Keywords
Therapeutic Drug Monitoring,Biosensor,Fabricated Biosensor
Therapeutic Drug Monitoring,Biosensor,Fabricated Biosensor
Administering the appropriate dosage of medication to patients is crucial. The correct dosage is crucial for effective treatment. Incorrect dosage can cause ineffective treatment or dangerous side effects. Therefore, monitoring drug levels in the body, known as therapeutic drug monitoring (TDM), is essential and necessary (Table 1).
| Biosensor type | Target | Transducer material(s) | Receptor | Detection method(s) | Limit of detections | Sample | References |
|---|---|---|---|---|---|---|---|
| Electrochemical printed electrode | Paracetamol, paroxetine, ethinylestradiol, uric acid | Carbon ink on plastic glove | - | DPV | Uric acid: mol/L | Artificial sweat and human sweat | 30 |
| Paracetamol acid: mol/L | |||||||
| Paroxetine: mol/L | |||||||
| Ethinylestradiol: mol/L | |||||||
| Electrochemical glassy carbon electrode | Ondansetron | Glass electrode | Plasticizer (NPOE and DOP), ion-exchanger (TPB, PT, PM, TKS, RK). and ionophore types (BCD, HPBCD, CMBCD, CX4, CX8) | SSM, one way ANOVA test | In Zofran tablet and phosphate buffer: M | Zofran Tablet in phosphate buffer and spiked human plasma sample | 35 |
| In human plasma: M | |||||||
| Fiber optic-based surface plasmon resonance | Infliximab (IFX) | Gold Nanoparticles (Au NPs) | Anti-IFX antibody | UV-Vis Spectro-photometry | <2 ng/mL | Dried blood spots | 14 |
| <1.5 ng/mL | |||||||
| Electrochemical pencil graphite electrode | Taxol | Pentel graphite leads | Salmon-sperm double-stranded DNA (ds-DNA) | DPV | M | Paclitaxel injection, urine and human blood serum | 32 |
| Electrochemical Microneedle | Levodopa | Hollow microneedles with carbon paste electrodes | Tyronase enzyme | SWV and chronoamperometry | 0.5-3 M | L-Dopa sample in ISF solution under artificial human skin and mice skin | 28 |
| SPR Optical Sensor ProteOn XPR36 from Bio-Rad | Infliximab (IFX), Anti-IFX antibody (ATI) | Thin gold film with bounded modified alginate-based polymer matrix | Tumor Necrosis Factor-alpha, Infliximab, IgG | SPR spectroscopy | For IFX: 0.20 g/mL | Undiluted blood sample of healthy object | 15 |
| For ATI: 2.5 g/mL | |||||||
| Piezoresistive microcantilever | Phenytoin | Polysilicon piezoresistive layer and gold surface reaction layer | Capture antibody | NI multi-channel acquisition system and the LabVIEW software | 9.5 g/mL | Phenytoin in de-ionized water solution | 24 |
| LSPR-based nanobiosensor | Digoxin | Gold Nanoparticles (Au NPs) | Anti-digoxin Antibodi | UV-Vis Spectrophotometry | 2 ng/mL | Digoxin soluble in PBS | 17 |
| Electrochemical FET-based | Tenofovir | Gold electrode connected to transistors’ gate | TFV-aptamer | EIS | 1.2 nM | TFV in PBS buffer | 46 |
| LSPR-based optical fibre sensor | Beta-lactam antibiotics | Gold Nanoparticles (Au NPs) | Bacteriolysis signatures of P. aeruginosa | UV-Vis Spectrophotometry | 0.01 to 1 g/mL | Tap water | 18 |
| 0.5 to 10 g/mL | Real human serum | ||||||
| Electrochemical Microneedle | Beta-lactam antibiotics | Gold electrode coated with iridium oxide | -lactamase | The open circuit potential measurement using in-house electronics. | 6.8 M in 10 mM PBS solution | Penicillin V potassium salt solution in PBS under human biopsy skin | 27 |
| Visual Colorimetric | Etimicin | - | Silver nanoparticles (AgNPs) | UV-Vis spectrophotometry, naked eye visualization | by UV-Vis: mol/L | Human urine | 21 |
| by visual: mol L-1 | |||||||
| Colorimetric Plasmonic Sensor | Fluoxetine | Citrate-capped silver nanoparticles (CIT-Ag NPs) | - | UV–Vis spectrometry, SEM, Fourier transform infrared spectroscopy | 0.18 g/mL | Blood serum sample and spiked human urine | 22 |
| Electrochemical Microfluidic Chip using Gate Effect Measurement | Warfarin Sodium | MIP modified Au–Ag alloy microwire (NPAMW) decorated on WE | - | CV, EIS | 8 pM | Warfarin sodium in real matrix | 31 |
| Electrochemical FET-based | Potassium ion | Ion-selective valinomycin-polyvinyl chloride (PVC) membranes coated on the floating electrode-based carbon nanotube (CNT) FETs | - | Semiconductor analyzer (4200-SCS, Keithley) | 1 nM | Nicotine solutions in the NMG buffer introduced to the cell media to stimulate the cells to produce potassium ions. | 47 |
| Colorimetric Plasmonic Nanobiosensor | Methotrexate (MTX) | Gold Nanoparticles (Au NPs) | Human dihydrofolate reductase (hDHFR) | UV–Vis spectrometry, TEM | 5 nM | MTX in PBS | 34 |
| Electrochemical-based wearable sweat band | Levodopa | Gold dendritic nanostructures decorated on WE | Tyronase enzyme | Amperometric, CV, SEM | 1 M | Sweat solution | 36 |
| Plastic Optical Fibre SPR Biosensor | Infliximab | Gold thin film functionalized with 11-MUA | Anti-IFX antibody | UV–Vis spectrometry | 73.7 ng/mL | 50-fold diluted human serum blood samples | 44 |
| Nanocellulose-based colorimetric assay kit for smartphone sensing | Deferoxamine | Nanopaper-based analytical device/curcumin-embedded in bacterial cellulose nanopaper (NAD/CEBC) bioplatform | - | Image captured by Smartphone Samsung Galaxy E5 (8.0 MP camera) then the mean color intensity measured using Adobe Photoshop CS5 | 8.2 nM | Human serum blood samples | 45 |
