Keywords
Ganoderma lucidum, chemical-induced corneal ulcer, bacterial-infected ulcer, anti-inflammatory, anti-infective
Ganoderma lucidum, chemical-induced corneal ulcer, bacterial-infected ulcer, anti-inflammatory, anti-infective
Corneal ulcers are estimated to cause 7.2% of corneal blindness1 and are thus a significant contributor to blindness. In Kenya, corneal injuries have been reported to account for an estimated 2.7% of corneal blindness.2 While corneal injuries are common, access to treatment and medical care is limited, and this may be due to a lack of awareness of treatment options, lack of accessibility and high cost.3 Lack of appropriate and prompt management of ulcers leads to poor wound healing and subsequent formation of scars, resulting in partial or total vision loss.
Antibiotics act by interfering with the metabolic processes or with the organism’s structures. However, there are growing concerns about the number of drug-resistant bacterial strains4 and many disease conditions are becoming more difficult to treat due to the emergence of antibiotic-resistant bacteria.5 In 2010, the World Health Organization (WHO) recommended that all countries implement control procedures to mitigate the effects of multi-drug-resistant bacteria.6 It emphasized the urgent need to identify alternative therapies against drug-resistant microorganisms in low- and middle-income countries, such as Kenya. In addition, Kenya has been reported to have a high utilization rate of unorthodox medical treatment due to challenges of access to orthodox medicine, cost and cultural beliefs.3
A mushroom is a fruiting body of fungi that is non-photosynthetic and feeds on organic matter and plant organisms.7 Ganoderma lucidum is a type of mushroom which has basidiocarp, mycelia and spores that contain approximately 400 different bioactive compounds.8 G. lucidum as a mushroom is in the family of Ganodermataceae, a family consisting of a large group of tree fungi of the genus Ganoderma. G. lucidum has been classified into Kingdom Fungi, Phylum Basidiomycota, Class Basidiomycetes, Sub-class Homobasidiomycetes, Order Polyporales, Family Ganodermataceae, Genus Ganoderma and Species lucidum.9,10 The mushroom has been reported to have several pharmacological effects, such as immunomodulation, analgesic, anti-atherosclerotic, anti-inflammatory and antibacterial, among other medicinal benefits to the human body.8 The antibacterial effect of G. lucidum has been shown to have a healing effect on non-ocular related inflammatory injuries and infective ulcers in other parts of the human body.11 Many ulcerative conditions are becoming difficult to manage due to the increasing numbers of drug-resistant bacteria strains such as Staphylococcus aureus, Pseudomonas pyocyanea, Streptococcus pneumoniae, Pseudomonas aureginosa.5,12 G. lucidum has been proven to be effective in managing bacterial infections that have shown resistance,13 with reports indicating it to be an effective alternative to treating bacterial infections and inflammatory injuries to the human body.14 G. lucidum is commonly available in Kenya.7 This study aimed to investigate the effects of G. lucidum on chemical-induced and bacterial-infected corneal ulcers in rabbits’ eyes. Furthermore, the study aimed to compare the effects of G. lucidum with those of standard orthodox treatment approaches to chemical and bacterial corneal ulcers to establish a possible relatively cheaper alternative and/or complementary treatment to these corneal injuries.
This randomized-controlled experiment was conducted in the animal house of Masinde Muliro University of Science and Technology (MMUST), Kakamega, Kenya, which is located in the western part of Kenya and 1 km from Kakamega central town. In this experiment, a pre-determined power analysis, assuming a large Cohen’s d effect size, 0.8, required a minimum total sample size of 15 to elicit 80% power for a 2-tailed test hypothesis at α=0.05, to detect a 10% mean difference between two homogenous groups in comparison. In this regard, 16 healthy New Zealand rabbits weighing 1.5 kg to 2.0 kg, without gender bias, were purchased from the local Kakamega animal market and were adapted for two weeks. These were randomly allotted to four groups of four animals each in separate housing. Each group of four animals were housed in a standard size cage with a floor area of 7.5 square feet and at least 18 inches high. These were cleaned on a daily routine, throughout the experiment. During the period of adaptation, they were dewormed with piperazine® (anti-helmintic drug), fed with Amilyte® food supplement – an anti-stress agent, as well as Bovitam® – a multivitamin. The animals were fed with pellets, elephant grass, cabbages (Brassica oleracea var. capitata L) and clean water for drinking. Before the commencement of the experiment, each animal was pre-tested for normal physiological functions, such as the eyelid, lacrimation and corneal statuses, and also re-tested 12 hours after the introduction of corneal injury for changes from the baseline physiological findings. The rabbits, before grouping, were labelled 1 to 16 and then randomly assigned to four groups of four animals each as follows: A: Experiment 1; B: Experiment 2; C: Positive control; and D: Negative control. This random assignment to groups was done using a computer-generated random system15 which was without bias to the gender or weight of the rabbits. All animals included in the experiment had equal physiological characteristics as per the baseline pretesting conducted.
