Keywords
ischemic stroke, middle cerebral artery occlusion model, filament method, intravascular modeling method
ischemic stroke, middle cerebral artery occlusion model, filament method, intravascular modeling method
The epidemiological study has shown that about 70% of all stroke patients worldwide suffer cerebral ischemic stroke (CIS),1 while ischemic stroke accounts for about 85% of all stroke cases in China.2 The study of reperfusion injury has become one of the hottest health topics in the world. Because the Sprague-Dawley (SD) rat vascular wall is very similar to that of humans in terms of structure and morphological changes during cerebrovascular disease,3,4 the SD rat middle cerebral artery occlusion (MCAO) model is the most commonly used animal model of stroke and can well simulate the process of ischemic stroke in humans.5 Zea Longa et al. reported a method for simulating ischemic stroke by wire embolization,6 which solves the issues of difficult surgery, long modeling time, and high mortality related to open brain simulations of ischemic stroke.7 As a result, the method is generally used in the establishment of ischemic stroke models. How to avoid thrombosis and vascular injury caused by misinsertion of the filament head into the pterygoid artery (PPA) and multiple embolus insertion is a difficult task in the preparation of the model. Zea Longa et al.6 proposed that ligation of the PPA could effectively avoid inadvertent insertion of the PPA. However, it is practically severe and detrimental to their postoperative survival because the location of the PPA closer to the posterior basal segment of the brain would make it more difficult to separate the vessels, and cause damage to the rats after separation.
To solve this problem, Kutluay Uluc et al. proposed bending the filament in a natural curve, so that they would be closer to the medial side during the intravascular alignment.8 This can partially avoid insertion into the PPA, but it is still not widely used because this method requires much experience and poor stability for the experimental operator and repeated practice to master. Although many researchers have subsequently improved this method in various ways, such as surgical technique, anesthesia, filament type, MCAO occlusion filament insertion depth, and occlusion time,9–13 there are still limitations such as long modeling time, high model mortality, and unstable model success rate.
In this study, we proposed to change the angle between the common carotid artery (CCA) and the PPA by pulling the vessel to avoid the inadvertent insertion of the filament into the PPA, and then advance the head of the filament to the middle cerebral artery, the ventral thalamic artery and the anterior choroidal artery. The MCAO is achieved by blocking the blood supply in the choroidal artery segment, avoiding damage to the rats caused by ligating the PPA and reducing operation time. At the same time, it avoids the long preparation time of the model caused by multiple filament insertion because it is more repetitive and stable compared to pulling the vessel to change the direction of the filament. By assessing the neurological function score and the stability of the cerebral infarct area, this method can significantly improve the model stability and modeling success rate.
The animals in this experiment were sacrificed according to the 2006 Guideline of the Chinese Ministry of Science and for the Care and Use of Laboratory Animals. Our study passed the ethical review requirements of Yunnan University of Traditional Chinese Medicine, ethical review no: R-062021002.
Seventy-five specific pathogen free (SPF) male SD rats, 8–10 weeks old, weighing 250–280g, were provided by Hunan Slack Jingda Biological Company. The breeding and modeling of experimental animals in this study were carried out in the SPF level experimental operation room of the university [License number: SYXK (Yunnan) K2017-0005]. The use of experimental animals followed the 3R principle and all experimental protocols were approved by the Experimental Animal Welfare Ethics Committee of Yunnan University of Traditional Chinese Medicine. Before the operation, four to five animals were housed in each cage, the rats were maintained under a 12h/12h light/dark cycle and could obtain food and water without restriction. The rats were randomly divided into1 no-surgery group (n=15),2 sham group (n=15),3 Zea longa group (n=15), and4 optimal group (n=15). At the end of the experiment, the animals were sacrificed by spinal cord dislocation after an intraperitoneal injection of sodium pentobarbital. All efforts were made to ameliorate any suffering of the animals.
1. MCAO bolt line purchased from Beijing Sinon Technology Co.
2. TTC (2%) staining reagents were purchased from Solebro Biotechnology Co.
3. Phosphate buffer solution was purchased from VivaCell.
4. Sodium pentobarbital (1%) was provided by the animal experimental center of Yunnan University of Chinese medicine.
5. 4% paraformaldehyde universal tissue fixative purchased from White Shark Biotechnology Co.