| Microfluidic Multiplexed Polymer-based Biosensor | SARSCoV-2 and Beta-lactam antibiotics | Platinum-patterned Polyimide Substrate | LbuCas13a and reRNA 20U | CRISPR | 2,000 and 7,520 copies/l | Human serum samples | 43 |
| FO-SPR Biosensor | Adalimumab | FO probes modified AuNPs | Anti-TNF antibody | FO-SPR readout system | 0.35 g/mL | Human plasma samples | 42 |
| Electrochemical biosensor | CPT-11 | Platinum electrodes | AChE/ChOx. | chrono-amperometry | - | PBS and FBS | 25 |
| Surface plasmon resonance (SPR) biosensors | Amikacin | Gold nanoparticles (AuNPs) | Anti-amikacin antibody | Homemade SPR biosensor based on the Kretschmann configuration under a prism-coupling scheme | 0.13 ng/mL | Amikacin in PBST buffer | 38 |
| Nanoplasmonic Biosensor/LSPR biosensor | Acenocoumarol | Gold Nanodisks (Ti/Au) decorated on WE | - | CCD spectrometry | 0.54 nM | Human plasma samples | 50 |
| Wearable electrochemical sensor monitoring | Acetaminophen | Screen-printed Carbon Electrode | Hydrogen-terminated boron-doped diamond | Cyclic voltammetry (CV) | 1 M | Human sweat and saliva | 41 |
| Electrochemical Biosensor | F17464 | Screen-printed Electrode | - | Differential Pulse Voltammetry (DPV) | 0.82 M | F17464 in PBS (pH: 7.4) | 37 |
| Electrochemical Biosensor | Doxorubicin | Glassy carbon electrode (GCE) | single-wall carbon nanotubes (SWCNTs) and ds-DNA | Electrochemical impedance spectroscopy (EIS) and Differential Pulse Voltammetry (DPV) | 0.6 nM | Doxorubicin in tris-HCl buffer solution pH 7.4 | 33 |
| Field-Effect Transistor-Based Biosensors | Calcium Ion | Gold electrode | Mammalian cardiomyocytes (H9c2) | Optical microscopy (OM) imaging and electrical measurements | - | Calcium Ion in buffer | 29 |
| Electrochemical biosensor | Level of i) two different -lactams and ii) piperacillin/tazobactam levels | Dry film photoresist (DFR) technology | PBP-3 and an ampicillin–biotin conjugate | Amperometry | - | Plasma, EBC, saliva, and urine | H.40 |
TDM is a process of monitoring the levels of drugs or chemicals in the body of patients to determine the appropriate dose and duration of treatment.1 This helps to minimize side effects and optimize treatment outcomes.2 TDM can also monitor substances produced in the body due to the treatment.
TDM is typically conducted by analyzing a sample of a patient’s bodily fluids, most commonly blood. The patient’s blood is analyzed using several tools, with high-performance liquid chromatography (HPLC-MS) being considered the gold standard.3 An immunoassay method can also be used to analyze samples with various kits. However, both methods are costly and have limited access to laboratories.4 As a result, there is currently a trend to develop small and flexible biosensors for TDM.
Biosensors are gaining attention as a cutting-edge technology for TDM. These sensors detect drugs and select optimal materials for the specific type of TDM being performed. Additionally, the trend in TDM is towards cost-effective solutions, which biosensors are well-suited to provide. Their compact size and flexibility also make them ideal for use in internet of things (IoT) based TDM systems. Furthermore, biosensors can potentially aid in the real-time monitoring of deadly diseases, greatly assisting medical experts in their treatment.5
In this paper, we review biosensor research for TDM. We explain the biosensors used for TDM, the types of drugs and chemicals detected using biosensors in the TDM process, and the future directions for developing biosensors for TDM. The review analyzes scientific articles from various research journals published in the last 15 years.
In this section, we explain the purpose of TDM in general, the considerations for using it in patients’ treatment, its history, and guidelines.
The purposes of TDM in the therapeutic process are the following1:
1. Assessing compliance;
2. Customizing treatment (during early therapy and/or dosage changes);
3. Recognizing under-treatment;
4. Preventing toxicity;
5. Keeping track of and identifying drug interactions;
6. Assisting with therapeutic discontinuation;
All the processes must be conducted from the start to the end of the treatment. Figure 1 shows the process details of determining the dose in treatment.
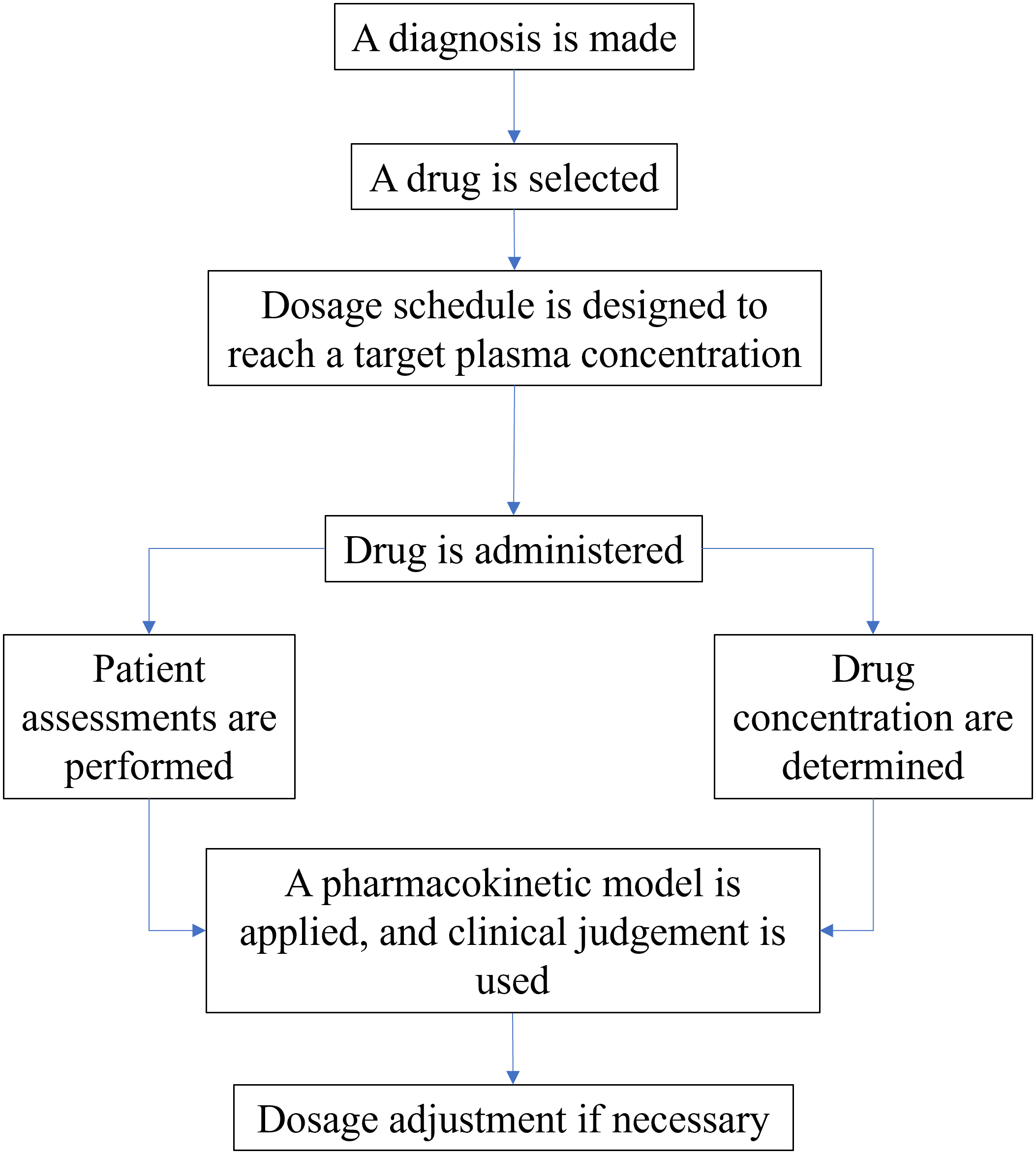
There are also several additional things to consider in applying TDM for patients. Some of these things are as follows:
Efforts by medical personnel to monitor therapy with drugs have been carried out since ancient times. However, the first recorded history of chemical substance or drug concentration calculation in a patient’s body was in 1932. Erik M.P. Widmark measures the concentration of alcohol in the human body to analyze the chemical components in the human body’s blood.6 Researchers have developed many technologies for therapeutic drug monitoring (TDM) since then.
