Keywords
systemic lupus erythematosus, zinc chloride, aging, pro-inflammatory cytokines, hematopoietic stem cells
systemic lupus erythematosus, zinc chloride, aging, pro-inflammatory cytokines, hematopoietic stem cells
Aging is a biological process that not only affects functional homeostasis but also drives pathological conditions in many diseases. Aging involves the senescence of many tissues and cells, including both innate and specific immune cells.1 Cellular senescence not only disrupts the homeostasis of biological processes but also affects disease progression through inflammaging, resulting in increased rates of morbidity and mortality.2 A previous study found that immune aging is associated with an increased risk of autoimmune disease in older individuals due to excessive autoantibody production and increased levels of autoreactive T-cells.3 Interestingly, low-grade chronic inflammation in the absence of infection is also common in older individuals, possibly owing to cellular senescence and dysregulation of innate immunity.4
Systemic lupus erythematosus (SLE) is an autoimmune disease of unclear etiology characterized by immune system dysregulation in which the body’s own tissues are attacked and chronic inflammation persists. SLE has various clinical manifestations, mimicking other diseases in some cases; thus, it is difficult to diagnose and treat.5 Aging is associated with autoimmune diseases such as SLE, reflected by similar immunosenescence characteristics observed in elderly individuals. From a clinicopathological perspective, patients with SLE share common clinical features with elderly individuals, e.g., they are prone to infection and exhibit an increased incidence of tumors and cardiovascular disease.6 Previous studies have found evidence of immunoaging and inflammaging in patients with SLE, particularly in respect of innate and adaptive immunity, e.g., dysfunctional phagocytic capacity, NETosis granulocytes, abnormal T-cell IL-2 production, and T-cell aging.6,7 However, information on higher level immune cells, e.g., hematopoietic stem cells (HSCs), in relation to aging and SLE is currently limited. HSCs have been mainly discussed in the context of lupus as an autologous HSC transplantation treatment in patients with active SLE,8,9 even though the clinical utility of this treatment remains unclear.10
Various cell aging–related biomarkers have been identified. The American Federation of Aging Research (AFAR) defines a true biomarker of aging as a biomarker that can predict a person’s physical and cognitive function in an age-related manner, is testable and not harmful to the test subjects, and is applicable to laboratory animals as well as humans. The expression of p16, a cyclin-dependent kinase inhibitor that acts on CDK4/6 kinases to prevent the phosphorylation of Rb family proteins and promotes G1 cell cycle arrest, fits the AFAR definition of an effective aging biomarker. The expression of p16 increases with age, and expression levels can be measured quantitatively to detect senescence, a central mechanism encompassing environmental, genetic, and lifestyle damage.11 Patients with SLE are more susceptible to immunosenescence than those without SLE, and immunosenescence increases the susceptibility of patients with SLE to infection, malignancies, and organ damage related to immune system impairment. Immunosenescence also decreases the tumor surveillance ability of the human body, which might explain why the probability of developing malignancies increases with age.12
Interestingly, immune dysregulation in SLE is also associated with nutritional deficiency, particularly elemental zinc deficiency.13 Zinc is an essential trace element involved in many biological processes in almost all living organisms. The key functions of zinc in these processes include enzyme modulation and cell communication, proliferation, differentiation, and survival. Furthermore, zinc plays a role in immunoregulation underlying many pathological conditions and inflammation, in the conditions where zinc deficiency is present, such as in autoimmune diseases.13 Previous studies have found that dietary zinc, along with other macronutrient and micronutrient supplements, has potential benefits as an immunomodulatory agent that can improve the physical and mental well-being of patients with SLE.14,15 Although some evidence suggests that dietary zinc can benefit the health of elderly individuals,16 evidence that zinc supplementation can prevent age-related degeneration is limited.
In the present study, we investigated the expression of the senescence marker p16 in circulating HSCs as well as proinflammatory cytokine production following zinc chloride treatment in vitro. We hypothesized that HSCs in patients with SLE are more aged than those of healthy individuals and that zinc can prevent age-related degeneration in patients with SLE.
This study was ethically reviewed based on World Medical Association Declaration of Helsinki, and approval was granted under approval number 400/117/K.3/102.7/2022 granted June 1st, 2022 from the Medical Research Ethics Committee, Saiful Anwar General Hospital, Malang, Indonesia.
The study was fully explained to all participants before they were enrolled, and written informed consent for publication of the participants’ details was obtained from the participants.
