Keywords
Eaosinophilic ureteritis, ureteric stricture, hydronephrosis, ureteroscopy
Eaosinophilic ureteritis, ureteric stricture, hydronephrosis, ureteroscopy
The term “hydroureteronephrosis” refers to a condition in which the renal pelvis, calyces, and ureter enlarge due to restriction of the free flow of urine from the kidney, which causes the renal cortex to gradually atrophy.1 It can be caused by a structural or physiological process that prevents urine from flowing normally.1 This can occur anywhere from the kidneys to the urethral meatus.1 The etiology, location, and duration of the obstruction may affect the symptoms. If the etiology is ureteral calculus and occurs suddenly, the symptoms often present with colicky pain in the flanks.1 The-non calculus etiology of hydroureteronephrosis differs depending on the patient’s age and gender.1 The most frequent cause in children is congenital obstruction of the pelvi-ureteric junction (PUJ) and posterior urethral valves, whereas the most frequent causes in adult males are benign prostatic hypertrophy (BPH), urethral/ureteric strictures, bladder cancer, and obstruction of the bladder outlet.1 It also happens secondary to pregnancy in childbearing females, but in older people, the major causes are cervix and ovary carcinomas.1
Eosinophilic ureteritis is a very rare etiology of ureteric obstruction that leads to hydroureteronephrosis, and to date the diagnosis of these findings can only be made through pathologic examination.2 We have only so far discovered a few case reports discussing eosinophilic ureteritis and the exact cause of this condition is poorly understood, but there may be some association with atopy, hypereosinophilic syndrome, and prior ureteral interventions.2 Here, we report a very interesting case of a 71-year-old patient presenting with bilateral hydroureteronephrosis caused by recurring ureteric strictures of eosinophilic ureteritis etiology.
A 71-year-old Indonesian housewife presented to our private hospital located in Centre of Jakarta, Indonesia with complaints of general weakness and oliguria. No previous episodes of fever, hematuria, dysuria, or flank pain were noted. The patient had already been diagnosed with bilateral ureteric stenosis six years ago and has had a double-J (DJ) stent for about four months. Since then, the patient had a record of recurrent urinary tract infections with only fever and incomplete emptying when urinating as symptoms. The patient had a history of lung tuberculosis 50 years ago that was treated until they recovered completely, unintentional weight loss of more than 10 kilograms last year, multinodular non-toxic goiter treated once daily with thiamazole 10 mg, and right breast tumour that has not been further diagnosed. There was no relevant family and psycho-social history that can cause the current symptoms. The physical examination, upon admission, revealed both conjunctival were pallor and no tenderness in both renal angles. According to a urine analysis that we collect from a mid-stream urine put in a sterile cup, there were packed pus cells per High Power Field (PHF), 1 red blood cell/HPF, 1+ protein, 1+ bacteria, and 1+ leukocyte esterase. The patient’s baseline investigations, upon admission, revealed red blood cell count was 6.3 g/dL (normal range 12.0-14.0 g/dL) with mean corpuscular volume (MCV) 85.8 fl (normal range 82.0-92.0), mean corpuscular haemoglobin (MCH) 29.4 pg (normal range 27.0-31.0), mean corpuscular haemoglobin concentration (MCHC) 34.3% (normal range 11.5-14.5%), white blood cell count was 6560/mm3 (normal range 5000-10000/mm3), with 3% eosinophils (normal range 1-3%). Thrombocytes was 361000/μL (normal range 150000-400000/μL). Thyroid stimulating hormone (TSH) was 1.8270 μIU/mL (0.3500-4.9400 μIU/mL) and Free T4 (FT4) was 0.79 ng/dL (0.70-1.48 ng/dL) which indicated the pateint’s thyroid level was within normal limit. A renal function test showed urea was 58 mg/dL (normal range 14-38 mg/dL) and serum creatinine was 3.39 mg/dL (normal range 0.55-1.02 mg/dL). The urine culture sample was obtained from the catheter using surface streak method. It showed escherichia coli infection with >100000 Colony-forming unit (CFU) (normal range <10000 CFU). Gene-Xpert (sample taken from patient’s sputum during hospitalization) and Interferon-gamma release assay (IGRA) (sample taken from blood obtained during hospitalization) showed negative signs of tuberculosis infection. Galactomannan test (sample taken from blood obtained during hospitalization) also revealed negative sign of aspergillosis infection.