The most rapid development period for TDM was in the mid-1950s to mid-1960s. Rosalyn Sussman Yalow developed a technique that uses radioactive materials to investigate the human body for small amounts of substances in 1959, called radioimmunoassay. She received the Nobel Prize in Physiology or Medicine for her discovery in 1977. Then, in the mid-1960s, Joseph Jack Kirkland discovered a method that would later become the gold standard of TDM, namely high-performance liquid chromatography (HPLC).6
The first time TDM was performed on treated patients was also in the mid-1960s. Frits Buchthal did a measure to find a clinical correlation between plasma concentrations of phenytoin in epileptic patients. In the following decades, researchers performed many TDM trials on various types of drugs. Carolyn Bertozzi introduced the term “biorthogonal chemistry”, which later became popular in determining drug dosages and the continuity of the TDM process in 2003, which led her to win the Nobel Prize in Chemistry in 2022. In 2019, TDM was used for the first time in a continuous treatment process. In recent years, there has been an increasing trend of producing cheap and flexible biosensors to assist the TDM process in patient care.7
In conducting TDM, there are guidelines that one needs to follow. These guidelines ensure drug concentrations in plasma or blood are within a desired range. Dealing with significant interpatient pharmacokinetic variability, therapeutic concentration-related effects, unclear therapeutic concentration ranges, and challenging-to-manage desirable therapeutic outcomes are a few things that must be considered when performing TDM.8
Several things must be considered in conducting TDM to ensure it runs smoothly, which are6:
1. Administering drugs to patients requires TDM: The psychiatry of the human body is complex, and the effects of drugs on patients can cause unexpected reactions. Clinical TDM currently targets a wide range of pharmaceuticals, including antineoplastic, anti-infective, immunosuppressive, cardioactive, respiratory-acting, neuropsychiatric, and mood-active agents.
2. Matrix selection: After determining the type of drug to be monitored, the next step is to select the type of sample containing the molecule of interest, with common choices being blood, urine, and saliva.
3. Interpretation of the results: The final step in TDM is to interpret the measurement results, which are monitored over a specific period based on the characteristics of the drug administered.
Analytical techniques are crucial for accurate TDM. Current standard practice for TDM involves separation, mass spectrometric analysis, and immunoassays on specialized equipment. A more recent advancement in chromatography, combining mass spectrometry and chromatography, has created a new potential instrument for TDM services. LC-tandem MS (LC-MS/MS) is currently the preferred technique among recently developed technologies for measuring therapeutic drugs in bodily fluids. This is due to its increased selectivity compared to single MS detection methods. Additionally, multi-component approaches can be used with LC-MS/MS, such as developing and successfully applying LC-MS/MS for simultaneous analysis of multiple immunosuppressive medications.9
While immunoassays require several steps to quantify therapeutic medicines, the ELISA (Enzyme-Linked Immune Sorbent Assay) approach can run numerous tests simultaneously. The widely used ELISA, a double-antigen test, cannot assess antidrug antibodies (ADAs) in the presence of medicines because the drug competes with the detection antigen. Therefore, this assay is only useful when trough levels are undetectable.10 The ability to multiplex is an advantage of ELISA technology. High multiplexing has its benefits, but it can also necessitate big enough samples to guarantee an analysis’s cost-effectiveness. The homogeneous mobility shift assay (HMSA), based on high-pressure liquid chromatography, is more promising than ELISA. The fact that HMSA separates drug-ADA complexes allows it to quantify both medicines and ADAs independently. The widely used ELISA has been used to validate this method. There was a significant link between drug levels and ADA titers, and HMSA also found ELISA-based false-positive ADA results.11
However, the infrastructure required for therapeutic drug monitoring using chromatographic, mass spectrometric, and immunoassay methods are expensive and often unavailable in smaller healthcare facilities and communities. When using separation techniques, the analysis is labor- and time-intensive because blood samples must be thoroughly prepared for analysis and the time required for separation on the device itself. Biosensors and nano biosensors are well suited to deliver a quick response for therapeutic drugs since they require less infrastructure and sample preparation.