This was an experimental study with a cross-sectional setting in which a consecutive sampling method was employed. In total, 73 participants were enrolled in the study, including 38 patients with SLE who attended the rheumatology outpatient ward at Internal Medicine Department, Saiful Anwar General Hospital, Malang, Indonesia in 11/07/2022 to 31/08/2022.36 SLE was diagnosed using SLICC criteria for SLE. Eligible participants included patients diagnosed with SLE that frequently attended regular medical visits. The exclusion criteria for SLE participants included patients who had been prescribed medication for nonlupus-related diseases. We also excluded patients with leukopenia (<4000 white blood cells/mm3) and patients with a positive direct agglutination (Coombs) test.17 In total, 35 healthy volunteers were recruited among health workers who met the criteria for participant enrollment, i.e., not being diagnosed with SLE or other autoimmune diseases and having normal results in complete blood count (CBC) tests as well as a negative antinuclear antibody test.
The participants enrolled from the rheumatology outpatient ward were grouped into the SLE group, whereas the healthy volunteers were grouped in the healthy control (HC) group.
CBC tests were conducted on the blood samples of each participant using a Sysmex XN-1000 hematology analyzer (Sysmex-Japan) to determine the hemoglobin level, white blood cell count, and platelet count.
To determine the levels of HSCs and p16 expression, 2 mL of EDTA-anticoagulated blood sample was added to red blood cell lysis buffer (1:5 ratio) and incubated at room temperature for 15 min in a dark room, after which the sample was centrifuged at 1500 rpm for 5 min and washed twice using cell staining buffer (BioLegend, USA; Cat# 420201). Subsequently, the pellet was resuspended with 100 μL of cell staining buffer and stained with PE/Cyanine7 anti-human CD45 antibody (BioLegend Cat# 304016, RRID:AB_314404), APC anti-human CD34 antibody (BioLegend Cat# 343510, RRID:AB_1877153), and p16INK4a antibody (F-12) Alexa Fluor® 488–conjugated (Santa Cruz Biotechnology Cat# sc-1661, RRID:AB_628067) according to manufacturers’ protocols.
Following 72 h of incubation, suspension cells were analyzed using flow cytometry to determine the intracytoplasmic expression of interleukin 6 (Alexa Fluor® 488–conjugated IL-6, Santa Cruz Biotechnology Cat# sc-28343, RRID:AB_627805), transforming growth factor beta (Alexa Fluor® 488–conjugated TGFβ1, Santa Cruz Biotechnology Cat# sc-130348, RRID:AB_1567351), interleukin 17 (Alexa Fluor® 488–conjugated IL-7, Santa Cruz Biotechnology Cat# sc-374218, RRID:AB_10988239), and senescence marker p16 (Alexa Fluor® 488–conjugated p16INK4a antibody, Santa Cruz Biotechnology Cat# sc-1661, RRID:AB_628067). Regulator T-cells were detected using FOXP3 Alexa Fluor® 488/CD4 PE-Cy5/CD25 PE (BioLegend Cat# 320027, RRID:AB_10120925). A Beckman Coulter Navios Flow Cytometer (Beckman Coulter, USA, RRID:SCR_014421) and BD FACSMelody Cell Sorter, (RRID:SCR_023209) were used in this study.
The pooled peripheral HSCs of selected participants from the SLE and HC groups were analyzed in vitro. CBC tests and coagulation tests were conducted before and after the procedure to ensure each participant’s safety. Participant data such as height, weight, and hematocrit were inputted into the leukapheresis unit (Haemonetics MCS+ System, USA), and afterwards, we selected the PBSC protocol to run.37 The procedure was performed with the following settings: 16–17 cycles, a 1:6 recirculation ratio, and a target plasma volume of 50 mL. For the initial experimental setup, the frequency of HSCs before and after leukapheresis was analyzed using flow cytometry and CD45 and CD34 markers to verify the leukapheresis efficiency in collecting HSCs (Figure 1).