Computerized tomography (CT) urogram without contrast was performed to investigate further the cause of patient’s complaints as lab results doesn’t point to a specific diagnosis.23 CT Urogram revealed the thickening of the right proximal ureteric wall up to the right mid-ureteric wall (Figure 1A) and thickening of the left proximal ureteric wall (Figure 1B) suggestive of ureteric stricture with severe bilateral hydronephrosis (Figure 1A,B). There was no diagnostic challenge during hospitalization. A pre-operative working diagnosis of bilateral ureteric stricture and Grade 4 hydronephrosis was made. Before the patient underwent surgery, 750 cc of packed red blood cells were transfused until the patient’s red blood cell count was 10.8 g/dL (normal range 12.0-14.0 g/dL). Then, bilateral diagnostic ureterorenoscopy (URS) under spinal anesthesia was performed with semi-rigid ureteroscope. Urine cytology revealed milky urine in the urinary tract that contains lots of neutrophils, lymphocytes, macrophages, and urothelial cells the (Figure 2), no malignant cells was found. Tuberculosis-polymerase chain reaction (TB-PCR) of urine showed no trace of tuberculosis. Retrograde Pyelography (RPG) showed multiple bilateral ureteral strictures (Figure 3) and then the scope was negotiated up to the right-left ureter to confirm our findings and tissue samples were obtained for biopsy (Figure 4). Subsequently, laser fulguration was done to release ureteral strictures from both ureters (Figure 5). Microscopy of the excised specimen revealed dominant eosinophilic infiltration along with atypical stromal cells (Figure 6). After that, a DJ stent was successfully inserted both in the left and right ureters (Figure 7). A diagnosis of eosinophilic ureteritis was made based on this pathological finding. We decided to give the patient iv meropenem 3×1 gram for seven days during hospitalization and oral mirabegron 1×50 mg to overcome patient’s overactive bladder. After seven days, the patient made a complete recovery with no sign of general weakness, oliguria, and urinary tract infection from the patient’s second urine analysis. We followed-up the patient’s condition for another week and discovered there were no sign of overactive bladder and oliguria.
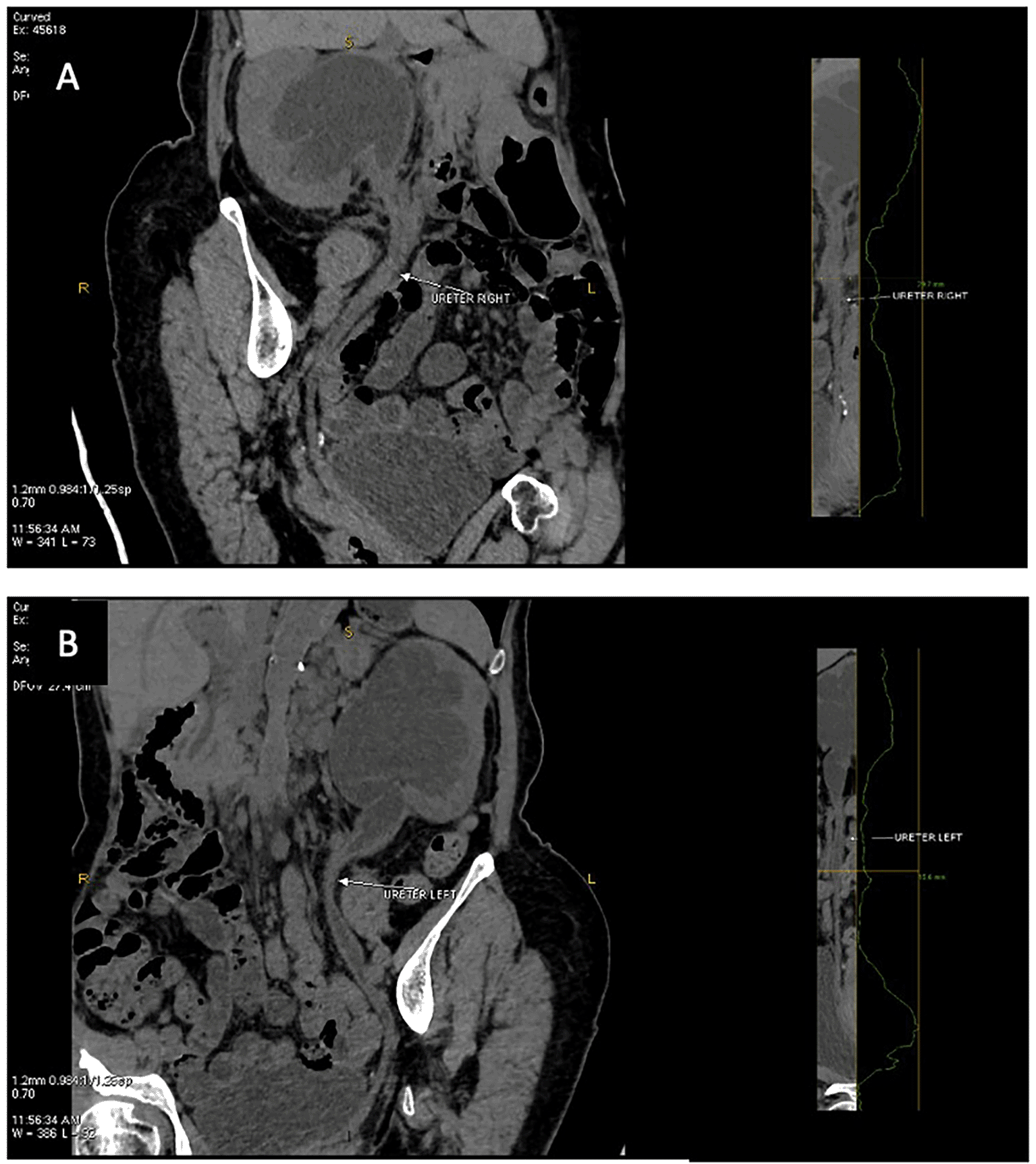
(A) Coronal view, thickening of the right proximal ureteric wall up to right mid ureteric wall (white arrow); (B) Coronal view, thickening of the left proximal ureteric wall (white arrow).
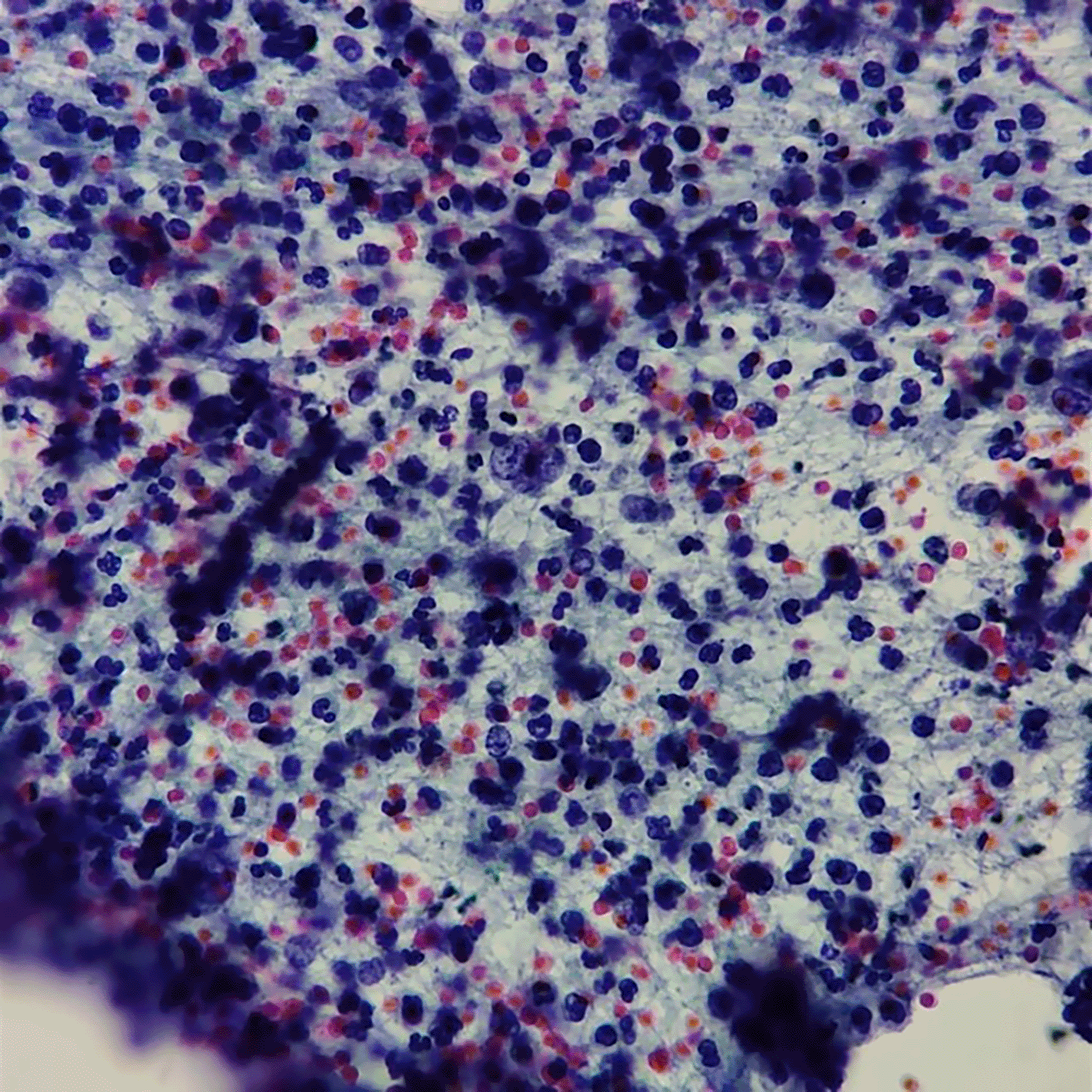
No sign of malignancy cell with lots of neutrophil, lymphocyte, macrophage, and urothel.