Group A: Experimental group 1. The rabbits were treated with G. lucidum extracts following exposure of their cornea to a chemical-induced injury.
Group B: Experimental group 2. The rabbits were treated with G. lucidum extracts following exposure of their cornea to a chemical injury and the subsequent infection with P. aeruginosa.
Group C: Positive control group. The rabbits were treated with a standard treatment approach for corneal ulceration, that being two hourly drops of fluoroquinolones (ciprofloxacin), eight hourly atropine sulfate and four hourly betamethasone ophthalmic formulations.
Group D: Negative control group. The rabbits received eight hourly drops of atropine sulphate, both as a placebo and to relieve the pain, without the intention of providing normal treatment.
Instruments for data collection included the pen torch, a magnifying loupe, Keeler® ophthalmoscope, 2-mL hypodermic syringes and needles, sample tubes, micropipettes and pipettes. Others included beakers, spatulas, a weighing scale, a water bath, a mortar and pestle, a sterilized laboratory grinder, Turtle stand, What-man® filter paper, Dettol®, gloves, a small bucket with a cover, as well as Methylated spirit, Vaseline® jelly, a razor blade and cotton wool. Dean–Stark apparatus, clean distilled water, double pocket condenser, a 5L-3-necked round-bottomed flask, Ganoderma mushrooms and a camera were also required. Drugs used for this experiment were: freshly prepared crude aqueous extract of G. lucidum, tetracaine hydrochloride 0.5%, atropine sulfate 1%, Probeta-N® (betamethasone-neomycin), Ceprolen® (ciprofloxacin), Nepuscein® (fluorescein) strips, Bovitam®, Amilyte®, Piperazine®, ketamine hydrochloride and 1 Molar sodium hydroxide.
The experimental procedure employed in this study commenced with two weeks of acclimatization of all 16 animals as described previously above. Additionally, in other to minimize pain and suffering among the animals during the procedure, both systemic and topical anesthetics were used and each animal was taken to a separate room, out of sight of the other animals during each experimental procedure. Generally, the experimental procedure commenced with the use of systemic anesthesia (1 mL/50 mg of ketamine hydrochloride) which was injected intramuscularly into each of the rabbits in groups A, B, C and D. Five minutes later, two drops (1 mL of 0.5%) of tetracaine hydrochloride (topical anesthesia) was instilled into the right eye only, of each rabbit. Three to five minutes later, a corneal sensitivity test was performed on each rabbit using the cotton swab test to ensure that the right eye cornea of each rabbit was fully anaesthetized. For confirmation, the same procedure was done on the left eye to verify their response to the cotton tips rubbed on the cornea. Following confirmation of corneal anesthesia in the right eye, a drop of 0.2 mL/1 Molar sodium hydroxide (NaOH) was gently instilled on the central corneal surface using a cotton swab. At the same time, the corneal periphery and conjunctiva areas were guided from the spread of the chemical with another set of cotton wool swabs. The cotton swabs were placed around the limbus of the right eye, while the chemical was being allowed to soak into the corneal epithelium.
This procedure was performed under minimal magnification, using a 10× magnifying loupe. The affected central cornea area was then assessed for successful induction of injury by applying a stain with a swipe of fluorescein strip and observing with the blue illumination of a Keeler® professional ophthalmoscope under 20× magnification. The right eyes were padded and left for 12 hours and then observed again. Ocular manifestation (signs of inflammations and possible infections) following the induction of chemical injury was observed after 24 hours. The following parameters were used to assess for ocular manifestations: eyelid and conjunctival status, lacrimation, epithelial disruption, corneal fluorescein staining, photophobia (using a flashlight), corneal oedema and infiltrates. The ocular manifestations were consistently graded on a scale of 0 to 5 (0: normal/no change observed; 1: very mild; 2: mild; 3: moderate; 4: severe; and 5: very severe).16 The rabbits in groups B, C and D were then infected with two drops of a laboratory-prepared solution of P. aeruginosa and padded for another 24 hours, after which they were examined for signs of active inflammation and infection, with heavy mucopurulent discharges and stuck eyelids being evident.
The G. lucidum extracts used for the treatment of the animals in groups A and B were prepared by dissolving 65 g of cleaned pieces of the mushroom, ground to fine powder, in 5 L of distilled water in a hydro distillation process, using the Dean–Stark apparatus. The process was set at 40°C and left to run for 9 h to obtain the saturated crude extract which was then stored at −10°C before bioassay. After 24 hours, treatment commenced with the animals in groups A and B with the fresh crude aqueous extract of G. lucidum now formulated into ophthalmic suspension at a concentration of 40 μg/mL.13 All the rabbits in the two groups were treated with G. lucidum extracts. Two drops of the suspension were instilled every two hours at the start, and then gradually tapered through seven days. Observed ocular changes following commencement of treatment were both photographed and recorded according to the stated grading system, both being taken systematically in an order of 1st hour, through the 12th, 24th, 48th, 72nd, 96th, 120th, 144th up to the 168th hour. Treatment was tapered following noticeable improvement (possible healing) on the ocular inflammations and infections.
