Keywords
Atorvastatin, Nitric Oxide, Clopedogrel, Serum Lipids, Coronary Artery Disease.
Atorvastatin, Nitric Oxide, Clopedogrel, Serum Lipids, Coronary Artery Disease.
Statins is a widely known medication which is used to reduce serum lipids. These medications are used to lower the production of endogenous cholesterol via reducing the activation of hydroxymethylglutaryl CoA reductase enzyme. This represents the rate-limiting step in cholesterol synthesis by the liver.1 This results in decreased low-density lipoprotein (LDL) deposition in the vascular walls by upregulation of hepatic LDL-C receptors, an essential event in the pathogenesis of atherosclerosis.2
Atorvastatin is a widely used statin which augment nitric oxide (NO) activity and production through several ways, which eventually lead to increase expression and activity of endothelial NO synthase enzyme (eNOS). A previous study revealed a significant rise in eNOS levels following atorvastatin therapy in mice model.3 In addition, atorvastatin can reduce NO elimination through its effects on reactive oxygen species (ROS).4
The benefits above exert several clinical implications. Especially considering the essential activity of NO as vasodilator, inhibiting platelet aggregation, and it promote endothelial function.5 Increased NO production by endothelium could furthermore offer protection against stroke.6
Clopidogrel is a known platelet inhibitor, which has been widely used for the prevention of major complications of heart disease following percutaneous coronary intervention (PCI).6 Previous studies have shown that there was some inter-individual difference in clopidogrel response6,7 and resistance to clopidogrel is related with an augmented risk of major adverse cardiac events (MACE).6,7 Among the several risk factors that affect the response of platelet to clopidogrel therapy, smoking is linked with enhanced clopidogrel responsiveness.8 Although various processes have been proposed (including those regarding cytochrome P450 enzyme system), the exact mechanism is still unclear.8
Antiplatelet therapy is the backbone for the management and prevention of heart disease, including coronary heart disease, transient ischemic attack or minor stroke, and acute coronary syndrome (ACS). Clopidogrel which is P2Y12 inhibitors was the first medication in this category, play an essential role in antiplatelet therapy and consequently in the treatment and prevention of CVD.9 However, there are several factors which may alter personal response to clopidogrel therapy. This is in addition to drugs.10
The main objective of the following project is to measure the efficacy of 2 known doses of atorvastatin (daily intake of 20 mg, and 40 mg oral supplementation for 16 weeks) on serum nitric oxide level and lipid profile in patients with heart disease, with and without clopidogrel resistance.
This was a comparative retrospective study performed at Al-Najaf Heart Surgery and Catheterization Center, Al-Najaf, Iraq, for the period between January 2019 - Jan 2021. Two hundred forty-eight patients were included in this study (202 males and 82 females), aged between 18-65 years clinically diagnosed with CAD (via angiography). As per the center guidelines, CAD diagnosis was confirmed by two cardiologists based on the clinical findings of 70% or more coronary artery stenosis affected at least one coronary blood vessel.
All CAD patients in this study had platelets reactivity tests to verify the level of clopidogrel resistance, then they were allocated into one of the two study groups: Clopidogrel resistant group (CR) and Clopidogrel non-resistant group (non-CR). All patients had atorvastatin therapy at one of the following two doses (20 mg or 40 mg, oral daily supplement) plus clopidogrel 75 mg/day for 16 weeks.
This research was approved by “Human Research Ethics Committee”, College of Medicine, Kufa University (KUM457). Patients provided their oral and written approval to participate in this study. Eighty-eight patients were included in the CR group as compared to 196 participants included in the non-CR group. Data were taken from all the participants in this study including history and physical examinations (Table 1).
Participants were excluded if they have been recently diagnosed with acute coronary syndrome; peripheral artery disease; cancer; history of bleeding diathesis; unstable angina class I to III; or if they were aged ≥65 or ≤18; or pregnant or lactating mothers.
Eight milliliters of venous blood samples were taken into an EDTA tube from each participant. Blood samples were collected to measure serum levels of nitric oxide, platelet function test, and serum lipids.
Serum lipid profile (TG, TC, LDL-C, HDL-C) were measured enzymatically using ELIZA kits from BIOLABO, France, kit reference number 95516.
Nitric oxide determined colormetrically in sera, based on that fact that both nitrate and nitrite exist in various regions of the cells. These chemical compounds can be produced via nitric oxide pathway without excessive uptake of nitrite and nitrate. Nitrite is able to interact with chromogenic agents to produce light-red azo compound. This can then be calculated by measurement of optical density at 550 nm.11
Five milliliters of venous blood sample were taken from the participants. Samples were placed in test tubes for 1 hour, then underwent centrifugation at 3000 revolutions per minute for ten minutes at 25 °C. The platelet reactivity test was assisted by means of Multiplate analyzer® (Verum Diagnostic, GmbH, Munich, Germany). The assay was quantified by the area under the aggregation curve (AUC) in units (U). In this study, the cutoff point for the ADP test that is used to determine the clopidogrel effect is value of UAC is 50 U, so any value ≥50 U is considered as clopidogrel resistance or non-responder, and values <50 U is non-clopidogrel resistance or responder.11
All data of the study were examined by using SPSS (version 25, IBM, USA). In this study, descriptive statistics were used as numbers and percentages. To analyze categorical data or variables, we used the “Chi-Square test”. The independent variables student’s test was applied for comparing the results between the clopidogrel resistant group (CR) and clopidogrel non-resistant group (non-CR). Significant P values ≤0.05.