6. Xylene and anhydrous ethanol (75%) were purchased from Tianjin Zhiyuan Chemical Reagent Co.
7. Toluidine blue staining solution (1%) and glacial acetic acid were purchased from Wuhan Google Biotechnology Co.
8. Neutral gum was purchased from Sinopharm Chemical Reagent Co.
After seven days of adaptive feeding, the rats were diet fasted for six hours before surgery. Each rat was kept in a separate cage after surgery and the wound was disinfected with iodophor twice a day to prevent infection. The food was softened with cow's milk before the rats were fed.
The operation was performed under general anesthesia (2% isoflurane in 30% O2 and 70% N2O). Heating pads and rectal thermometers were used to detect and maintain the body temperature of the rats between 36.5–37.0°C. The MCAO standard procedure Zea longa approach for the surgery was as follows: After 75% ethanol skin disinfection, a 2 cm long median incision was made in the neck, and the right side was dissected CCA, external carotid artery (ECA), internal carotid artery (ICA), occipital artery (OA), superior thyroid artery (STA), and PPA. The distal ends of OA, STA, PPA, and ECA were ligated, and CCA and ICA were temporarily clamped. Insert the filament from the ECA into the CCA and finally insert the ICA to block the main MCA and ICA. The filament was pulled out after one hour and the muscle skin was sutured. The wire lifting method of this study is specifically as follows: After skin disinfection with 75% ethanol, a 2 cm long median incision was made in the neck, and the right CCA, ECA, ICA, and carotid artery are dissected. The proximal end of the common artery was ligated to block the blood supply, the ICA was temporarily clamped. In addition, pulling thread a (Figures 1 and 2) was tied around the ECA above 2–3 mm of the bifurcation of the ICA and ECA and a 45° incision was made on the CCA below 5–6 mm of the bifurcation of the ICA and ECA. The filament was placed into the ICA through the incision access. Then the ECA arteriole clamp was loosened, the pulling thread b was tied on the CCA, and the pulling thread a, b was lifted left with the index finger and middle finger (raise the CCA by about angle 20°–30° between the ICA and PPA to avoid the filament passing into the