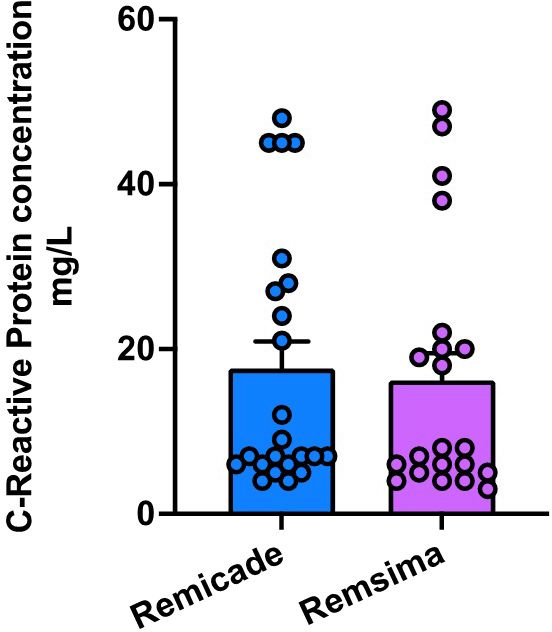
The mean CRP value measured just before the second dose of treatment was [17.65±15.61] mg/dl in the Remicade group and [16.19 ± 15.15] mg/dl in the Remsima group. There was no statistically significant difference in serum calprotectin levels between the two treatment groups (p = 0.7546) Figure 6.
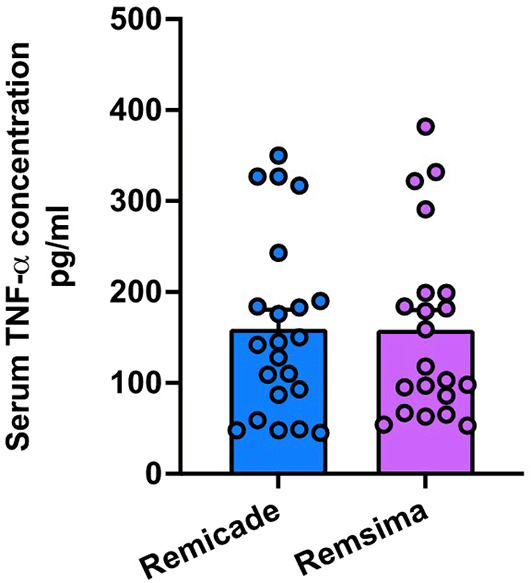
The mean serum TNFa level measured was [159.5 ± 98.34] pg/ml in the Remicade group and [158.5 ± 99.75] pg/ml in the Remsima group. There was no statistically significant difference in serum calprotectin levels between the two treatment groups (p = 0.9179) Figure 7.
The mean serum Oncostatin M level measured was [107.3 ± 81.5] ng/ml in the Remicade group and [107.5 ± 80.07] ng/ml in the Remsima group. There was no statistically significant difference in serum calprotectin levels between the two treatment groups (P = 0.9919) Figure 8.
Overall, the results of this study suggest comparable treatment outcomes between Remicade and Remsima in ulcerative colitis patients. There were no significant differences in infliximab trough levels, development of anti-infliximab antibodies, and inflammatory markers (serum calprotectin, ESR, CRP, TNFa, and oncostatin M) between the two treatment groups.
In recent years, the treatment of inflammatory bowel disease has been significantly improved because of the introduction of monoclonal antibodies (MAbs).27 Infliximab (IFX) revolutionized the pharmaceutical approach to treating Crohn’s disease and ulcerative colitis.28 MAbs, on the other hand, have hurdles in terms of their immunogenicity, efficacy, and safety because of the intricate interaction that exists between pharmacology and immunology. Inflammatory bowel disease (IBD) and other serious disorders have led to the development of biosimilars, a substitute for the original medicine that is more affordable.29
According to the most recent findings, it has been shown that biosimilar versions of IFX in patients with IBD are just as safe and effective as the original versions of these medications.30 However, the use of these products is accompanied by several misunderstandings about their efficacy and safety, in addition to disputes surrounding the interchangeability of these products. As a result, there is a need for empirical evidence on the efficacy and safety of biosimilars when used over extended periods of time, at least until a scientific consensus can be reached.31
This study aimed to compare the treatment outcomes between Remicade and Remsima, focusing on key parameters, including infliximab trough levels, anti-infliximab antibodies, serum calprotectin, ESR, CRP, TNFa, and serum oncostatin M.