Optical biosensors detect changes in light’s properties, such as refraction index, absorption, fluorescence, or scattering, when a receptor comes into contact with an analyte. These sensors are useful for identifying affinity or catalytic receptors and are more sensitive and versatile than other methods. They consist of an optical transducer system and a biorecognition element, such as surface plasmon resonance (SPR), localized SPR (LSPR), surface enhanced Raman spectroscopy (SERS), visual plasmonic colorimetric sensors, or SPR imaging (SPRI).8
An optical biosensor aims to provide a signal corresponding to the concentration of the studied analyte. It uses a variety of biological substances, such as enzymes, antibodies, antigens, receptors, nucleic acids, cells, or tissues, as biorecognition elements.12
Surface plasmon resonance spectroscopy
SPR utilizes the oscillations of free electrons at the interface of a metal and dielectric to detect changes in the refractive index at a surface. Light reflects off surfaces at different angles, each with a distinct refractive index. The absorbed light causes a reduction in the intensity of the reflected light. As the refractive index changes, the angle of minimum shifts, enabling monitoring.13
SPR is widely used in biosensing applications due to its versatility and the ability to detect a wide range of molecules. However, detecting and measuring small molecules, such as most drugs, can be difficult as they do not significantly alter the refractive index. Secondary detection methods using antibodies or functionalized nanoparticles can be employed to overcome this limitation to enhance the SPR response. Many biosensors for therapeutic drug monitoring are based on competitive assays, but these methods can have lower sensitivity. SPR is susceptible to nonspecific binding in complex matrices like many universal detectors. This occurs when molecules from the matrix produce a signal by depositing on the sensor’s surface without being recognized by a specific receptor. This can mask the signal from the analyte, making detection impossible. To address this issue, researchers have developed various surface chemistries to protect the SPR sensors from fouling agents commonly found in complex biological matrices.4
SPR has been used to find medicines in the past. One example is infliximab (IFX) detection employing fiber optic SPR with anti-IFX antibodies and AuNPs coupled to detection antibodies. Compared to ELISA, Pearson correlation coefficients of 0.997 were attained, demonstrating a substantially comparable outcome of SPR sensing technology with ELISA as TDM standard practice. This was demonstrated to be applicable in diluted whole blood, dried blood spots, and IFX-treated patients.14
SPR is effective in multiplex measurements, for example, simultaneous detection of IFX and the anti-IFX antibody. This is achieved by attaching tumor necrosis factor, used for detecting IFX, and IFX, used for detecting the antibody, to the flow channels of a microfluidic device. The concentration of both analytes can then be determined by comparing the SPR shift from the ligand strips to the control strips.15
Localized surface plasmon resonance spectroscopy
The use of gold and silver metal nanoparticles as small, highly sensitive label-free optical sensors has gained attention in recent years. These nanoparticles act as transducers by translating changes in the local refractive index into shifts in the LSPR band. This general LSPR-shift assay technique can test any chemical’s binding affinity or rate that alters the local refractive index near the metallic nanostructures. By adding molecular recognition components, such as chemical or biological ligands, to the nanostructures, specificity and selectivity to the target analyte can be achieved. The main difference between LSPR and SPR is that, in LSPR, the molecular receptor is immobilized using noble metal nanoparticles rather than a thin gold film as in SPR.16
In contrast to SPR, LSPR’s electromagnetic field is much stronger and close to the nanoparticle. It decays 40–50 times faster as it moves away from the surface, making it less sensitive to bulk changes in the refractive index but more sensitive to the adsorption of small molecules. This high intensity near the surface enables direct detection of small molecules with LSPR, making it well-suited for therapeutic drug monitoring, but most of these investigations are conducted in buffer or diluted matrices before they can be used in a clinical setting for the study of drugs.4
LSPR, using gold nanoparticles (GNP), has been used to detect therapeutic drugs with a limited therapeutic range, such as digoxin, by evaluating the interaction between digoxin and its monoclonal antibody from UV-Vis’s absorption spectrum, resulting in a limit of detection as low as 2 ng/mL and is still in digoxin’s pharmaceutical range with higher sensitivity at higher digoxin concentration indicating the biosensor works properly.17
LSPR has also been used to quantify third-generation cephalosporins in environmental samples and human serum. LSPR-bacteriolysis signals of bacteria on optical fiber probes have been used for rapid beta-lactam susceptibility testing, which can be used directly for TDM. These measurements were made using bacterial lysis signatures of Pseudomonas aeruginosa immobilized on gold nanoparticles modified optical fibers, which offer a rapid replacement for the time-consuming Kirby–Bauer disk diffusion tests typically used in tertiary care institutions and community health settings for drug susceptibility testing and faster, less expensive, and point-of-care antibiotic quantification alternative to chromatography coupled mass spectroscopic approaches with applications in therapeutic and environmental monitoring.18
Surface enhanced Raman spectroscopy
SERS utilizes metal nanoparticles or nanostructures to enhance the power of the Raman phenomenon. When two nanoparticles are placed close together, or if a metallic nanostructure has a rough surface, the localized electromagnetic field can be significantly increased, resulting in a significant amplification of the Raman scattering on the molecule adsorbed on the nanostructure, allowing researchers to track the number of molecules adsorbed on the metal, which depends on the drug concentration in the test solution.4
The metal surface significantly impacts the presence of very concentrated areas of extreme local field enhancement caused by surface plasmon resonance, which typically occurs in interstitial cracks in metal structures. Researchers create SERS substrates, or metallic nanostructures capable of causing a SERS effect, using a variety of approaches. These substrates, typically metallic nanoparticles coated with a Raman reporter and functionalized with specific receptors that recognize the analyte, make this technology appropriate for various biosensing applications. The therapeutic drug must promote interparticle connections for the aggregation process to take place.19
Many medications can be detected and quantified in simple solutions, but the process becomes more difficult when dealing with biofluids. SERS is a technique used to quantify target analytes in biofluids. Still, it can be difficult to do so in samples like a human serum because other biomolecules can interfere with the SERS spectra. A new study has found that using noble metal thin films derived from self-assembled NPs created using pulsed laser ablation (PLA) procedures can improve SERS by producing unique nanostructures with good film-level SPR stability and reproducibility, which allows for better control of the chemical enhancement route in SERS.20
Visual plasmonic colorimetric sensor
Visual colorimetric is a quick and easy label-free detection method involving observing color changes resulting from molecular recognition events. Metal nanoparticles, such as gold and silver, are commonly used as probes in colorimetric studies due to their unique electrical and optical properties. When these nanoparticles aggregate and approach one another, the surface plasmon band shifts to a longer wavelength, changing the color of the nanoparticles. The size, shape, distance, and medium of metal nanoparticles can significantly affect their surface plasmon resonance absorption.21
Colorimetric assays offer great potential for remote clinical effects and toxicity monitoring to optimize drug dosage and patient response at the point of care. In a research, a colorimetric sensor was used to detect the concentration of antidepressants in biological fluids. Citrate-capped silver nanoparticles (CIT-Ag NPs) were used as a probe to monitor fluoxetine concentration. The fluoxetine caused the CIT-Ag NPs to aggregate, resulting in a color change from yellow to dark brown and a shift in the absorbance peak and surface plasmon band. This demonstrates the potential of colorimetric assays to monitor drug concentration in real-time at the point of care.22