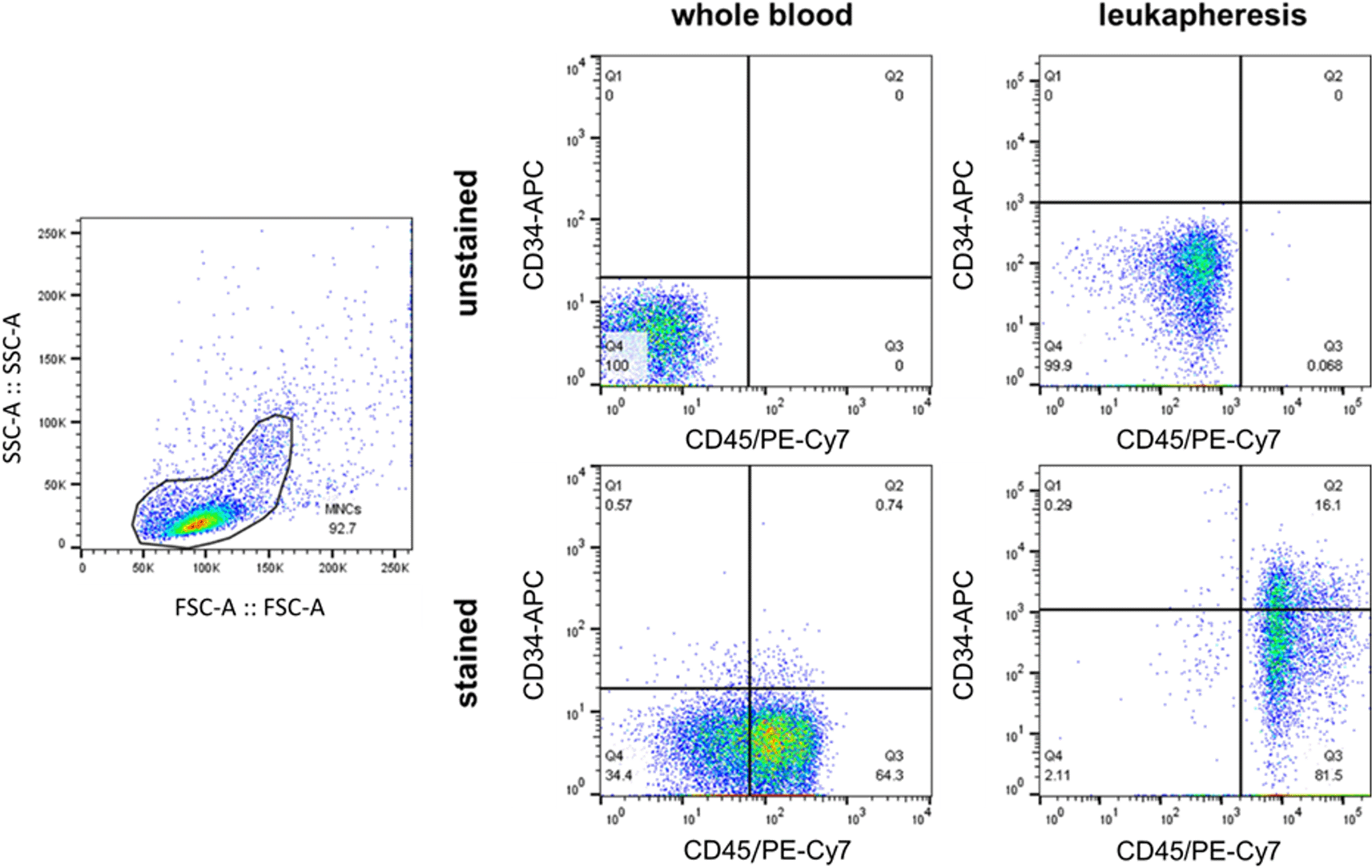
A flow cytometry analysis determines the frequency of HSCs (CD45+CD34+) in peripheral blood before the leukapheresis procedure and in the blood product of leukapheresis. A different quadrant gating setting was done due to a different flow cytometer that was being used (Beckman Coulter Navios for before leukapheresis-blood and BD FACS Melody for leukapheresis-blood product). The leukapheresis increased the frequency of HSCs approximately by 16 times (from 0.74% to 16.1%).
Leukapheresis blood products underwent further mononuclear cell (MNC) separation using a Ficoll gradient (Lymphoprep, Serumwerk Bernburg AG; Cat#04-03-9391/03) for separation from red blood cells and platelet contaminants. Pooled peripheral HSCs were incubated using Stemline® II Hematopoietic Stem Cell Expansion Medium (Sigma-Aldrich; Cat #S0192) with 10% fetal bovine serum (Sigma-Aldrich; Cat #F7524) and 1% penicillin–streptomycin (Sigma-Aldrich; Cat#P4333) and a cell density of 106 cells/mL in 5% CO2 at 37°C for 72 h. The cultures were then divided into three groups: untreated, treated with 50 μM ZnCl2, and treated with 100 μM ZnCl2 (Sigma-Aldrich; Cat#Z0152). All experiments were conducted in triplicate.