Treatment of the animals in group C also commenced 24 hours following the induction of ulcers on the right eye cornea of each animal. A standard treatment protocol for chemical (alkaline) induced ocular injury and bacterial-induced corneal ulcers was employed. This consisted of two hourly drops of fluoroquinolone (ciprofloxacin), six hourly drops of corticosteroid (betamethasone) initiated after 24 hours of treatment with fluoroquinolone, and eight hourly drops of atropine sulphate 1%.17,18 These were gradually tapered following the observation of significant reductions in ocular inflammations and infections. As with the experiment groups, photo shoots and recordings according to the stated grading system were done starting after the 12th hour of treatment with the standard protocol, through every 24 hours up to the 168th hour. While no positive active treatment was given to the rabbits in group D, in line with the treatment schedule in groups A, B and C, cycloplegic agent (atropine sulfate 1%) was instilled topically (1 drop), four hourly on the right eye cornea of each rabbit. The use of the cycloplegic agent applied to the animals in group D was an interventional protocol introduced in other to minimize pain, suffering and distress for animals in this group that served as a negative control in the experimental design. Observations (photographs and recording of parameter changes) were done according to the schedules of groups A, B, and C.
In keeping with ARRIVE guidelines on providing a humane endpoint for animals used for this experiment, a Veterinary expert from a local Agrovet store, was professionally engaged to euthanize all the rabbits at the end of the experiment period.