SPR imaging
SPRI is a technique that determines the spatial distribution of RI in individual cells. This method has been used to measure responses in various cells, such as rat mast cells, mouse keratinocytes, human epidermal carcinoma cells, and human basophils. Additionally, this technique can differentiate between the responses of different cell types grown together on the sensor chip.23
Piezoresistive sensors
Piezoresistive sensors convert mechanical events into resistance values. They can be modified with biomolecular receptors to interact with specific molecules, which causes a change in resistance due to molecular recognition. This makes them useful in various applications such as biosensing, drug delivery, and medical diagnosis.24
Microcantilevers
Microcantilever-based sensors have many advantages, such as being lightweight and not requiring fluorescent labeling, making them highly compatible with integrated circuits. Label-free microcantilever biosensors allow for downsizing, high sensitivity, and real-time analysis. A cantilever is a structural element that is supported at one end and carries a load on the other. Cantilever deflection can be detected using electrical or optical-level techniques. Electrical detection is more portable and suitable for point-of-care or individualized diagnostics as it does not require additional space and alignment for a laser, optics, and detectors.24
Electrochemical biosensors are devices that use the principle of an oxidation or reduction reaction to detect specific molecules. These sensors typically involve a coating of enzymes, which catalyze the reaction of the target molecule. This causes a change in the current flow, which can be measured and used to determine the concentration of the target molecule. Electrochemical biosensors are highly specific and sensitive, making them suitable for detecting a wide range of biomolecules. They are also relatively simple to use and can be made portable for point-of-care applications. However, they may require frequent calibration and maintenance to ensure accurate results.25
Electrochemical biosensors are a type of biosensor that use electrochemical methods for detection. These biosensors can be divided into conductivity measurement biosensors and potential measurement biosensors. Both types use electrodes as signal transducers, which convert biological changes into electrical signals. Recent advancements in electrical devices have made it possible to shrink these biosensors down to on-chip devices. This allows for in vivo monitoring, where the biosensor is placed inside the body or portable devices for on-site monitoring. This miniaturization has made it possible to use electrochemical biosensors in various applications, including medical diagnostics and environmental monitoring. There are a few ways the electrodes can be introduced to the desired sample26:
Microneedle based
Transdermal microneedle-based electrodes are used to detect various analytes in bodily fluids. These electrodes are receiving attention due to their ability to quickly and painlessly sense biomarkers in interstitial fluid. Additionally, they can be used for medication administration.27,28
Field effect transistor based
The electrodes are connected to the transistors’ gate and alter the conductivity of the transistor to change the drain current (Id). Researchers have increasingly focused on field-effect transistors (BioFETs) as biosensors over the past two decades because of their noninvasive detection, simple pretreatment, ultrasensitive response, and real-time readout. BioFETs have enabled molecular and cellular assays to shift towards point-of-care (PoC) diagnostics.29
Printed electrode based
Electrodes are created from conductive ink and printed onto a substrate. Traditional electrochemical sensors and biosensors used in transdermal microneedles (TDM) require specialized labs with expensive equipment and trained personnel, making them inefficient. Printed electrode-based technology offers a new alternative for sensors that are robust, repeatable, and sensitive to target molecules. These sensors are also low cost and easy to fabricate, making them non-labor intensive. This technology has the potential to revolutionize the field of TDM and make sensing and diagnostics more accessible and affordable. Printed electrode-based biosensors may also be proven helpful in the field of point-of-care diagnostics.30
The working electrode is decorated using the molecular imprinting technique (MIT), which uses synthetic materials obtained by polymerizing functional monomers and crosslinker molecules in the presence of a template. The measurement strategy based on the “gate-controlled effect” is then used, which has several benefits, such as anti-disturbance ability and adaptability. The MIP film has specificity for the analyte similar to an antibody, ensuring more accurate and trustworthy analysis. The strategy is independent of the analyte’s electrochemical activity, broadening the range of medicines detected by TDM. Ultrasensitive and selective electrochemical sensors have already been developed using MIT and gate effect basis, with MIP membrane serving as a suitable sensing agent due to its exceptional recognition capabilities toward target molecule.31
Pencil graphite electrode based
The working electrodes used are pencil leads, a more practical, quicker, and straightforward method of modifying the electrode surface compared to other methods. Electrochemical pre-treatment of the pencil-graphite electrodes (PGE) eliminates the need to modify the electrode surface with harmful substances. Utilizing pencil-graphite electrodes provides several benefits, such as avoiding sample contamination and being simple to use as they do not require any pre-treatment. They also meet ongoing sensitivity, selectivity, and repeatability requirements. The pencil lead electrodes are cost-effective, and the graphite material is easy to obtain. Additionally, pencil lead electrodes are a sustainable alternative to other electrodes as they can be easily replaced and recycled.32
Cancer is a major non-communicable disease that causes millions of deaths each year. One of the most commonly diagnosed types of cancer is breast cancer. Doxorubicin is a widely prescribed medication for treating breast cancer, as it has shown effectiveness over time. However, due to its potential side effects, it is important to administer it in controlled doses during chemotherapy. Monitoring and assessing its effects in biological contexts after therapy is crucial for ensuring its safe and effective use. Continual monitoring and assessment are necessary for safe and effective treatment.5
A highly sensitive and potent electroanalytical biosensor was successfully developed for detecting doxorubicin. Single-wall carbon nanotubes (SWCNTs) and ds-DNA was subsequently modified the surface of the glassy carbon electrode (GCE). Additional evaluation on the effectivity of the Doxorubicin-DNA complex also evaluated through molecular docking using several bioinformatic tools. The receptor molecule was kept rigid on the carbon surface while the target molecule (doxorucubin) allowed moving on its simulation freely. The simulation confirmed the intercalation of adenin-guanine bases from ds-DNA. Low detection concentration of 0.6 nM was reported using differential pulse voltammetric (DPV) technique.33
A plasmon-coupling assay has been used to detect methotrexate at clinically relevant doses using a naked-eye assessment. This test involved free methotrexate and folic acid gold nanoparticles competing for gold nanoparticles functionalized with human dihydrofolate reductase (hDHFR) (Au NP). The hDHFR-functionalized Au NPs were fixed on a glass sensor that was put into a portable 4-channel LSPR reader. This allowed for estimating the concentration of methotrexate quickly (in minutes) and sensitively (in the nanomolar range) using total internal reflection plasmonic spectroscopy. The assay caused massive bathochromic shifts resulting in spectacular color changes that were visible to the naked eye for methotrexate at clinically meaningful concentrations. The results indicate that therapeutic drug monitoring of standard chemotherapeutic treatment is possible through eye inspection.34
A new method for identifying potential anticancer drugs is being researched using electrochemistry to study DNA-drug interactions. One powerful naturally occurring anticancer medication is paclitaxel, derived from the Western yew tree. However, its effectiveness is limited by a narrow therapeutic window and patients’ varying elimination half-lives. A fast and reliable method for measuring paclitaxel is needed to address these issues. This method uses a pencil-graphite electrode and differential pulse voltammetry to study the interaction of paclitaxel with salmon sperm DNA. The reduction of guanine and adenine oxidation signals in response to paclitaxel contact was used as an indicator for paclitaxel detection. The experiment used a typical three-electrode cell with Ag/AgCl/KCl, platinum wire, and pencil-graphite electrodes as a reference, auxiliary, and working electrodes. The pencil-graphite electrode was prepared by sonication, rinsing, drying, and pre-treatment with ds-DNA in an acetate buffer solution.32