PPA). The filament was moved slowly forward in the ICA till a hindrance was felt indicating that the filament had approached the front end of the MCA. After ischemia of one hour, the filament was removed then the muscle skin was sutured, and the wound was disinfected. The filament was not inserted in the sham group, but the other operations were the same as those in the Zea longa group. The no-surgery group did not do have any intervention. Please see underlying data (Video file).14
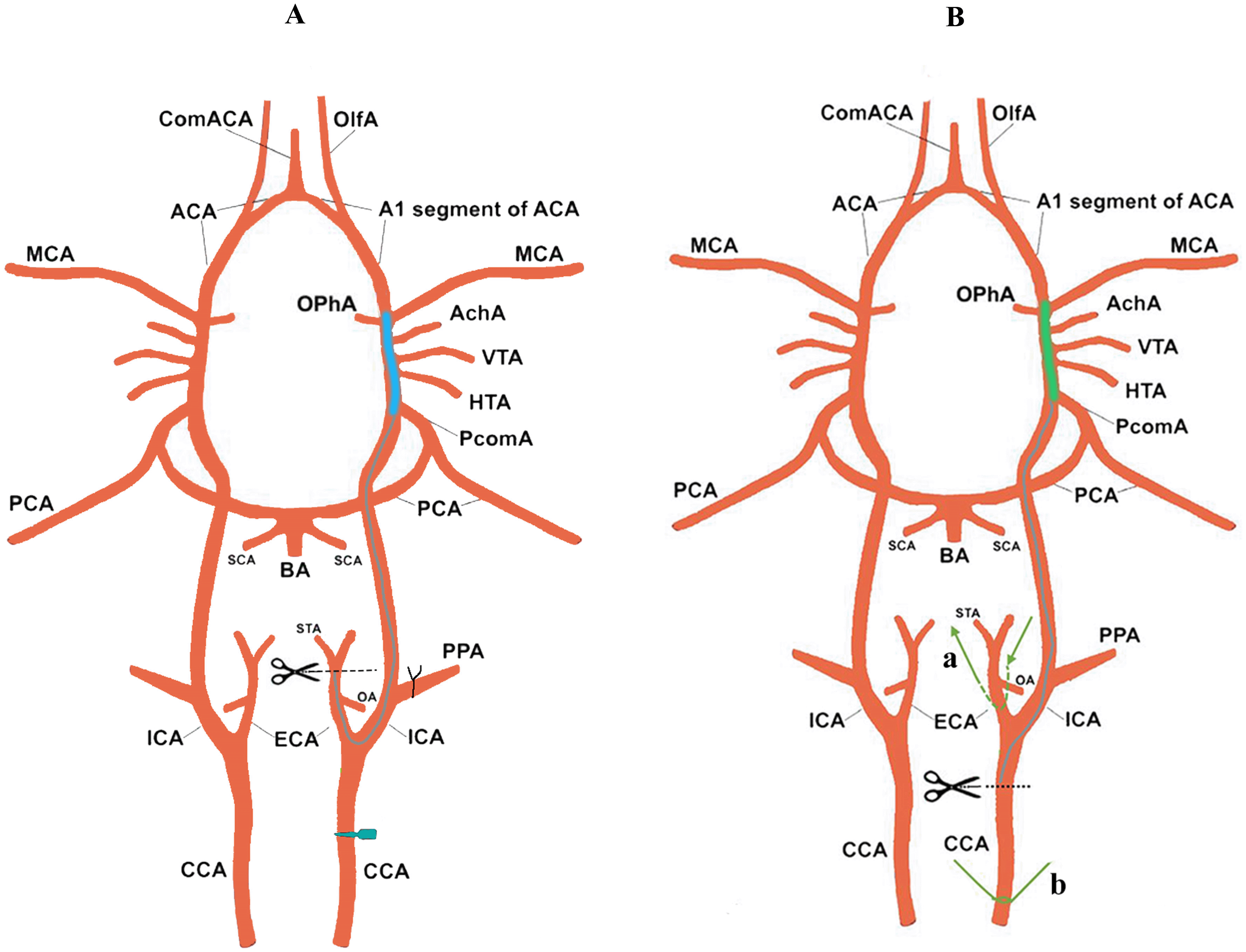
A. In the Zea longa method. B. The optimization method of this study.
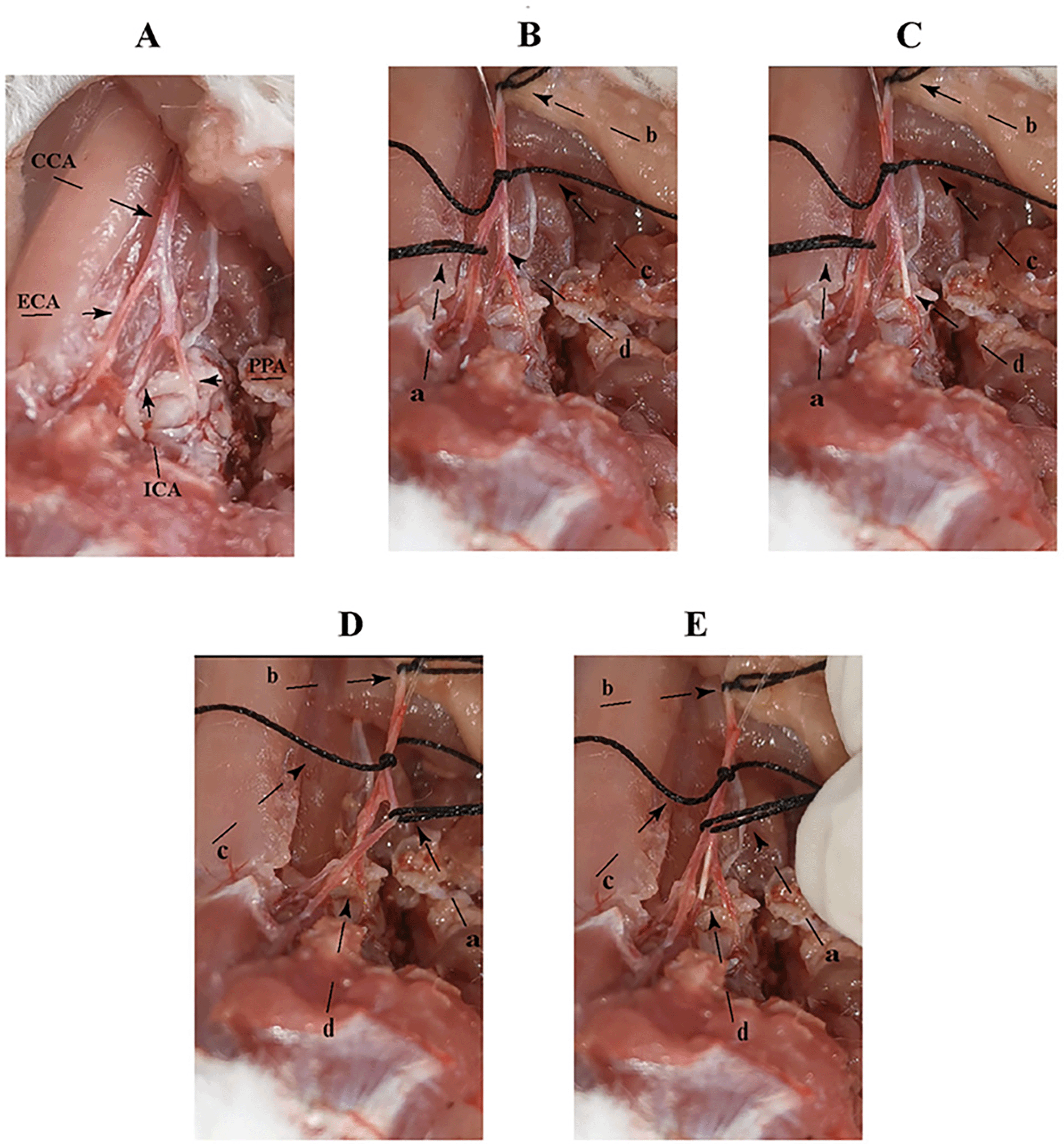
A. The anatomy of the rat neck vessels: common carotid artery (CCA), external carotid artery (ECA), internal carotid artery (ICA) and pterygoid artery (PPA). D and E are the threaded paths, in which the angle between CCA and PPA is changed by pulling (a and b) two threads to the left, and CCA and ICA tend to form a straight line at the same level, and the head of the thread is easily inserted into ICA within a short period of time (a is the pulling thread, b is the pulling thread, c is the fixed thread, and d is the head of the thread).
After the rats had been awake for 24 hours, the behavior of the rats was scored according to the Zea longa 5-level 4-point neurological scoring principle. The Zea longa 5-level 4-point system scoring principles: 1. 0 points, indicates no signs of neurological abnormalities. 2. 1 point indicates unable to fully extend the upper limb on the contralateral side of the lesion. 3. 2 points, indicates the rat rotates to the opposite side while walking. 4. 3 points, indicates a fall to the opposite side while walking. 5. 4 points, indicates no spontaneous activity with reduced consciousness. A score of 1–4 points is a successful model; rats that died accidentally during the operation, death within 24 hours of reperfusion, subarachnoid hemorrhage, and 0 points were excluded. Please see underlying data (Data file 2).14