All data collected pre- and post-experiment were entered for groups A, B, C, and D into a statistics software (SPSS, version 23) for analysis according to within and between groups comparison. The findings for ocular morphologic changes were entered quantitatively and coded on the grading scale of 0 to 5 based on severity,16 with the findings in all groups being presented in tables. Considering the small sample size of this experiment, alternative non-parametric tests to paired t-tests and independent t-tests were used for statistical analysis of the results. All comparisons were made at α=0.05 (95% CI) using Wilcoxon signed-rank test for correlated (within groups) variables and the Mann–Whitney U test for independent (between groups) variables.
Data were collected from 16 healthy New Zealand rabbits weighing an average of 1.4±0.42 kg. Ocular parameters for various observations following treatment for 168 hours after the introduction of chemical injuries and subsequent infection with P. aeruginosa were coded according to the grading scale of 0 to 5 and these are presented in Tables 1 to 4. Table 1 shows a comparison of the healing effects of G. lucidum extract on various ocular parameters following induction of chemical injury, using 1 Molar sodium hydroxide, for animals in group A (experiment group 1) at two-time points: at the 24th and 168th hours of treatment. The statistical presentation shows that although treatment with G. lucidum extract had a significant anti-inflammatory effect on just a few ocular structures: eyelid and conjunctival status changes, reduced lacrimation, reduced corneal staining and oedema, the clinical effect of treatment with the extract in all ocular structures observed were highly significant, particularly with the reduction in corneal infiltrates (effect size=74%). These pictural findings are shown in Figure 1.
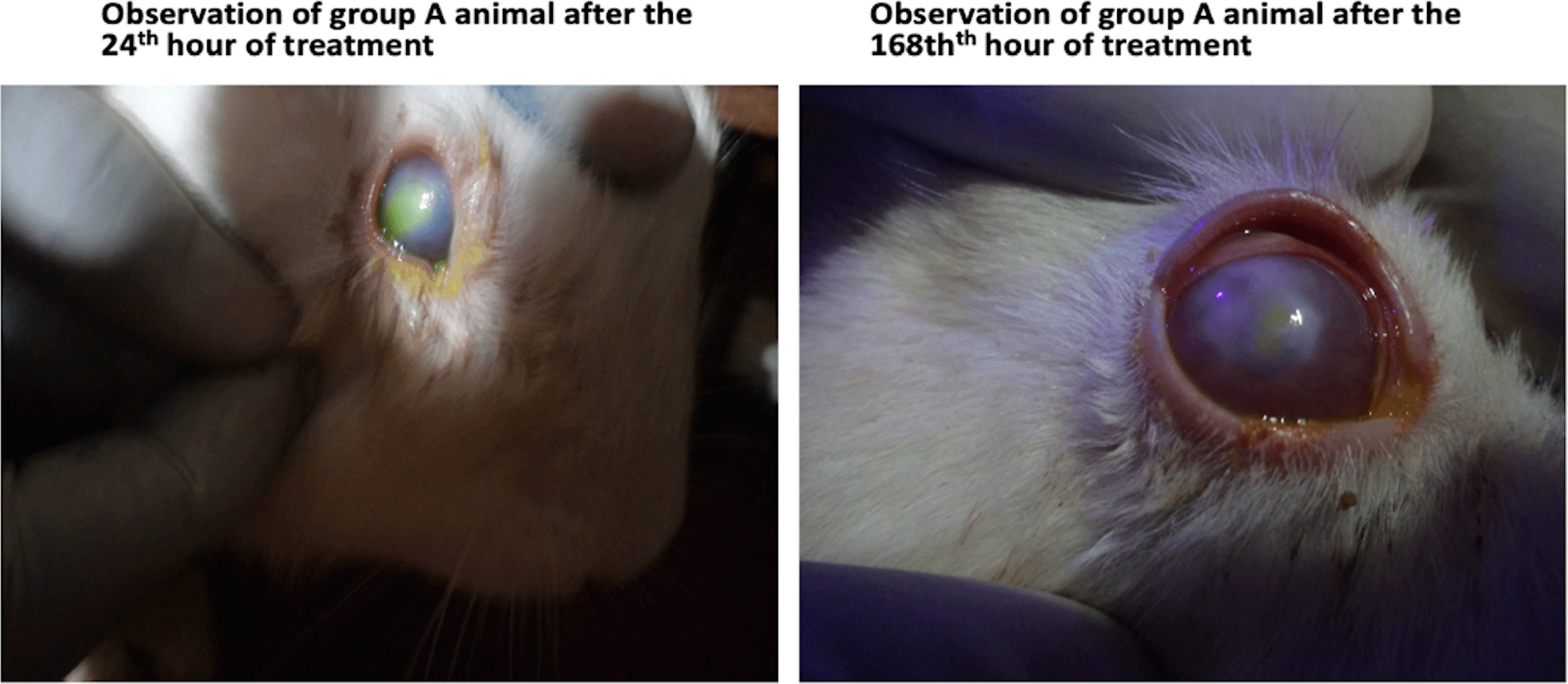
As shown, animals in group A (right) appeared to have healed, with fewer discharges, lacrimation and better corneal epithelial changes, after 168 hours of treatment with G. lucidum.