Chemotherapeutic drugs are used to treat various types of cancer, but they can cause side effects such as nausea and vomiting. To limit these negative effects and ensure patient compliance, it is essential to treat patients effectively. The primary treatment for chemotherapy-induced nausea is ondansetron (OND). However, traditional OND-monitoring methods can be expensive and require a well-equipped laboratory. To adjust its dose during chemotherapy, monitoring of OND is necessary. An ion-selective electrode potentiometry sensor is a low-cost, easy-to-use, non-destructive approach for OND monitoring. The sensor’s membrane design includes three variables: plasticizer, ion exchanger, and ionophores. The sensors are calibrated by measuring the potential differences between the working electrode and the counter electrode when immersed in OND solution, and pre-treated human plasma sample.35
Parkinson’s disease (PD) is the second most common neurological disorder. It causes disability, impacts the quality of life and financial stability of patients and their families, and increases healthcare costs. It is estimated to affect 7 to 10 million people worldwide, roughly equivalent to half the population of New York City.28
Levodopa (L-DOPA) has been the most effective treatment for Parkinson’s disease since it was first introduced in the 1960s. It has been the gold standard for treating the condition for over 50 years. L-DOPA is a precursor to dopamine, a neurotransmitter that is essential for motor pathways and is lost in Parkinson’s disease. By boosting dopamine levels, L-DOPA helps to alleviate the symptoms of Parkinson’s disease.28
There is a need for a wearable device to continuously monitor L-DOPA levels in the blood or other bodily fluids of Parkinson’s patients. Continuous monitoring requires a device that can be worn for long periods and frequently measure L-DOPA levels. Studies have been conducted on minimally invasive and non-invasive methods for monitoring L-DOPA in PD patients. One example is a wearable chemical sensing platform using a microneedle electrode array to monitor L-DOPA continuously. This device uses a unique dual-mode sensing approach that combines electrochemical measurements and redox and biocatalytic processes. This multimodal sensing approach improves the information capacity of the microneedle sensor array and increases redundancy. The device has excellent analytical performance when tested in a phantom gel simulating skin and in the skin of mice, with high sensitivity, a significant linear dynamic range, outstanding stability, and high selectivity. Such efficient L-DOPA monitoring in real-time would allow for appropriate dosing and better control of Parkinson’s disease.28
However, the effectiveness of L-DOPA can vary depending on the individual’s dietary habits, age, gender, and drug administration history. If the dosage is not adjusted accordingly, it can negatively impact the patient’s motor and cognitive abilities. Monitoring L-DOPA levels is important for the effective treatment of Parkinson’s disease. The best way to determine the correct dosage is to evaluate the patient’s motor abilities, but this approach can be challenging and make dosing uncertain. Point-of-care testing is difficult as it requires clinicians to assess the patient’s motor abilities.36
Human perspiration is a convenient and non-invasive alternative to blood as it contains many biomolecules. Like other pharmacological compounds, L-DOPA is excreted through sweat, and its concentration in sweat may be correlated with human plasma. A study designed a wearable sensor packaged into a sweatband (s-band) based on electrochemical detection, incorporating gold dendritic nanostructures onto the electrodes, resulting in a remarkable enhancement of the L-DOPA sensor’s detection limit to approximately 1 M in sweat solution. The sensor is produced on a polyethylene terephthalate (PET) substrate using a typical three-electrode configuration with a functionalized L-DOPA sensing electrode as the working electrode, an Ag/AgCl top layer as the reference electrode, and an Au top layer as the counter electrode. The tyrosinase enzyme can convert L-DOPA secreted in sweat during amperometric measurement to dopaquinone, generating a Faradaic current that can be calibrated to estimate the L-DOPA concentration in the resulting sweat.36
Biosensors are a crucial tool in disease detection and medication monitoring. Patch-type devices, which detect biomarkers in biofluids such as sweat, are particularly popular. These non-invasive devices allow for real-time and continuous analysis of biomarkers. Recent technological advancements have led to the development of smart e-patches for monitoring schizophrenia medication, and electrochemical sensors for continuous monitoring of drug intake through perspiration measurement.37
Pierre Fabre Pharmaceuticals created a wearable electrochemical biosensor called the Smart e-Patch to monitor a new medicinal molecule called F17464, a D3 antagonist that binds to dopamine D3 receptors. The research was completed using Nova 1.11 software, AUTOLAB PGSTAT204 compact, screen-printed electrodes (SPE) from Dropsense, a sweat collection patch from PharmChem, and chemicals from Sigma. The Smart e-Patch has three layers: a porous sweat adhesive, a sweat-collecting patch containing a biosensor for medication measurement, and a protective textile layer. It is based on a commercially available sweat-collecting patch with a 3-electrode configuration. An electrochemical biosensor is positioned close to the drug release patch and examined using differential pulse voltammetry (DPV) to detect the concentration of the B compound. Ascorbic and uric acid are used as interfering substances for sensor selectivity tests, while parameters that must be addressed include concentration, temperature, and pH. The Smart e-Patch is a wearable electrochemical biosensor that can detect the presence of F17464 in sweat and potentially transmit the data to a mobile device. The study has shown that the sensor is selective and capable of detecting F17464 and can be designed to continuously measure and monitor F17464 in sweat without interference from other pharmaceuticals.38
Epilepsy is a complex disorder caused by neurological circuits, cells, and molecules dysfunction. Antiepileptic drugs (AEDs) can effectively reduce the frequency of seizures. Still, the type and dosage of these medications are crucial due to their direct interaction with the central nervous system and brain. Currently, around 30 targeted AEDs available, but challenges such as medicine resistance, unpleasant side effects, and precise monitoring remain. Examples of AEDs include phenobarbital, primidone, phenytoin, carbamazepine, oxcarbazepine, valproate, ethosuximide, gabapentin, and lamotrigine. TDM of AEDs is necessary to optimize drug administration due to the unpredictability of pharmacokinetics, limited target range, and difficulty in separating toxicity symptoms from laboratory findings. Utilizing nanoparticles can facilitate the examination of these compounds.26
A nanocomposite sensor of reduced graphene oxide and Pt nanoparticles immobilized on a modified glassy carbon electrode (nPtGN/GCE) has been developed. The electrochemically catalyzed phenobarbital (PB) reactions with the modified electrode showed exceptional activity. This sensor was used to develop a mediator-free PB detection sensor, which has proper LOD and linearity, and can reliably detect PB in actual samples.26
Biosensors are becoming increasingly crucial in anti-epileptic drug (AED) monitoring due to their ability to simultaneously measure multiple AEDs and their metabolites in biological samples. These new techniques are expected to simplify, quicken, and measure AED levels in the blood more cost-effective. They can be used in pharmacokinetic research, bioequivalence testing, and therapeutic drug monitoring. One of the AEDs, phenytoin, has a narrow therapeutic dose range that requires therapeutic drug monitoring. Sensors based on piezoresistive microcantilevers have been developed for detecting chemical interactions and biomolecular recognition in a liquid environment. These sensors can detect surface tension or deflection changes and associated resistance changes. They can detect phenytoin by using immobilized antibody molecules.24
TDM of antiepileptic and antipsychotic drugs has been done for decades, but TDM for antidepressants is still relatively new. Assessing treatment safety and efficacy through TDM of antidepressants is particularly valuable in primary care settings.