The first day after the successful model, the rats were sacrificed after spinal cord dislocation under pentobarbital sodium anesthesia to remove the brain. The brain was washed twice with normal saline, and frozen in a refrigerator at -20°C for 10 min. After removal, the brain was placed in the brain groove and cut into five pieces at intervals of 2 mm with a coronal plane slice. The first brain cut was between the optic chiasm and the anterior pole of the brain, the second cut was on the optic chiasm, the third cut was on the pituitary gland, and the fourth place was between the pituitary gland and the posterior pole of the brain. The pieces of brain were incubated in a 0.08% TTC-0.01 mol/L pH 7.26 phosphate buffer solution stored in the dark at 37 °C for 30 min, turning once every 5 min. The brain slices were washed three times with 0.08% TTC-0.01 mol/L pH 7.26 phosphate buffer solution and the solution was then discarded. Then they were put into 40 g/L paraformaldehyde solution for 24 hours. Finally, these brain slice images were captured by a Nikon D3100 camera and ImageJ v1.8.0 analysis software was used to calculate the cerebral infarction volume of the brain slice images (Cerebral infarction volume (%) = total cerebral infarction area/total brain slice area × 100%). Please see underlying data (Data file 1).14
On the first day after the successful modeling, and after the rats were sacrificed, the heart was perfused with physiological saline and then perfused with 4% paraformaldehyde 0.1 mol/L phosphate-buffered saline (PBS, pH = 7.4). Paraffin embedding took place after brain extraction. For the paraffin section, the thickness of the clean, glass slide was about 4 μm, and the paraffin section was deparaffinized with water. The sections were placed sequentially into xylene I for 20 min; xylene II for 20 min; absolute ethanol I for 5 min; absolute ethanol II for 5 min; 75% alcohol 5 min, then washed with distilled water. For the Toluidine blue staining: the tissue sections were placed in the dye solution for 5 min, washed with water, and slightly differentiated with 1% glacial acetic acid. The reaction was terminated by washing with distilled water. The degree of differentiation was controlled under a microscope (Olympus BX43, 10X). After washing with distilled water, the sections were placed in an oven (DHG Series, Shanghai Yiheng Biological Company) and dried. Transparent mounting was performed by slicing into clean xylene for 5 min and sealing with neutral gum sealer. The pathological changes of Nissl-stained tissues in the cerebral cortex and hippocampus were observed under a mounting microscope (Olympus BX43, 10X, 40X) Please see underlying data (Data file 3).14