Descriptive data of the HC and SLE groups are shown.36 Independent t-tests were used to compare the variables between participants in the HC and SLE groups when appropriate according to a data normality test. Nonparametric tests (e.g., Mann–Whitney test) were used if data transformation failed. All flow cytometry data were analyzed using FlowJo (Beckton-Dickinson, USA RRID:SCR_008520), whereas statistical analysis and graph production were performed using GraphPad Prism version 9 (RRID:SCR_002798); an open-access alternative that can perform an equivalent function is R (RRID:SCR_001905). Differences in data between the HC and SLE groups were considered statistically significant at p<0.05. This manuscript has been checked against the STROBE checklist.38
Of the 38 and 35 participants in the SLE and HC groups, no participants dropped out, and all participant data were analyzed. The mean age (and age range) of the participants was 31.18 (18–53) and 32.82 (20–58) years in the SLE and HC groups, respectively. Patients with SLE exhibited the characteristics shown in Table 1 for disease duration, SLEDAI scores, and treatments. In total, 27 patients (71.05%) had the disease for >2 years, whereas 7 (18.42%) and 4 (10.53%) patients had the disease for 1–2 years and <1 year, respectively. Low SLEDAI scores were observed in 21 (55.26%) patients, whereas 9 (23.68%) and 8 (21.05%) patients had mid and high SLEDAI scores, respectively.18 The patients had received six types of treatment for their diseases, including hydroxychloroquine (37 patients; 97.37%), methylprednisolone (8 patients; 21.05%), methotrexate (1 patient; 2.63%), mycophenolic acid (14 patients; 36.84%), azathioprine (10 patients; 26.32%), and cyclophosphamide (1 patient; 2.63%) (Table 1).
Among 38 systemic lupus erythematosus (SLE) patients, most of them (71%) are having the disease for between 1–2 years, have non-active disease (45%) as shown by low Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score, and are routinely treated with drug regimens for SLE.
The CBC test results and HSC profiles of both treatment groups are shown in Table 2. The CBC parameters were hemoglobin, white blood cells, absolute neutrophil count, absolute lymphocyte count, and platelet count. Hemoglobin parameters differed significantly between the SLE and HC groups [11.50 (5.60–14.30) versus 12.92 (9.00–14.50) g/dL, respectively; p=0.0005] as did platelet count [250.5 (71.0–388.0) vs. 307.5 (191.0–412.0) × 103 cells per microliter of blood, respectively; p=0.0007)]. However, the white blood cells per microliter did not differ significantly between the two groups [SLE: 6,727 (2.72–18.20); HC: 7,234 (3.97–11.59); p=0.0596].
Patients with SLE tend to have lower hemoglobin (11.5 vs 13.0 g/dL, p<0.0001), lower platelet count (250.5 vs 306.1 × 103 cells per microliter, p=0.0006), lower absolute lymphocyte count number (1.438 vs 2.151 × 103 cells per microliter, p<0.0001), and a higher percentage of mononuclear cells (45.85% vs 32.43%, p=0.0068).
| Parameters | Healthy | SLE | p-value |
|---|---|---|---|
| Hemoglobin (g/dL) | 13.0 (9.0-14.5) | 11.5 (5.6-14.3) | <0.0001* |
| Platelet (×103/μL) | 306.1 (191.0-412.0) | 250.5 (71.0-388.0) | 0.0006 |
| WBC (×103/μL) | 7.114 (3.97-11.59) | 6.727 (2.72-18.2) | 0.0778 |
| ANC (×103/μL) | 4.316 (2.358-7.890) | 4.712 (1.310-18.26) | 0.5046 |
| ALC (×103/μL) | 2.151 (0.988-4.498) | 1.438 (0.280-4.410) | <0.0001 |
| MNC (%) | 32.43 (13.9-69.7) | 45.85 (2.49-95) | 0.0068 |
| MNC (×103/μL) | 2.267 (1.072-6.084) | 3.031 (0.454-15.523) | 0.7641 |
| HSC (%) | 1.6 (0.11-5.73) | 1.261 (0.0-5.33) | 0.2932 |
| HSC (cells/μL) | 11.74 (0.89-57.86) | 11.20 (0.0-86.76) | 0.1653 |
Neither the percentages nor cell numbers of HSCs differed significantly between the SLE and HC groups (p=0.3210 and p=0.1685 for the percentage and absolute number of HSCs, respectively). Although the absolute number of MNCs did not differ between the two groups (p=0.9930), the percentage of MNCs was higher in patients with SLE (p=0.0150) (Table 2).