All the demographic data for the participants included in this study are shown in Table 1. No significant differences were noted in the mean levels of age, BMI, gender, hypertension, smoking status, and family history were observed between the study groups except for DM (P=0.054), Table 1.
The effects of atorvastatin on nitric oxide level
No significant relationship was identified between nitric oxide level in the resistant and the non-resistant groups for both the 20 mg and the 40 mg of atorvastatin (Table 2). However, 40 mg/day atorvastatin induced a more significant increase in NO levels across both study groupings, Table 2, P≤0.05.
| Atorvastatin | Resistant group (n=88) | Non-Resistant group (n=196) | P-value |
|---|---|---|---|
| Nitric oxide level (uM) | Nitric oxide level (uM) | ||
| Baselines | 16.3±2.5 | 17.98±7.5 | 0.136* |
| 20 mg | 19.82±1.39 (n=37) | 24.33±2.1 (n=94) | 0.195† |
| 40 mg | 27.08±2.32 (n=51) | 30.65±1.85 (n=102) | 0.251† |
| P-value | 0.016# | 0.24# |
The correlation between the serum nitric oxide level, and platelet aggregation
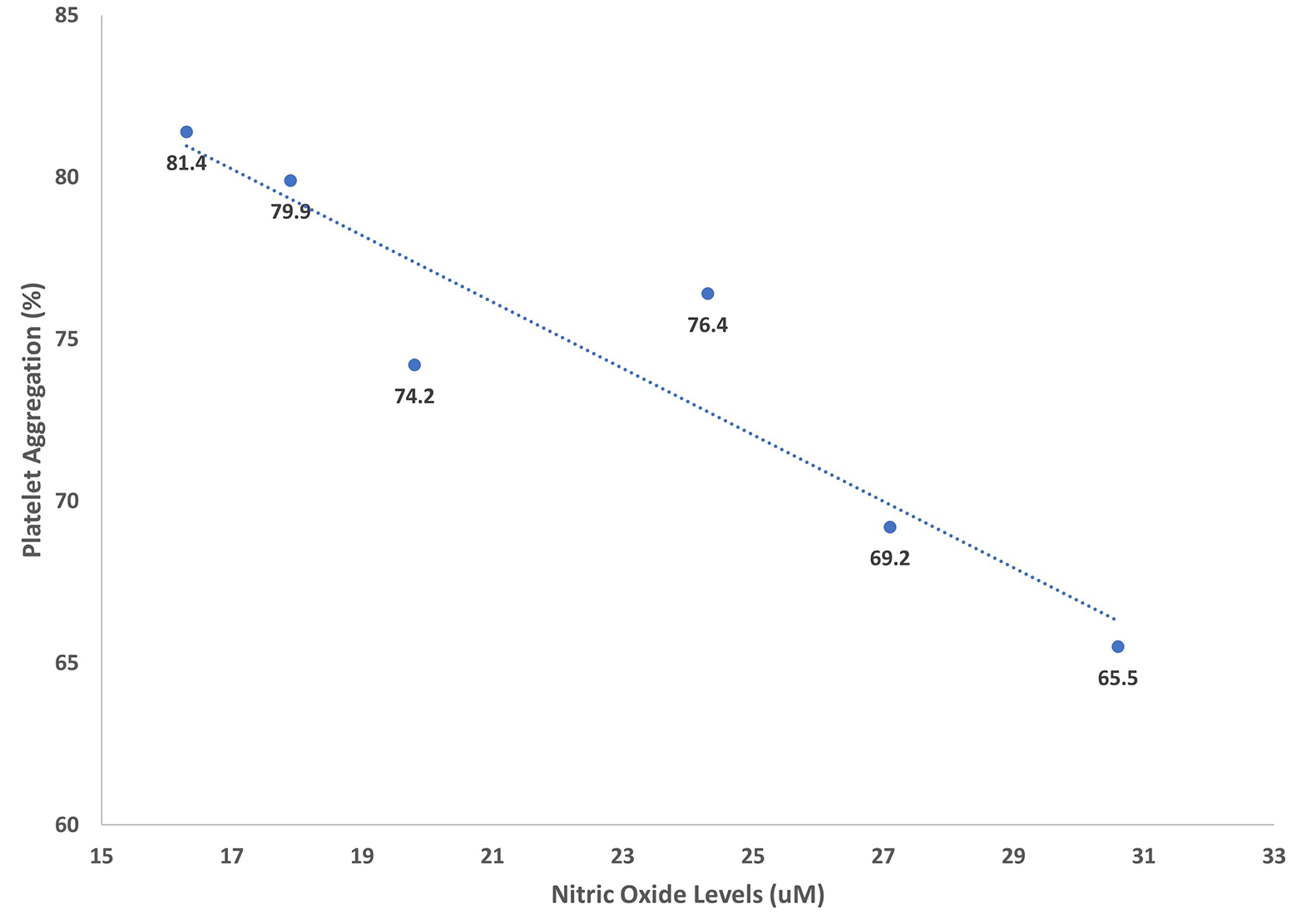
A significant negative association between serum nitric oxide level and the percentage of platelet aggregation in all the participants of this study, Figure 1, P<0.05.