Antidepressant medication is used during the acute phase of the condition to produce remission without creating adverse effects and to prevent recurrence or relapse during ongoing or maintenance therapy. To achieve these objectives, medicine selection and dosing must be individualized for each patient. TDM may be helpful in primary care for monitoring adherence, adjusting medication, preventing toxicity, and documenting idiosyncratic reactions.39
Wearable sensor technology can meet the need for precision medicine, but on-body devices for quick, targeted medication screening are still a relatively new concept. A wearable sensor that is adaptable, deformation-resistant, and non-invasive is incorporated into gloves to selectively and non-invasively measure therapeutic drugs and biomarkers in sweat samples. Using a screen-printing process and carbon ink for the working and auxiliary electrodes, the sensors were manually printed on the fingertips of the gloves using a squeegee. The sensor inserted in the glove can simultaneously monitor paracetamol, paroxetine, ethinylestradiol, and uric acid as significant health indicators, highlighting the potential for pharmacological and biomarker monitoring in patients with bipolar disorder, depression, hormone replacement therapy, and early menopause to improve therapeutic efficacy with minimal adverse effects.30
Antibiotic monitoring is crucial to prevent antimicrobial resistance. A minimally invasive method, such as microneedle biosensors, is preferred for tracking the administration of antibiotics, including beta-lactam antibiotics. These biosensors can penetrate the stratum corneum and minimize pain and blood drawn, as they do not reach blood vessels or nerve endings in the dermis. Research has shown that a microneedle-based real-time continuous antibiotic detection method using a pH-sensitive iridium oxide layer can detect local pH changes caused by -lactam interaction with -lactamase.27
Etimicin (ETM) is a drug with a limited therapeutic index, making monitoring its use crucial for clinical use. Different analytical methods for determining ETM have been proposed, but there are still unsolved problems. AgNPs, with their unique electrical and optical properties, possess higher absorption coefficients than AuNPs, allowing for sensitive colorimetric detection. A test tube containing an appropriate concentration of ETM in a urine sample matrix and cit-capped AgNPs can be transferred to a spectrophotometric cell to measure the absorbance of various ETM concentrations, and the sensing system’s color change can also be captured by a digital camera.21
A biosensor is a valuable tool for monitoring the concentration of digoxin, a drug with a narrow therapeutic range, in human blood serum. Surface plasmon resonance (SPR) technology is an effective method for detecting immediate chemical contact, and gold nanoparticles (GNPs) have proven helpful in SPR-based analysis. The LSPR property of GNPs allows for developing sensors with high detection accuracy. The GNPs were synthesized using the Turkevich method and the concentration was determined using UV-Vis spectrophotometry. The anti-digoxin monoclonal antibody was then immobilized on the functionalized GNPs, and the interaction between digoxin and the antibody was evaluated using the UV-Vis absorption spectrum.17
An SPR biosensor has been developed for therapeutic medication monitoring amikacin (AK) in blood serum or plasma. Due to its toxicity risks and narrow therapeutic range, AK is an antibiotic that requires TDM. The sensor is highly sensitive and desirable for TDM, with a sensitivity of 1.4 ng/mL and a limit of quantification of 0.33 ng/mL. The sensor is based on an indirect competitive immunoassay with AK immobilized on a gold surface and has good reproducibility and reusability.38
A microfluidic biosensor, called miLab, has been developed for simultaneous monitoring of piperacillin/tazobactam and beta-lactam antibiotics in various biological samples such as plasma, bronchoalveolar lavage fluid, saliva, and urine. The biosensor was fabricated using dry film photoresist technology, and it consists of two zones: an immobilization area and an electrochemical cell, separated by a hydrophobic barrier to prevent contamination. The platform uses electrochemical sensing to detect specific antibiotics, and it has been validated using HPLC (high-performance liquid chromatography). The results of this study were in good agreement with the gold-standard analysis and showed that the antibiotics’ concentration and clearance behavior were accurately monitored.40
Several autoimmune disorders are caused by tumor necrosis factor-alpha (TNF). Adalimumab (ADM), which has a high specificity and affinity for TNF, makes anti-TN agents safe and efficient in biological treatments. To address such problems, tailored TDM is crucial since it can guide dosing by capturing the dynamic pharmacokinetic profile of the patient’s prescription medication during therapy.41
A portable sensor for detecting ADM in plasma samples has been developed to provide quick, accurate results within 12 minutes. The sensor combines a fiber-optic (FO) sensing probe for bioassay implementation, an FO-SPR readout device, and a disposable iSIMPLE microfluidic cartridge. The device can also be used for TDM of other biological medications at the point of care. The sensor is highly sensitive, provides real-time monitoring, and is versatile, compact, self-powered, and mobile.42
TDM has been developed for COVID-19 monitoring to help track the efficacy of antibiotic therapy for bacterial co- or superinfections that may occur during SARS-CoV-2 infection, which can compromise the immune system. A microfluidic multiplexed polymer-based electrochemical biosensor combined with CRISPR/Cas-powered assays for nucleic acid testing was able to identify SARS-CoV-2 from diverse biomolecule classes and detect the presence of protein-based -lactam antibiotic. This method is able to identify individuals infected with variants of SARS-CoV-2, and can be done without cross-contamination.43
A label-free biosensing device for TDM has been developed to detect infliximab therapeutic antibodies using plastic optical fiber (POF). The device uses the SPR sensing technique to detect specific binding between anti-IFX antibody immobilized on the POF sensing region and IFX diluted in buffer and human serum. The results showed that the device could detect IFX in a concentration-dependent and specific manner, with LODs of 23.5 and 73.5 ng/mL for buffer and human serum, respectively. However, the LOD obtained by this POF biosensor is insufficient for measuring the lowest clinically relevant concentrations of IFX. This limitation can be improved by conjugating the SPR-POF sensing region with gold nanoparticles, which have been previously demonstrated to increase sensitivity through excitation of LSPR.44
A study has developed a decentralized and affordable method for TDM by using smartphones and portable spectrometers. The method uses a colorimetric change in a nano paper-based analytical device/curcumin-embedded bacterial cellulose nano paper (NAD/CEBC) bio platform to detect Fe(III) and deferoxamine. This is achieved by creating a Fe(III)-curcumin complex, which liberates curcumin from the bio-platform in Fe(III) presence and reduces color intensity. The smartphone camera is placed in a homemade light control box to observe the color changes and the average color intensity of images captured with a smartphone camera is determined. This method produced a LOD for deferoxamine of 8.2 nM with an RSD value of 3.6% and is highly equivalent to conventional spectrometers.45