We developed a gating strategy for flow cytometry analysis to determine the mean fluorescence intensity of p16 expression in selected HSCs (Figure 2). Intracytoplasmic p16 expression was significantly higher in the circulating HSCs of the SLE group than in those of the HC group (p=0.0043) (Figure 3), suggesting that the level of senescence in circulating HSCs is higher in patients with SLE than in healthy individuals, although the number of circulating HSCs did not differ significantly between the two groups (p=0.1685).
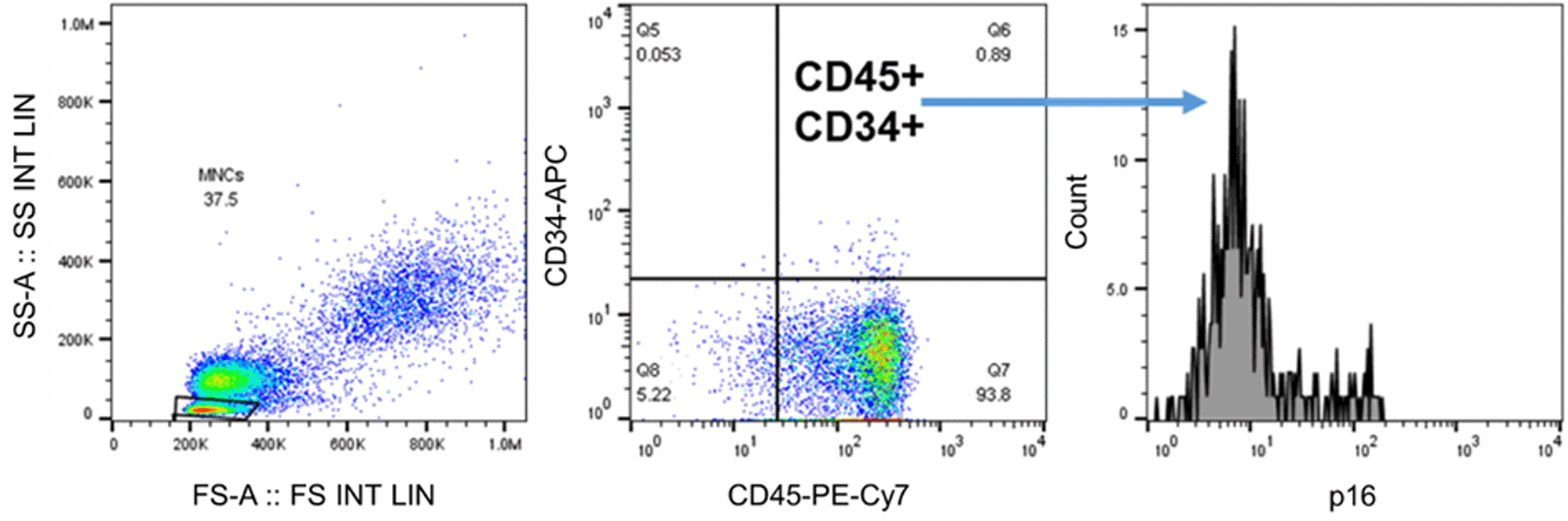
The population of HSCs in peripheral blood is detected as CD45+CD34+. Further analysis of p16 expression is done by measuring the mean fluorescence intensity of p16 on CD45+CD34+ cells.

Representative scattergram data showed the lower frequency of circulating HSCs (CD45+CD34+) in a systemic lupus erythematosus (SLE) patient in comparison with a healthy subject (2.26% vs 3.75%) (A). Further analysis showed no significant difference in HSCs number between SLE patients and healthy subjects (p=0.1653) (B). Representative histogram analysis of p16 expression showed that an SLE patient has a higher expression of p16 in comparison with a healthy subject, the red dashed line showed p16 expression in a healthy patient while the blue line is from an SLE patient (C). Further analysis showed a moderately higher level of p16 on HSCs from SLE patients than in healthy subjects (p=0.0140) (D).