Table 2 shows the manifestations of various ocular parameters for animals in group B (experiment group 2) at the first 24th hour following injury with 1 Molar sodium hydroxide, and subsequent infection with P. aeruginosa 12 hours thereafter (to induce bacterial ulcer), and at the last 168th hour of treatment with G. lucidum. The results show that treatment with G. lucidum extract had no significant difference (p>0.05), in the statuses of most of the ocular parameters after the 168th hours, except for a significant reduction in photophobia and lacrimation (p<0.05). However, the clinical effects of treatment with the extract of G. lucidum for bacterial infected corneal ulcers in all ocular parameters observed were clinically significant (effect size>5%), with reduction of ocular discharges being most significant (effect size=93%). This finding is also shown pictorially in Figure 2.
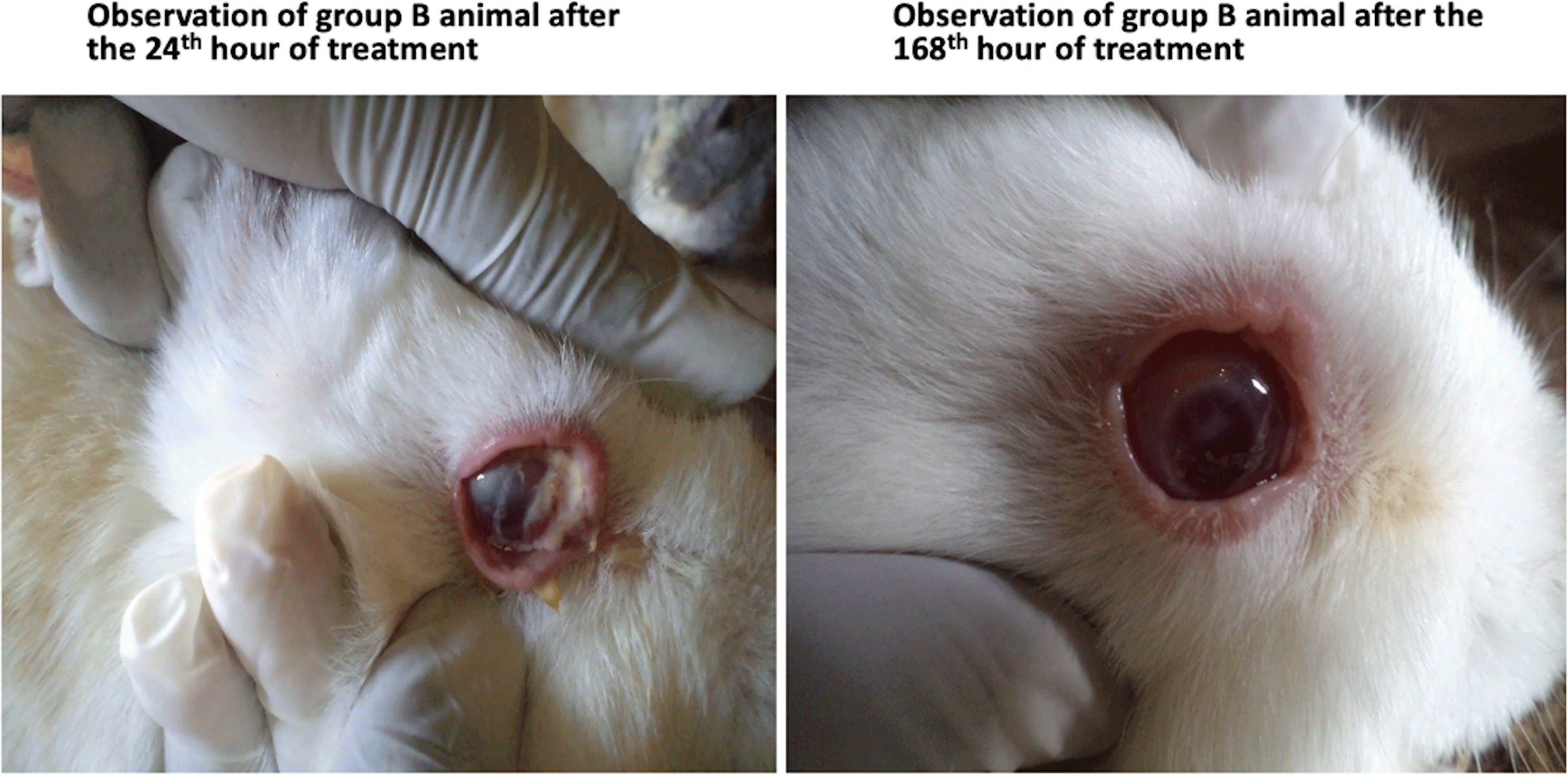
As shown, animals in group B (right) appeared to have healed better, with fewer discharges, lacrimation and better corneal epithelial changes, after 168 hours of treatment with G. lucidum.
Table 3 shows a comparison between manifestations of various ocular parameters for the animals in groups B and C after 168 hours of treatments with G. lucidum in group B (experimental group 2) and the standard treatment protocol in group C (positive group). The results show that although treatment in group C had better clinical effects than in group B, the difference in the treatment was, nonetheless, not statistically significant (p>0.05). This finding is also shown pictorially in Figure 3.
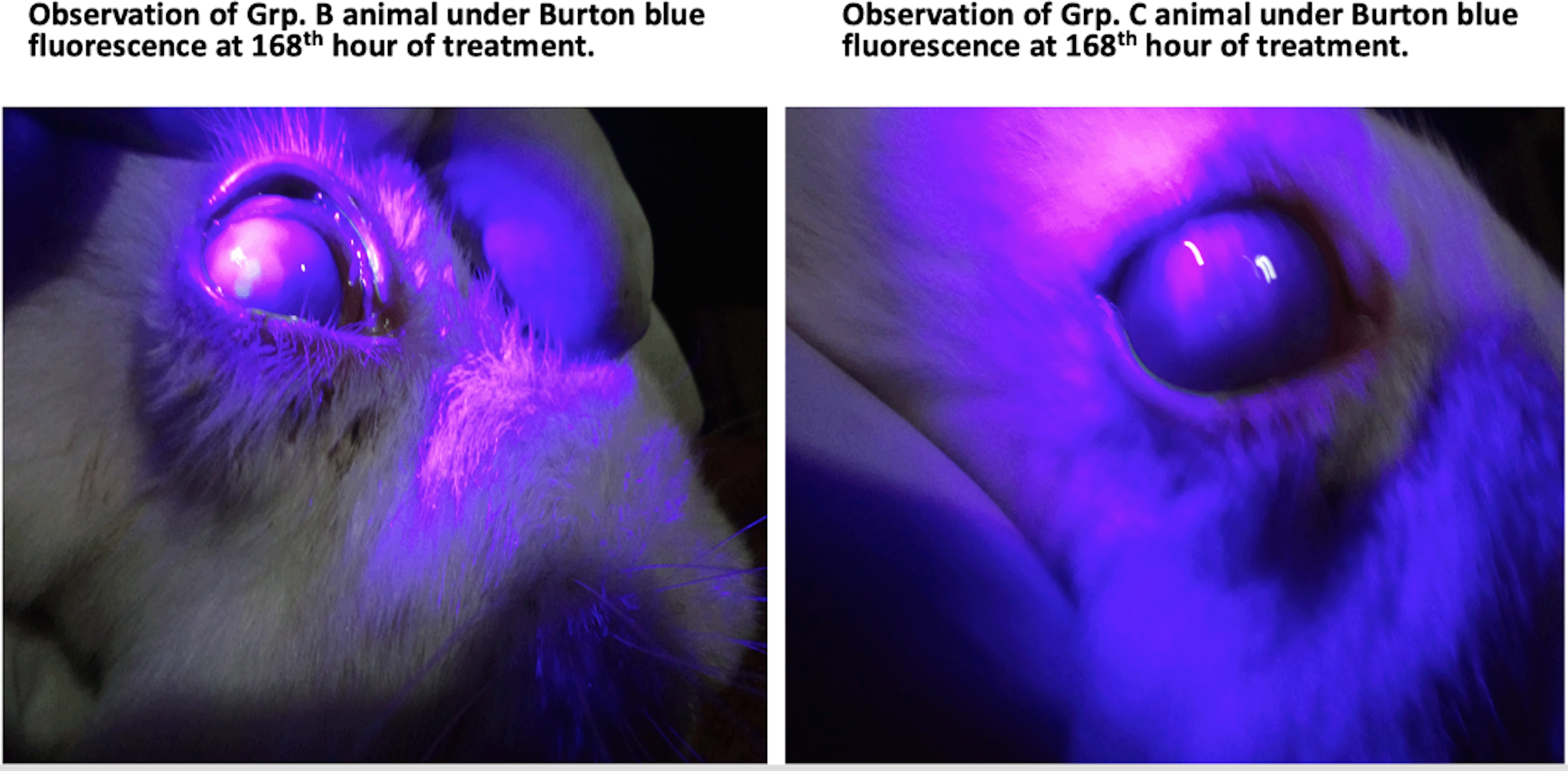
As shown, animals in group C (right) did not physically appear (observation on burton blue lighting) to have healed better than group B animal (left), after 168 hours of treatment with G. lucidum.