Our results demonstrate comparable infliximab trough levels between the Remicade and Remsima treatment groups, indicating similar drug exposure and pharmacokinetics. This finding suggests that Remsima, as a biosimilar of Remicade, effectively delivers an equivalent concentration of infliximab to patients, supporting its role as a cost-effective alternative in UC treatment. These findings demonstrate similarities to those published in an earlier Norwegian study that aimed to evaluate the trough serum levels of CT-P13 in patients with ulcerative colitis. The study utilized an automated immunofluorometric assay on the automated dissociation-enhanced lanthanide fluorescent immunoassay platform, and the results indicated comparable serum drug levels.32
The development of anti-infliximab antibodies can impact treatment efficacy by neutralizing the drugs therapeutic effect. In our study, the incidence of anti-infliximab antibodies was comparable between the Remicade and Remsima groups. This suggests that both medications have similar immunogenic potentials, further supporting the bio-similarity of Remsima to Remicade. The findings align with a prior study by Ben-Horin et al., which indicated roughly comparable immunogenicity and the presence of shared immunodominant epitopes on both infliximab agents.33
Serum calprotectin is a non-invasive biomarker used to assess intestinal inflammation in UC patients. Our study revealed no significant difference in serum calprotectin levels between the Remicade and Remsima groups. This finding suggests that both treatments effectively reduced inflammatory activity at the time of the second dose, as indicated by similar levels of this inflammatory marker. However, the lack of multiple serum calprotectin measurements throughout the treatment course limits our ability to assess the dynamic changes in inflammatory activity over time. Future studies incorporating serial measurements of serum calprotectin would provide a more comprehensive understanding of the long-term efficacy of Remicade and Remsima in managing UC. Nikkonen et al. have reported a similar outcome when examining the calprotectin levels, no significant disparity was observed between the original drugs and biosimilar drugs during the initial treatment phase or after one year in the management of patients diagnosed with inflammatory bowel disease.34
ESR and CRP are commonly used markers of systemic inflammation and disease activity in UC patients. Our study showed no significant differences between the two treatments. This suggests that both medications effectively mitigated systemic inflammation, as evidenced by improvements in these markers. The levels of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) did not exhibit any significant differences between the groups in the EXTENDED REPORT, which investigated the efficacy and safety of transitioning from reference infliximab to CT-P13 in patients with rheumatoid arthritis; these results endorse our findings.35
Furthermore, the reduction in serum TNFa and oncostatin M levels following treatment initiation in both groups suggests that Remicade and Remsima effectively modulate the inflammatory response in UC patients. However, no significant differences were observed between the treatments, indicating comparable mechanisms of action in targeting these pro-inflammatory cytokines.25
It is important to acknowledge several limitations of our study. Firstly, the relatively small sample size and a single measurement of serum calprotectin restrict the generalizability and ability to assess longitudinal changes in inflammatory activity. Further prospective studies with larger cohorts and multiple measurements of inflammatory markers are warranted to validate our findings.
In conclusion, our study provides valuable insights into the comparison of Remicade and Remsima in UC patients. The comparable infliximab trough levels, the incidence of anti-infliximab antibodies, and reductions in serum calprotectin, ESR, CRP, TNFa, and oncostatin M levels suggest that Remsima, as a biosimilar to Remicade, holds promise as a cost-effective alternative in UC management. However, future studies with longer follow-up periods and comprehensive assessments of treatment outcomes are needed to establish the long-term efficacy, safety, and cost-effectiveness of Remsima in the management of UC.
Zenodo: Evaluation of selected serum biomarkers levels in response to infliximab reference product (Remicade®) versus it’s biosimilar (Remsima®) in a sample of ulcerative colitis patients: a cross-sectional study. DOI: https://doi.org/10.5281/zenodo.8164655. 36
This project contained the following underlying data:
- Article data.xlsx (Evaluation of selected serum biomarkers levels in response to infliximab reference product (Remicade®) versus it’s biosimilar (Remsima®) in a sample of ulcerative colitis patients: a cross-sectional study).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY ).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: inflammatory bowel diseases
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 06 Dec 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)