Expression levels of the proinflammatory cytokines IL-6 and IL-17 and the senescence marker p16 were significantly higher in the SLE group than in the HC group (p=0.0025, p<0.0001, and p=0.0003, respectively). However, TGF-β expression levels did not differ significantly between the two groups (p=0.9816). Furthermore, IL-6, IL-17, TGF-β, and p16 expression levels in patients with SLE decreased significantly after they were treated with zinc chloride, reaching expression levels similar to those of their healthy counterparts. Interestingly, IL-17 and p16 expression levels were significantly reduced only in the SLE group after zinc chloride treatment, whereas IL-6 expression levels exhibited a similar tendency in the two groups, although patients with SLE were more sensitive to zinc chloride treatment, as indicated by the gradual reduction in expression levels in line with higher zinc chloride concentrations (Figure 4). In summary, compared with the HSCs of healthy volunteers, HSCs collected from patients with SLE exhibited higher levels of proinflammatory cytokine and p16 expression and were more sensitive to cytokine reduction after long-term zinc chloride exposure.
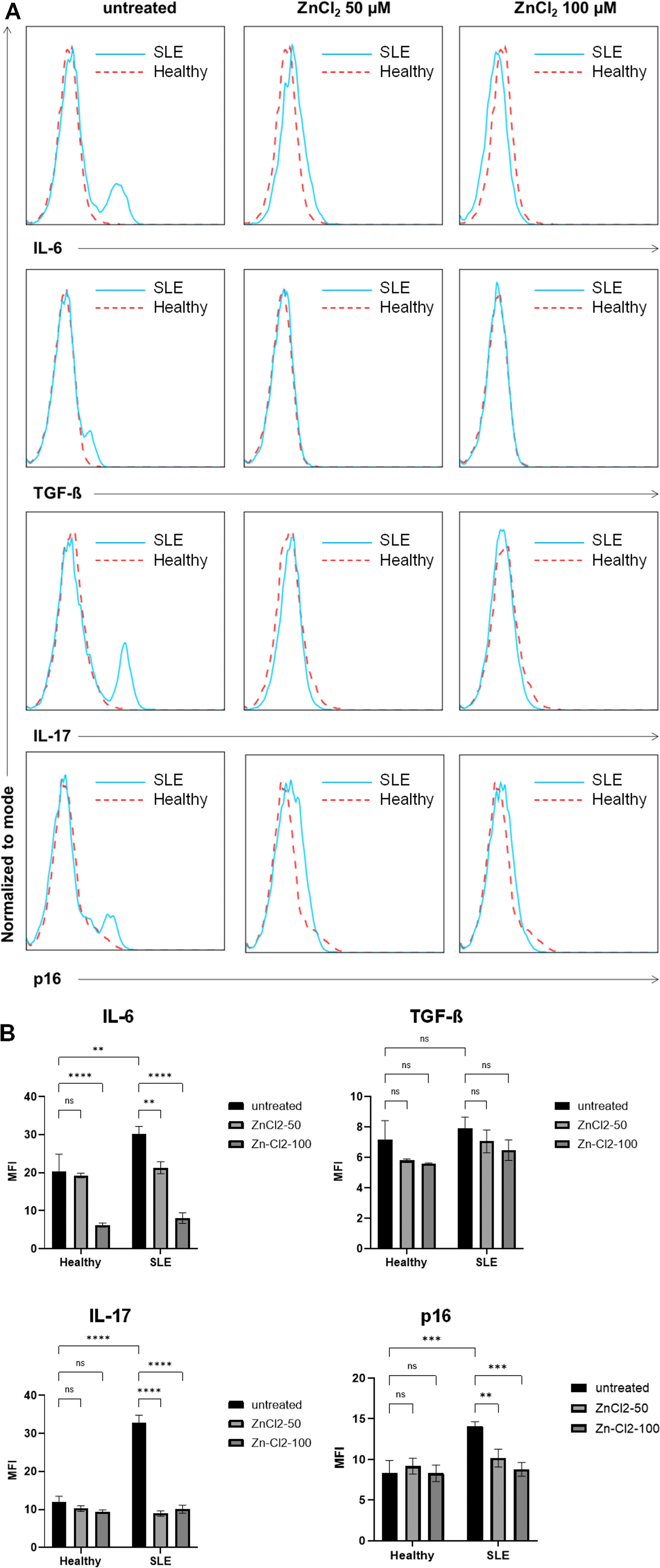
Histogram analysis of IL-6, TGF-β, IL-17, and p16 expression showed that SLE patient has a higher level of IL-6, IL-17, p16, and a slightly higher level of TGF-β than the healthy subject (p=0.0025, p<0.0001, p=0.0003, and p=0.9816 respectively); treatment of 50 μM and 100 μM of zinc chloride effectively reduced the expression of IL-6, TGF-β, IL-17, and p16 in SLE patient into becoming similar to its healthy subject’s counterpart (A). Mean fluorescence intensity (MFI) of each cytokine was analyzed using two-way ANOVA to determine the difference among the treatment groups (B).