Table 4 shows a comparison between the manifestations of various ocular parameters for animals in group B (experiment group 2) and those in group D (negative control group) after 168 hours of treatments with G. lucidum on the former (group B) and atropine alone for the latter (group D). The results show that the difference in the treatment in both groups was not statistically significant (p>0.05). This finding is also shown pictorially in Figure 4.
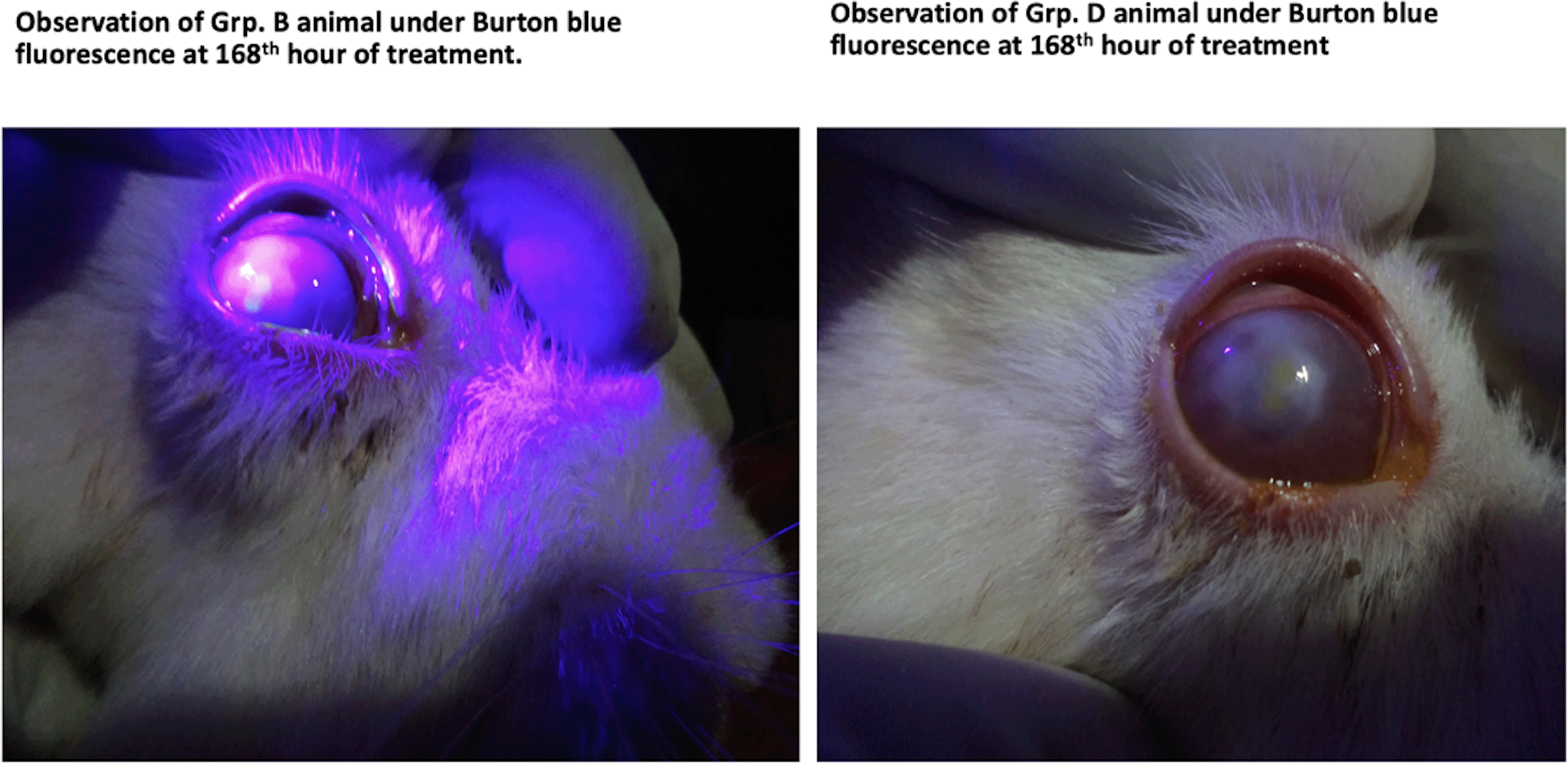
As shown, Group D animals (right) appeared to have healed better.