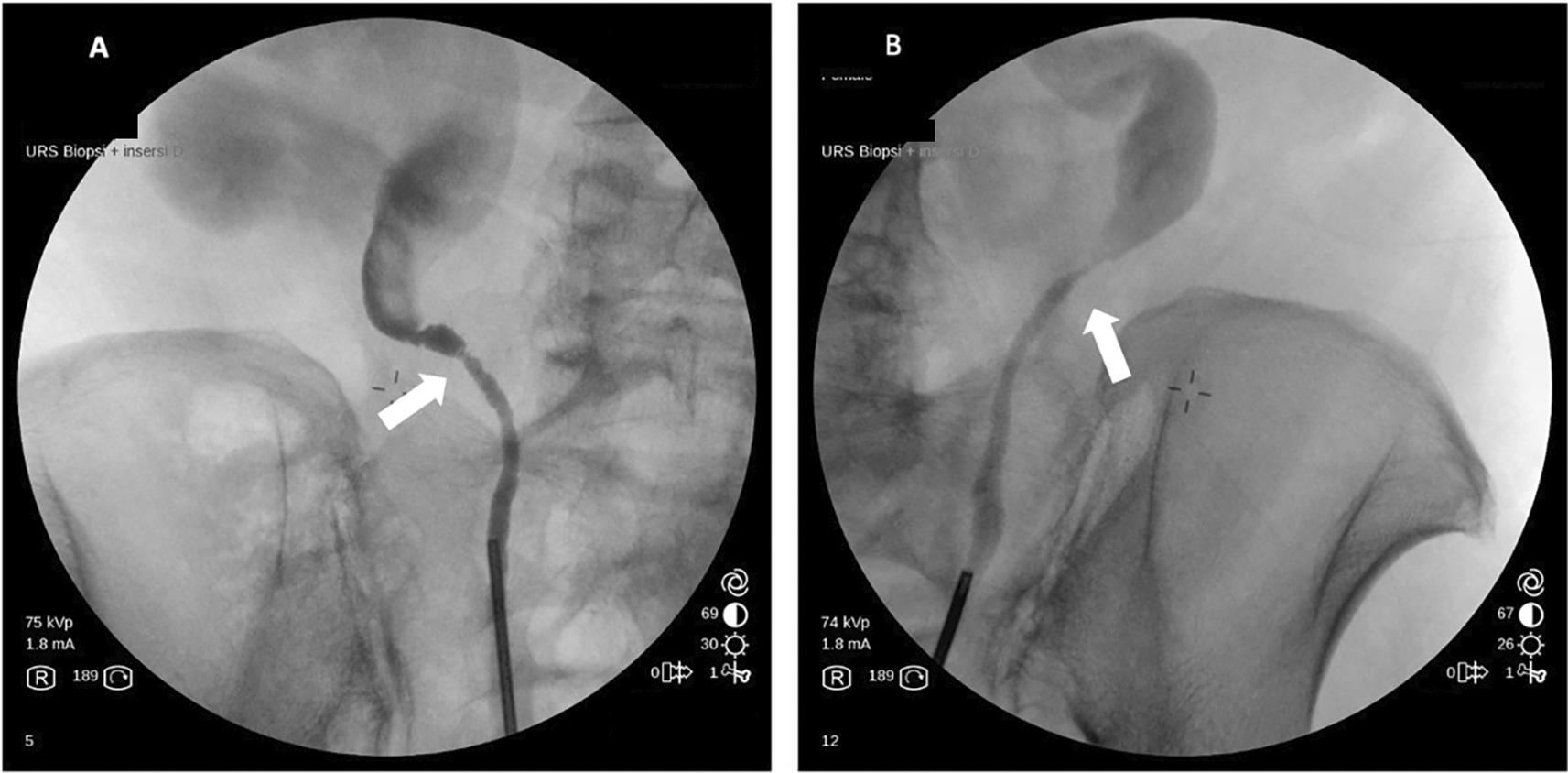
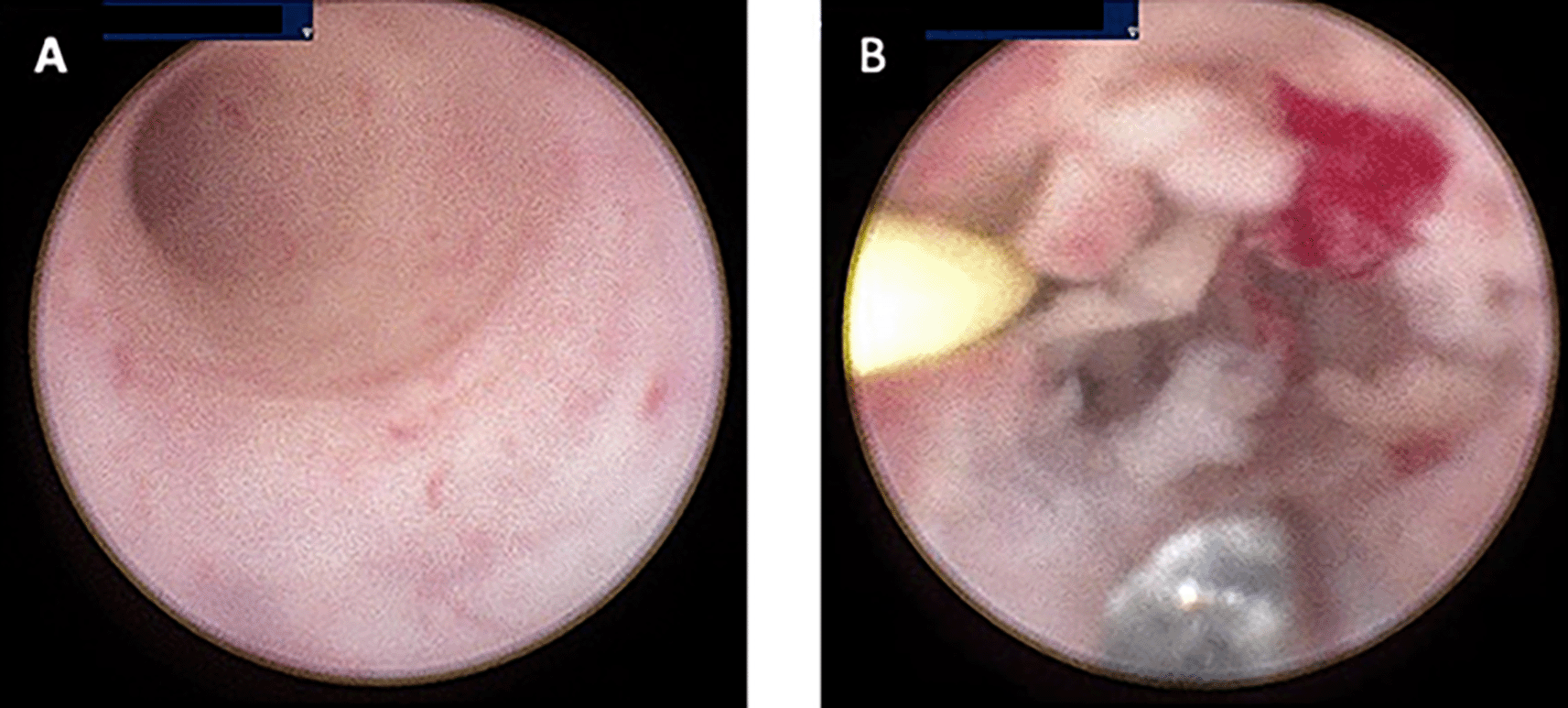
(A) Visible Milky Urine in the bladder and ureter; (B) Mass that causing stricture in ureter bilateral.