The frequency of regulatory T-cell (Treg) populations in pooled HSCs was comparable between the treatment groups (p=0.3997); however, long-term zinc chloride treatment induced a decrease in the Treg populations of both groups. Interestingly, following a 100 μM zinc chloride treatment, the Treg population of patients with SLE was significantly depleted relative to that of healthy volunteers (p=0.0001). Therefore, patients with SLE were more sensitive to Treg population reduction after long-term zinc chloride treatment (Figure 5).
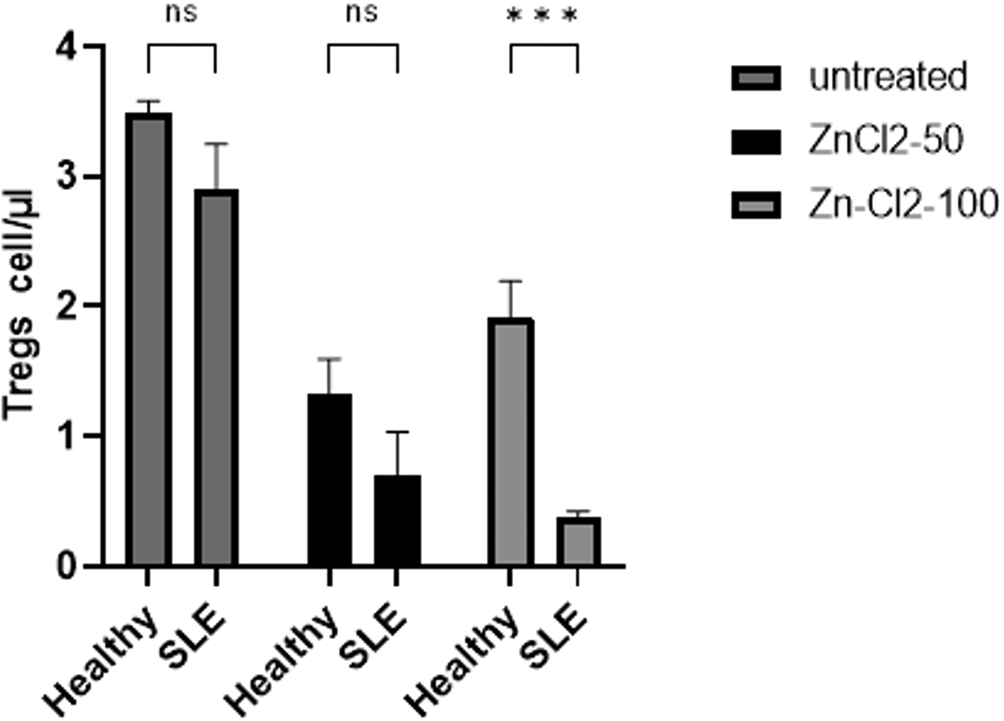
Analysis of Treg frequency as detected as CD4+CD25+Fox-P3+ by flow cytometry in both groups given selected treatment (untreated, 50 μM and 100 μM of zinc chloride) for 72 hours. The frequency of Treg population in pooled hematopoietic stem cells (HSCs) in both groups was comparable (p=0.3997). And at a concentration of 100 μM, Treg population from systemic lupus erythematosus (SLE) patients has been further depleted significantly in comparison with healthy volunteers’ Treg (p=0.0001).
Immunoaging occurs naturally in elderly people, characterized by their inability to overcome infections and respond to vaccines in typical manner. Thymic involution–driven loss of naïve T-cell production further reduces cellular responses to foreign antigens, altering self-tolerance and hampering naïve T-cell populations.19 Importantly, aged CD4+ T-cells cannot produce a sufficient level of IL-2, which is required for responding to antigen stimulation through T-cell receptors. These features of immunoaging lead to poor Th1/Th2 polarization, disrupted Th17 differentiation, and a state that favors an inflammatory and autoimmune phenotype.19 Furthermore, the number and functionality of Treg populations decrease during aging, particularly in respect of low IL-10 production and the contribution to Th17 bias (i.e., production of IL-17, IL-21, and IL-22 at higher levels).19 Another unique phenotype in immunoaging associated with autoimmune disease is the shortening of telomere length, which indicates excessive cell replication in response to the autoimmune-related inflammation process.19 Studies on immunoaging at the level of precursor cells rather than mature immune cells are limited; nevertheless, autologous HSCs are increasingly being used to treat active SLE.