After the completion of the operation, the survival time and the number and weight of the rats in each group were recorded each day.
The experimental data include at least three independent and repeated experimental values, and the values are described by the mean + standard deviation. Student’s-t Test was performed in a two-group comparison, <0.05 was considered statistically significant (SPSS 22.0, SPSS Inc, Chicago, IL, USA)
It is well known that the MCAO animal model is the main method of studying cerebral stroke in human beings. In recent years, many researchers have improved the Zea Longa MCAO method in various ways9–13 since Zea Longa first performed it.5–6 But there are still some limitations such as long modeling time, high model mortality and unstable model success rate. It is urgent to find an MCAO model that can be time saving, cause less mortality and provide a more stable model success rate than before. Efficient MCAO would facilitate the research of cerebral stroke in humans and massively contribute to the field that causes one of highest morbidity and mortality rates in cardiovascular disease. In our study, the MCAO method had a significant time saving, less mortality and a stable model success rate compared to other methods by changing the angle between the CCA and the PPA by lifting the vessel left of the CCA and ECA to avoid the inadvertent insertion of the filament into the PPA.
To compare the success rate of each group, neurological function score was adopted to evaluate the function damage of the rats. According to the model success criteria, our result indicated there was no significant difference in the neurological function scores in the sham-operated group and no-surgery group. A total of 45 rats were used in the Zea longa group and our optimal group. Ten models failed and 35 succeeded in the Zea longa method group, with a success rate of 77.77% (Figure 3A); five models failed and 40 succeeded in the optimal group, with a success rate of 88.88% (Figure 3B) and the difference was statistically significant (Figure 3C).