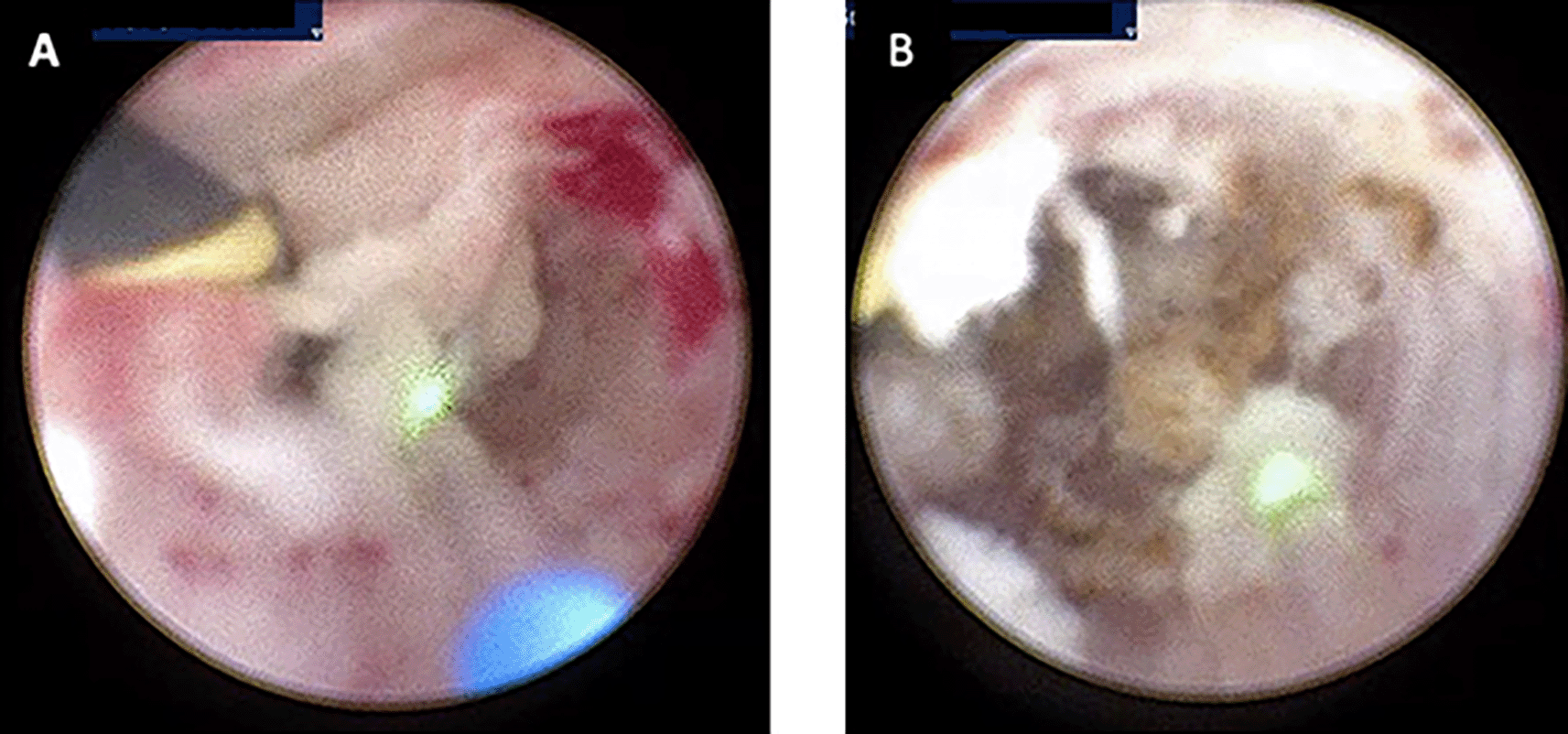
(A) Laser fulguration in left ureter for biopsy; (B) Laser fulguration in right ureter for biopsy.