In this study, we found a progressively improved healing effect of the extract of G. lucidum, from the first 24th hour to the 168th (seven days of treatment). The indices adopted for the study to show improvement in the healing process of a deliberately induced corneal injury and then subsequently infected using P. aureginosa, a common, normal floral, but opportunistic microorganism, were eyelid status, conjunctival status, lacrimation, epithelial eruption, corneal staining, photophobia, ocular discharge, corneal oedema, and corneal infiltrates. In group A animals (Table 1), there were observable changes in this first experimental group exposed to chemical injury alone using 1 molar sodium hydroxide. Our findings showed that treatment with crude aqueous extract of G. lucidum had significant healing effects on just a few ocular parameters: eyelid status change, reduced lacrimation, reduced corneal staining and oedema. Nonetheless, there were improved clinical effects of treatment with the extract in observed ocular parameters as seen in Figure 1. This thus demonstrates that treatment with G. lucidum after the 168th hour improved the corneal healing process following an induced chemical (alkaline) injury. However, as can be seen from Figure 1, and as is typical with most chemical injuries, specifically from sodium hydroxide that causes corneal damage through pH change, ulceration, proteolysis and collagen synthesis defects to the cornea, healing from such injuries takes time, often longer than 7 days to achieve significant physically observable signs of healing.19 This time limitation was a key challenge in this study. Still, and as noted by Singh,20 chemical injuries, particularly those of alkaline origin, to the cornea are potentially blinding ocular injuries and constitute a true ocular emergency requiring immediate assessment and initiation of treatment.20 Our finding demonstrates that instituting treatment with an extract of G. lucidum as early as within the first 24th hour of occurrence has the potential to prevent extensive and penetrating damage to the cornea and other ocular surface tissues that present a risk of irreversible vision loss.
Furthermore, among animals in group B (experiment group 2), as shown in Table 2, our finding demonstrates that treatment with crude extract of G. lucidum did not significantly change the ocular morphology of most of the animals after the 168th hours, except for a significant reduction in photophobia and lacrimation. Even though the differences in the quantitative morphological parameters at the two-time points, following the initiation of treatment with the crude aqueous extract of G. lucidum, compared were not significant, the converse (Table 2), was true for the clinical effects (effect size>5%). This, therefore, implies that the clinical effect of treatment with crude aqueous extract of G. lucidum for bacterial infected corneal ulceration is significant. Additionally, the clinical (healing) effects were found to be most significant with a reduction of ocular discharges, a good morphological indication of a reduction in microbial load.13 This finding, also shown pictorially in Figure 2, confirmed findings of previous studies,5,11,12 that the antibacterial effect of G. lucidum on non-ocular related inflammatory injuries and infective ulcers in other parts of the human body is clinically effective. In addition, our finding demonstrates that G. lucidum has proven to be effective in managing bacterial infections that have shown resistance,13 as well as an effective alternative to treating bacterial infections and inflammatory injuries to the human body,14 may as well be an effective alternative to the management of ulcerative conditions of the cornea resulting from bacterial infections.
Our study also compared the treatment effect of the crude aqueous extract of G. lucidum with the existing standard treatment protocol for chemical (alkaline) induced ocular injury and bacterial-induced corneal ulcers consisting of two hourly drops of fluoroquinolone (ciprofloxacin), six hourly drops of corticosteroid (betamethasone), and eight hourly drops of atropine.17,18 We found that after the 168th hour of treatments, while animals in group C had better clinical healing (effect size>5%), the difference between the two treatment protocols was not statistically significant (p>0.05) as can be seen in Table 3. The implication of this finding, therefore, is a demonstration that treatment with the crude aqueous extract of G. lucidum may be an effective approach to the treatment of bacterial-induced corneal ulcerative and inflammatory injuries. This further justifies a previous finding on the healing effects of extracts of G. lucidum on infective and inflammatory injuries to other non-ocular tissues of the human body.14
Finally, we sort to further demonstrate the healing effects of crude aqueous extract of G. lucidum on ulcerative injuries to the cornea and other ocular adnexa. To do this, we compared the results, after 168 hours of treatment, of animals treated with the crude extract to those of a negative control group that only received atropine treatment (Table 4). As shown, even though we found no significant difference (p>0.05) in the two groups, treatment with the crude extract showed less clinical healing effect (Figure 4). This was an interesting finding of our study that the animals in group D (negative control) that were administered with atropine sulphate alone showed better clinical improvement than those treated with G. lucidum. Atropine sulphate was purposively administered to control ciliary spasms in the eyes of the animals in the negative control group. It was interesting to observe that atropine (primarily a cycloplegic agent) also known to show anti-inflammatory secondary effects,21,22 resulted in significantly better healing effects compared with the crude aqueous extract of G. lucidum. This finding may explain the importance of introducing an anti-inflammatory treatment protocol as early as possible in the management of both chemical and bacteria-induced corneal ulcerations.