An n-channel metal-oxide-semiconductor field-effect transistor (n-MOSFET) is a reliable biosensor that can identify a single target and be used for continuous medication monitoring. The device has an enlarged gate and a drug-specific DNA aptamer that addresses the issues. This biosensor can detect the presence of the targeted pharmaceutical tenofovir (TFV) and can be employed for real-time drug monitoring by real-time monitoring impedance tests. The tests reveal the quality of TFV binding to the aptamer and the fewest non-specific interferences under flow conditions. The biosensor works by using an external gold electrode that is functionalized with a drug-specific aptamer and exposed to the measurement sample, which is physically connected to the gate of the transistor. When a voltage is applied, a current flows through the induced channel beneath the gate dielectric.46
An ion-selective sensor was developed to monitor drug effects on nicotinic acetylcholine receptors (nAChRs) in living cells. The sensor uses floating electrodes and a carbon nanotube field-effect transistor (CNT-FET) coated with a potassium ion-selective membrane. This allows the device to recognize potassium ions flowing through the membrane, which are released in the body by stimulating nAChRs on PC12 cells with acetylcholine or nicotine. This technology allows for quantitative monitoring of the effects of drugs on these receptors in real-time.47
Biosensor technology has the potential to greatly improve the therapeutic drug monitoring (TDM) process for patients. Biosensors are highly flexible and small in size, which allows for continuous, real-time TDM. Some future development directions for biosensors in TDM include incorporating multianalyte sensing to achieve thorough TDM evaluation, developing a sensing-therapeutic feedback system that utilizes drug delivery to enable a smarter healthcare platform, overcoming the obstacle of power by leveraging energy harvesting or storage methods to enable continuous operation, improving wireless communication methods to send real-time monitoring findings over long distances, developing integrated, multifunctional, and self-sustaining systems for wearable chemical sensors and incorporating point-of-care testing to make TDM more accurate and closer to the patient’s condition.
TDM is traditionally done in laboratory settings. However, recreating conditions that closely mimic the patient’s environment is difficult. The term “point of care” (POC) or bedside testing refers to diagnostic tests that are not performed in a laboratory but rather as close to the patient as possible. POC TDM is believed to improve patient care by providing more accurate results in a patient’s specific environment.48
Fiber optic surface plasmon resonance (FO-SPR) combined with self-powered microfluidics is one example of biosensor development towards POC testing, specifically for the therapeutic drug monitoring (TDM) of adalimumab. This study demonstrates the potential of FO-SPR biosensing in POC using adalimumab as a model case. It highlights the immense potential of FO-SPR biosensing in POC, and it also has promise for bedside TDM of other medications such as immunosuppressants, antiepileptic therapies, etc. This is because bioassays are very customizable.42
The internet of things (IoT) is rapidly advancing, and wearable physical sensors are becoming increasingly important for healthcare monitoring. These sensors, such as those used for therapeutic medication monitoring, allow for real-time patient health monitoring. In addition to physical sensors, chemical sensing technologies also show great potential for use in personalized healthcare. The future of wearable chemical sensors is expected to include integrated systems that can sense, treat, and sustain themselves. For these systems to be successful, chemical and physical sensors must be developed and integrated into the next generation of IoT sensors for therapeutic drug monitoring (TDM) practice. These sensors will be critical in advancing personalized healthcare through real-time monitoring and treatment.
Multianalyte sensing is a promising approach for thorough TDM evaluation. Developing a sensing-therapeutic feedback system that utilizes drug delivery is essential to achieve smart healthcare. Power is a significant obstacle for continuous TDM, but energy harvesting and storage methods can overcome this. Short-distance communication is a limitation of current wireless communication methods for real-time monitoring. For wearable chemical sensors to be effective, it is necessary to integrate software and hardware and develop wireless technologies for long-distance data exchange.49
One of the limitations of biosensors is their detection specificity, as they are typically designed to detect only one type of molecule. However, current research is focused on developing biosensors that can detect multiple analytes at once. This is known as multiplexing and is being applied in TDM. One way to achieve multiplexing is by creating a biosensor platform to detect multiple analytes on-site in TDM for antibiotics. This type of biosensor can provide fast, accurate, and cost-effective results. The multiplexed biosensors can potentially improve the treatment and monitoring of patients receiving antibiotics.40
Biosensors can detect multiple analytes, requiring various reagents and enzymes to be implanted. Current biosensor development is focused on detecting one type of molecule from multiple samples, such as blood, urine, saliva, and sweat. Research40 has shown that the detection accuracy of a specific molecule in plasma, EBC, saliva, and urine is high compared to the gold standard for TDM analysis, the HPLC. The use of biosensors in TDM analysis is a promising field of research.
In this article, a review was carried out on biosensor technology to assist the therapeutic drug monitoring (TDM) process. The development of TDM began in the early 1900s and continues to the present. Due to the need for TDM that is flexible, low-cost, can measure in real-time, and can be used comfortably by patients, biosensors are a promising technology for TDM processes.
This article explains several types of biosensors that are being developed for TDM. These types are optical sensors, mechanical sensors, and electrochemical sensors. The types of drugs that can be monitored by TDM are increasingly diverse every year. Currently, the types of drugs that TDM can do with biosensors are anti-cancer drugs, neurological diseases such as Parkinson’s and schizophrenia, antiepileptics, antidepressants, antibiotics, and diseases caused by viruses such as COVID-19.
With so much research on biosensors for TDM, several developments will come. Several things being developed in biosensors for TDM are enabling POC for TDM, combining biosensors with IoT systems for TDM, and enabling multiplexed on-site for TDM.
WS: Writing - Review & Editing, Supervision, Project Administration, Funding Acquisition. AS: Writing - Review & Editing, Supervision, Project Administration, Funding Acquisition, IA: Conceptualization, Methodology, Validation, Investigation, Resources, MFN, ACHA, DAS, FAN: Data Curation, Writing - Original Draft, Visualization, CS: Supervision, Project Administration, Funding Acquisition.
We thank the School of Electrical Engineering and Informatics, Institut Teknologi Bandung, and the School of Electrical Engineering, Telkom University for the research support.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the review discussed comprehensively in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
No
Is the review written in accessible language?
No
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology, TDM, chromatography
Is the topic of the review discussed comprehensively in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
No
Is the review written in accessible language?
Partly
Are the conclusions drawn appropriate in the context of the current research literature?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: TDM antibiotics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 13 Feb 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)