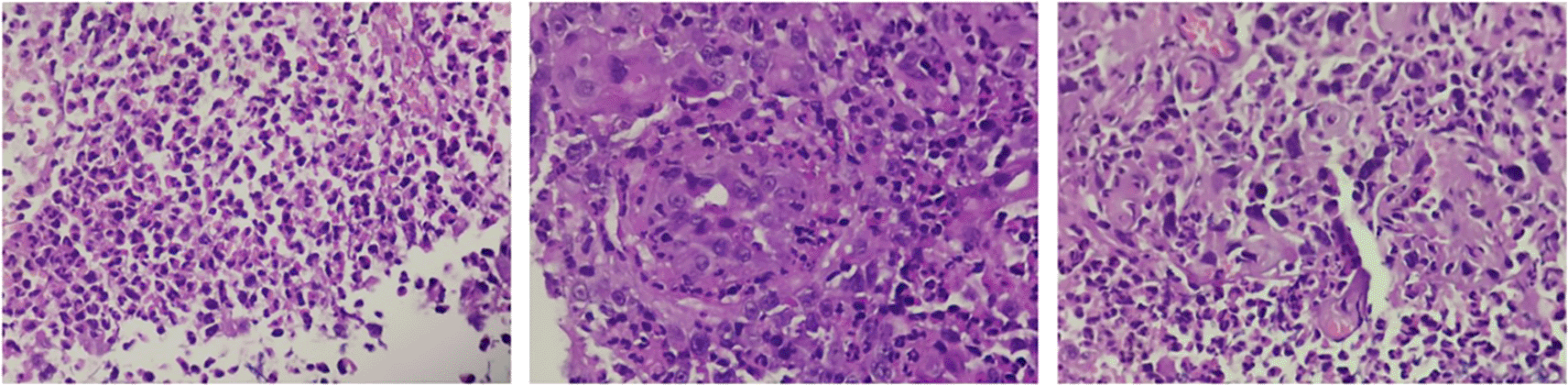
Wall of the ureter containing a dense mural infiltrate composed predominantly of eosinophils.
Eosinophil is one of blood granulocytes derived from bone marrow. Production of eosinophil required a complex interaction between IL-5, IL-3, and GM-CSF, which were mediated by T-helper 2 cells’ immune response. Eosinophil acts by secreting proteolytic enzymes which result in cell wall damage of targeted pathogens, mostly parasites.3,4 Eosinophils may reside in several tissues, mostly located in the respiratory and gastrointestinal tract. The presence of eosinophils in the urinary tract is deemed pathologic, such as bladder and ureter.5
Eosinophilic ureteritis is a very rare clinicopathological entity known to cause ureteric stricture.6 The symptoms may vary, although most of the patients reported the symptoms mimicking urinary stones with or without urinary tract infection (UTI). In our patients, the main symptoms were recurring UTIs despite no history of colicky pain suggesting urinary stone.7
To our knowledge, the present case is the second reported example of eosinophilic ureteritis causing bilateral upper ureteric strictures and bilateral hydronephrosis in adults. The first reported case was from Singh et al.7 who also reported bilateral upper ureteric strictures with hydroureteronephrosis.7 Another study conducted by Bajracharya et al.2 reported a case of unilateral ureterohydronephrosis also related to eosinophilic ureteritis.2
The exact etiology of the disease remains unclear. In our patient, we previously suspected a tuberculous infection as 50 years ago the patient was infected with this disease. However, there was no evidence of tuberculous infection in the ureter from the patient’s urine cytology, GeneXpert and IGRA tests were also negative. Previous studies reported the possibility of prior parasites infection such as Schistosoma, Toxocara, and Sparganum in the bladder as the disease etiology. Filarial infection has also been reported as a possible trigger of the condition.7 It is hypothesized that in parasite infections, activated eosinophils released cytotoxic cationic proteins which target surrounding tissues, further resulting in chronic inflammation with scattered eosinophils that lead to fibrous formation.8 Another possible pathophysiology is IgE-mediated release of eosinophilotrophic molecules.9 Presence of other organisms (Enterobacter aerogenes or fungal Candida albicans) has also been reported in other organs, such as eosinophilic cholangitis. In our case, the patient’s galactomannan test revealed to be negative and we didn’t find any stigmata of filariasis.10
Our patient is currently treated for multinodular non-toxic goiter with thiamazole. Hyperthyroidism has also been associated with peripheral eosinophilia. The link between hyperthyroidism with eosinophil-induced organ damage has not been clearly reported, although relative cortisol deficiency in thyrotoxicosis has been associated with high eosinophil count.11 Another possible connection in our patient was related to the breast tumour, in which 3.7% of patients with breast tumour has been associated with tissue eosinophilia.12 Although, further studies are needed to evaluate these possible etiologies of eosinophilic ureteritis due to lack of evidence.