To summarize, we note that there is a dearth of empirical knowledge on the ocular-related healing effects of extracts of G. lucidum in literature to compare our findings with. Nonetheless, our study demonstrates an observable reduction in the severity of damages to the ocular adnexa from induced ulcerative chemical injury and bacterial infection. These reductions are evident from the positive changes in the ocular parameters affected following the induction of chemical injury and subsequent bacterial infection of the cornea. Specifically, we note a significant reduction in eyelid swelling and lacrimation in animals treated with G. lucidum after 168 hours of treatment. This is evident with the animals in groups A and B, where the eyelid and conjunctival severity were significantly reduced progressively (Figures 1 and 2). Although no significant change was noticeable in a few parameters (epithelial eruption, epithelial staining, corneal fluorescein staining, and corneal oedema), we found the clinical healing effects of the crude aqueous extract of G. lucidum extracts to be highly significant, particularly for the reductions observed with ocular discharges, photophobia and corneal infiltrations. This we found, while not consistent with the healing effect observed in a study on paw oedema following intra-muscular instillation of G. lucidum,23 was nonetheless consistent with the healing effect observed in another study on gastric ulcers.24 Other studies that compared the clinical healing effects of extracts of G. lucidum with commonly used antibiotics, such as gentamycin sulfate13 and fluoroquinolones,22 found it to be equally effective in other body structures. We found this to not be the case with ocular healing effects, as standard treatment with a combination of fluoroquinolone, corticosteroid and atropine sulfate, and even with using atropine sulphate alone, turned out to show better clinical effect than treatment with the crude aqueous extract of G. lucidum alone.
Crude aqueous extracts of G. lucidum have shown the potential to be an alternative treatment approach for chemical (alkaline) and bacterial (P. aeruginosa) induced corneal ulceration. The healing (anti-inflammatory and anti-infective) effects of G. lucidum have shown clinically significant healing effects after 168 hours of treatment compared with that of 24 hours. Although the cornea of animals treated with standard protocol showed better clinical effects than those treated with G. lucidum, the current investigation has shown the importance of the early institution of the anti-infective and anti-inflammatory protocol in the management of corneal ulcers. Therefore, G. lucidum, where available, may be a useful anti-infective alternative for chemical-induced and bacterial-infected corneal ulcerative conditions. In this regard, therefore, our study notes that despite the limitation of time, crude aqueous extract of G. lucidum has bioactive compounds that have the potential to be used as an alternative treatment approach for chemical-induced and bacterial-infected corneal ulcerations in resource-constrained settings. Hence, the study recommends further research to explore the anti-inflammatory potentials of crude extract G. lucidum in population-controlled settings. The study further recommends research to characterize the bioactive compounds in the crude aqueous extract as well as cytotoxicity assay of the bioactive compounds in the crude aqueous extract, as with tests to demonstrate the effectivity levels of extracts of the mushroom from other less polar and non-polar solvents.
Approval for this study was obtained from the Institutional Ethics Review Committee of Masinde Muliro University of Science and Technology (MMUST, IERC) (MMU/COR: 403009 (VOL. 1). This study was conducted per the ethical guideline of the Prevention of Cruelty to Animals Act of Kenya.25,26 Additionally, all methods applied in this study adhered to the Committee on Animal Research and Ethics’ guideline for ethical conduct in the care and use of animals, including responsible and ethical disposal of animals during clinical and laboratory research.27 All procedures in this study were conducted and reported in accordance with ARRIVE guidelines.
EOV was involved in the study design, data collection, data analysis, drafting and reviewing of the manuscript. AKM was involved in the study design, data collection, data analysis, drafting and reviewing of the manuscript. POO was involved in data collection, drafting and reviewing the manuscript. AVD was involved in drafting and reviewing the manuscript to its final stage. KPM was involved in drafting and significant review of the manuscript to its final stage. All authors have read and approved the final version of the manuscript.
Figshare: Underlying data for ‘Effects of Ganoderma lucidum on chemical-induced and bacterial-infected corneal ulceration of rabbits’ eyes’. https://doi.org/10.6084/m9.figshare.21789026.v1.28
The project contains the following underlying data:
Data are available under the terms of the Creative Commons Zero ‘No rights reserved’ data waiver (CC0 1.0 Public domain dedication).
We acknowledge the support received from Masinde Muliro University’s IERC, the Department of Pure and Applied Chemistry, and the Department of Medical Laboratory Science both of Masinde Muliro University. We also acknowledge the efforts of the optometry students and other university staff who assisted both in the data collection for this study and the handling and care of the animals during the duration of this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infective keratitis, endophthalmitis, experimental animal corneal and ocular infections.
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Ocular microbiology, bacteriology, ocular host-pathogen interactions, molecular pathogenesis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 16 Feb 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)