Atorvastatin effect on lipid profile
Irrelevant to the study group, using 40 mg/day atorvastatin significantly reduced serum lipids (total cholesterol and LDL-C), when compared to 20 mg/day atorvastatin dose. However, both triglycerides and HDL-C did not exert similar significance, Table 3.
This is the first clinical study of its type to be conducted in Iraq and Middle East to highlight the therapeutic impacts of atorvastatin on nitric oxide levels and serum lipids and correlate the data with clopidogrel resistance. The study intended to evaluate the dose-response effects of two doses of atorvastatin (20 mg and 40 mg supplementation per a day for 16 weeks) on serum nitric oxide level and serum lipids in CAD patients, with variable clopidogrel resistance. All non-modifiable and modifiable risk factors were considered, including age, gender, obesity, family history of chronic illnesses (hyperglycemia & hypertension) and lifestyle factors. No significant differences between these factors and both doses of atorvastatin in resistant and non-resistant groups were found in exception to diabetes12,13 and inconsistent with findings from others.14 Incidence of diabetes mellitus was significantly associated with both doses of atorvastatin in the clopidogrel resistant and non-resistant groups,15 but contract with the findings of Pandey et al.16
We could not find a statistical difference in nitric oxide levels between the clopidogrel resistant and clopidogrel non-resistant groups using 20 mg/day atorvastatin. Similarly, there was no significant difference with 40 mg/day atorvastatin use in both the clopidogrel resistant and non-resistant groups. However, the levels of nitric oxide were significantly elevated by increasing atorvastatin to 40 mg/day in both clopidogrel resistant and non-resistant groups, Table 2. Having said that, we noted a significant adverse correlation between serum levels of nitric oxide and the percentage of platelet aggregation in this cohort. These data are in accordance with the findings from previous studies.17
Moreover, increasing the dose of atorvastatin to 40 mg/day significantly decreased the level of total cholesterol and HDL-C, but not triglycerides and HDL-C in both study groups (Table 3).14 There is no clear understanding of the mechanism behind this. One explanation may be related to the link between levels of nitric oxide and its effects on CVD. The elevation in production nitric oxide might be caused by a compensatory mechanism towards CAD, which can produce more nitric oxide from the vascular endothelium.18,19 This contradict the previous knowledge which hypothesized that a) the increased level of nitric oxide is not necessarily reflecting differences in nitric oxide activity, and b) racial differences between our current study participants (Iraqi Nationality) and other nationalities.20 The variation in the outcomes of previous studies may be related to confounding factors which may include inclusion/exclusion criteria, sample size, racial, geographical, and environmental factors.20–22 Hence, it is worth considering all these factors when assessing the effectiveness of atorvastatin therapy in CAD.
In conclusion, atorvastatin supplementation improves the serum levels of nitric oxide and serum lipids (TC and LDL-C) in patients with CAD with and without clopidogrel resistance.
Some of the potential limitations of this study include the fact that this study was taken from one region within Iraq, and it would be more reflective if it includes participants across all regions of Iraq. Second, nitric oxide measurement was assessed colormetrically using enzymatic technique which may possess medium sensitivity. It would be best to use alternative more automated techniques for future assay. Therefore, further, more inclusive, study would be good to have a better insight on the role of atorvastatin on serum nitric oxide level in patients with heart disease with and without clopidogrel resistance.
Conceptualization, A.A., A.R., B.M., and H.A.; methodology, A.A., D.J., B.M., and A.R.; investigation, H.A., A.R., A.A., and B.M.; writing—original draft preparation, A.A., B.M., and A.R.; review and editing, H.A., and D.J.; supervision, B. M., and A.R. Authors have read and agreed to the final version of the manuscript.
Figshare. Effectiveness of atorvastatin on the levels of nitric oxide and serum lipids in coronary artery disease patients: Response to clopidogrel resistance. DOI: https://doi.org/10.6084/m9.figshare.21944660.v1. 23
This project contains the following data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors thank members of the Pharmacology and Therapeutic Department of University of Kufa.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
At the request of the author(s), this article is no longer under peer review.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)