Studies regarding eosinophilic ureteritis are limited, although eosinophil-induced inflammation in other genitourinary organs such as the bladder has been reported.13 Many factors have also been associated with eosinophilic inflammation, including previous medication (such as warfarin, methicillin, intravesical mitomycin, thiotepa, and anthranilic acid), atopy (food or other allergens) or autoimmune diseases, previous operative procedure, and trauma. In our case, the patient never had any previous history of the above-mentioned medications.13,14
Eosinophil-induced organ damage has also been reported to be linked to one another, in line with a study by Kim et al.15 who showed an association between eosinophilic cystitis and enterocolitis.15 Similar pattern has also been shown by Platt et al.16 who showed an association between eosinophilic ureteritis and eosinophilic cholangitis.16 Eosinophilic ureteritis has also been associated with hypereosinophilic syndrome, marked by persistent blood eosinophilia (>1.5 × 109/L in six or more consecutive months) with eosinophil-induced organ damage without evidence of active allergic and parasitic causes, or malignant disorders.17 Examples of eosinophil-induced organ damage are eosinophilic gastroenteritis, esophagitis, dermatitis, pneumonia, and fasciitis. The etiology of this syndrome was also unknown, but cryptic cytogenetic abnormality in eosinophils has been identified. Since we couldn’t find any etiology linked to eosinophilic ureteritis in our patient, the main cause remained idiopathic. 17,18
Regarding the disease rarity, care needs to be taken into account while encountering patients with recurrent UTIs without marked laboratory parameters. Before eosinophilic ureteritis was suspected, prior exclusion to active parasitic infection, genitourinary malignancies, and calculus are needed. In eosinophilic ureteritis, the main diagnostic tool is a histopathological examination using biopsy.2,6,7,19
Radiologically, the patient presented a nonspecific pathology which shows ureteral wall thickening suggestive of stricture that can be found in eosinophilic ureteritis, 7 but these findings can also be found in other inflammatory or neoplastic cases such as in genitourinary tuberculosis or carcinoma of the urinary tract.20,21 As such, a radiological diagnosis is unclear and there is a need for pathological examination to diagnose the specific cause of the disease and guide the clinician to the right therapy.
In our patient, after confirmation of tissue eosinophilia suggesting the presence of eosinophilic ureteritis, double-J (DJ) stent was inserted to overcome the ureteric stricture. There has not been any accepted consensus regarding the disease’s management, although the use of oral corticosteroids (e.g., prednisolone) and non-steroidal anti-inflammatory drugs (e.g., diclofenac) has been reported as the initial conservative therapy with subsequent ureteral obstruction remission. Other oral drugs include antihistamines and antibiotics. Cyclosporine and azathioprine can be given in refractory cases. We decided to administer meropenem as an antibiotic as the patient already had recurrent urinary tract infections and a history of prolonged usage of antibiotics 13,19
More invasive treatment may be needed in patients with evident hydronephrosis. DJ stenting may be performed to evacuate the obstruction related to ureteral stricture. However, the insertion of stent or catheterization procedure has been paradoxically associated with a case of eosinophilic ureteritis in already present eosinophilic cystitis. This may also lead to potential recurrence due to persistent inflammatory reactions after instrument insertion in the genitourinary tract.22
Regarding the above-mentioned risks, surgical treatment has been shown superior in the management of eosinophilic ureteritis.13 Previous studies have performed variable surgical procedures, ranging from total nephroureterectomy to ureteral segmentectomy in the stenotic segment with subsequent end-to-end anastomosis. After this treatment, the patient refused further surgery after some considerations because they wanted to observe how the current treatment goes first. The patient is also scheduled for another URS evaluation in the next six months, then whether reconstructive surgery or conservative treatments using DJ stents are needed will be determined afterward. Routine monitoring should be done to evaluate the patient’s clinical signs and symptoms.2
This case report is limited by the short follow-up period and lack of information from patient’s past medical history as she was admitted not in our hospital. We also couldn’t find any literature discussing relationship between eosinophilic ureteritis and gender. However, its strength is that as we directly observed the patient rather than taking information retrospectively, this may reduce any potential bias. We also have excluded most of the potential etiology for ureteric strictures.
In conclusion, it is important to consider eosinophilic ureteritis in the differential diagnosis of any ureteric stricture because it is a highly uncommon entity with variable clinical characteristics. The gold standard procedure, which should be utilized to make the diagnosis, is a histological examination. For improved results, early detection, and prompt treatment are expected.
Zeonodo: A Rare Case of Eosinophilic Ureteritis in a Woman with Reccuring Bilateral Ureteric Strictures causing Hydroureteronephrosis: Supplemental CT Scan Data, https://doi.org/10.5281/zenodo.7622523. 23
This project contains the following extended data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pathology, renal disease, immunology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 07 Mar 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)