The present study revealed that precursor cells, i.e., circulating HSCs, in patients with SLE also suffer from aging, as indicated by the higher expression level of the senescence marker p16 in these cells. Although the number of HSCs did not differ between the SLE and HC groups, p16 expression levels were significantly higher in the HSCs of patients with SLE. These data support the findings of a previous study, i.e., that the upregulation of p16INK4a promotes the cellular senescence of bone marrow–derived mesenchymal stem cells collected from patients with SLE.20
Proinflammatory cytokines associated with immunoaging also play roles in SLE; two such cytokines, IL-6 and IL-17, are commonly found at higher levels in patients with active SLE.21–24 We determined the expression of IL-6 and IL-17 in pooled circulating HSCs collected using a leukapheresis procedure, finding that both cytokines were expressed at significantly higher levels in the pooled HSCs of SLE patients than in those of healthy participants. A previous study revealed that Th17 cells produce significant levels of IL-17 in the kidneys of lupus-prone mice and patients with SLE, and targeting IL-17–producing cells, such as anti-IL-12/23 p40 monoclonal antibodies, was highlighted as a promising treatment strategy for SLE.25 In the present study, the administration of zinc chloride effectively reduced the expression levels of not only IL-17 but also IL-6 in the pooled HSCs of patients with SLE.
We found no significant difference in TGF-β expression between the SLE and HC groups, and zinc chloride treatment had no significant effect on TGF-β expression. TGF-β is a regulatory cytokine that plays pleiotropic roles in regulating immune responses; thus, several studies have found lower serum levels of TGF-β in patients with SLE.26–28 Our study indicates the potential benefit of administering zinc chloride for reducing proinflammatory cytokine (IL-6 and IL-17) levels without hampering TGF-β production.
Zinc chloride also effectively reduced the expression of the senescence marker p16 in the pooled HSCs of patients with SLE. Zinc is involved in regulating immunoaging as well as controlling systemic cellular stress.29 The concept that zinc deficiency leads to an accumulation of senescent cells was proposed by Malavolta et al.30 Excessive zinc may also lead to increased ROS production and induce senescence in vascular smooth muscle cells.31 Consistent with these studies, we found that the high expression of p16 in the HSCs of patients with SLE was significantly reduced after the cells were treated with zinc chloride.32
In recent years, Tregs have been well-studied in autoimmune disease, particularly in SLE. Although some results are contradictory, most studies have indicated that the loss of Tregs plays a role in the pathogenesis of SLE.33–35 In this study, we found that Treg populations in patients with SLE were slightly decreased compared with those of healthy participants.
We acknowledge that this study has some limitations. First, we collected patient samples mostly from the outpatient rheumatology clinic, which has a considerably low rate of active SLE cases. Second, we were unable to control the influence of the drug regimens given to enrolled patients, which possibly altered the levels of biomarkers measured in this study. Therefore, our results may not reflect the actual conditions in active SLE.
In summary, we found that the circulating HSCs of patients with SLE exhibit more signs of aging than those of healthy individuals, as indicated by increased expression levels of intracytoplasmic p16. In addition, the production of proinflammatory cytokines, such as IL-6 and IL-17, was higher in the cells of patients with SLE, although levels of the regulatory cytokine TGF-β were similar in the two treatment groups. Zinc chloride treatment could be a promising therapy for not only preventing immunoaging in the HSCs of patients with SLE but also inhibiting proinflammatory cytokine–producing cells. However, a study with a larger cohort must be conducted to determine the appropriate dose of zinc chloride required to treat SLE.
Figshare: Dataset (Data Submit F1000.xlsx (Raw data in Excel format)) https://doi.org/10.6084/m9.figshare.21966263. 36
Figshare: Stem Cell Protocol (Stem Cell Protocol.pdf ) https://doi.org/10.6084/m9.figshare.21980618. 37
Figshare: STROBE checklist for ‘Zinc chloride may regulate hematopoietic stem cell aging and pro-inflammatory cytokines in systemic lupus erythematosus’ https://doi.org/10.6084/m9.figshare.22014911. 38
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We would like to thank the Saiful Anwar General Hospital and Medical Faculty of Brawijaya University for the use of their facilities and for providing the patients.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Human hematopoietic progenitor cells between young and aged individuals. Inflammation effects on hematopoieitic progenitor populations and mature immune cell populations in human aging. Previous projects on hematopoietic progenitor cells and mature immune cell populations between healthy donors and SLE patients. Immune development changes in mouse aging.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 06 Dec 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)