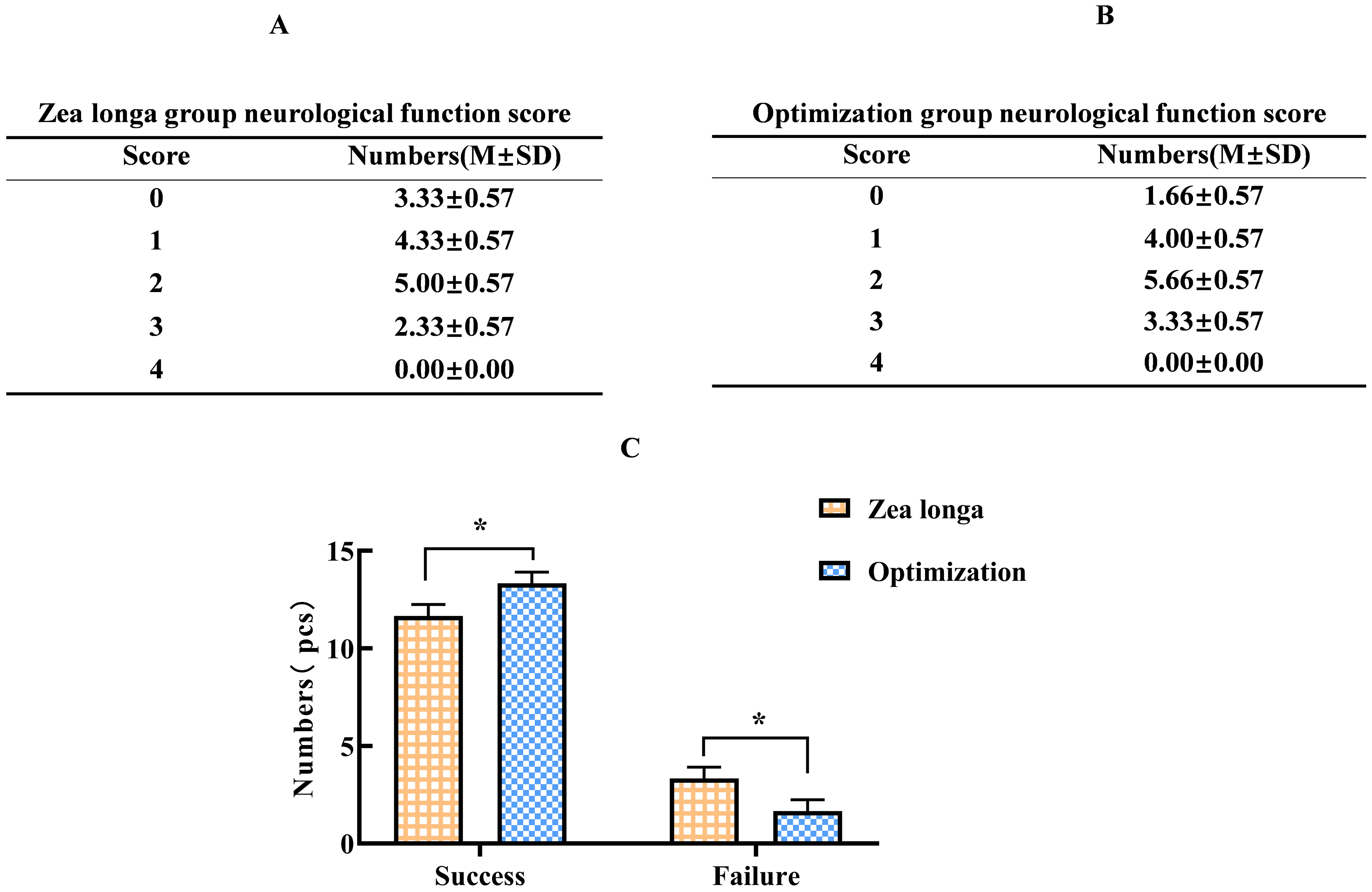
(score >1 means success). A and B. The neurological function scores of Zea longa group and Optimization group; C. The statistics of the number of successful modeling rats by different methods, and the differences are statistically significant (* indicates p <0.05).
TTC staining was performed on the brain tissue of rats in each group, and the results showed that the right and left cerebral hemispheres of the sham-operated group were evenly and symmetrically stained in coronal section, with red, gray matter and light red or porcelain white matter, and no pale infarcted areas. The ischemic side of the brain tissue of the Zea longa group and the wire group showed pale white infarcted areas in the cortex and subcortex, including the lateral basal nucleus, parietal and temporal cortex, hippocampus, striatum and other areas. The infarct volume was 18.36 ± 4.93% in the Zea longa group and 23.75 ± 2.96% in the optimization group, and the difference was statistically significant and apparently smaller within-group, indicating that the success rate and stability of the method were better than those of the traditional method (Figure 4).
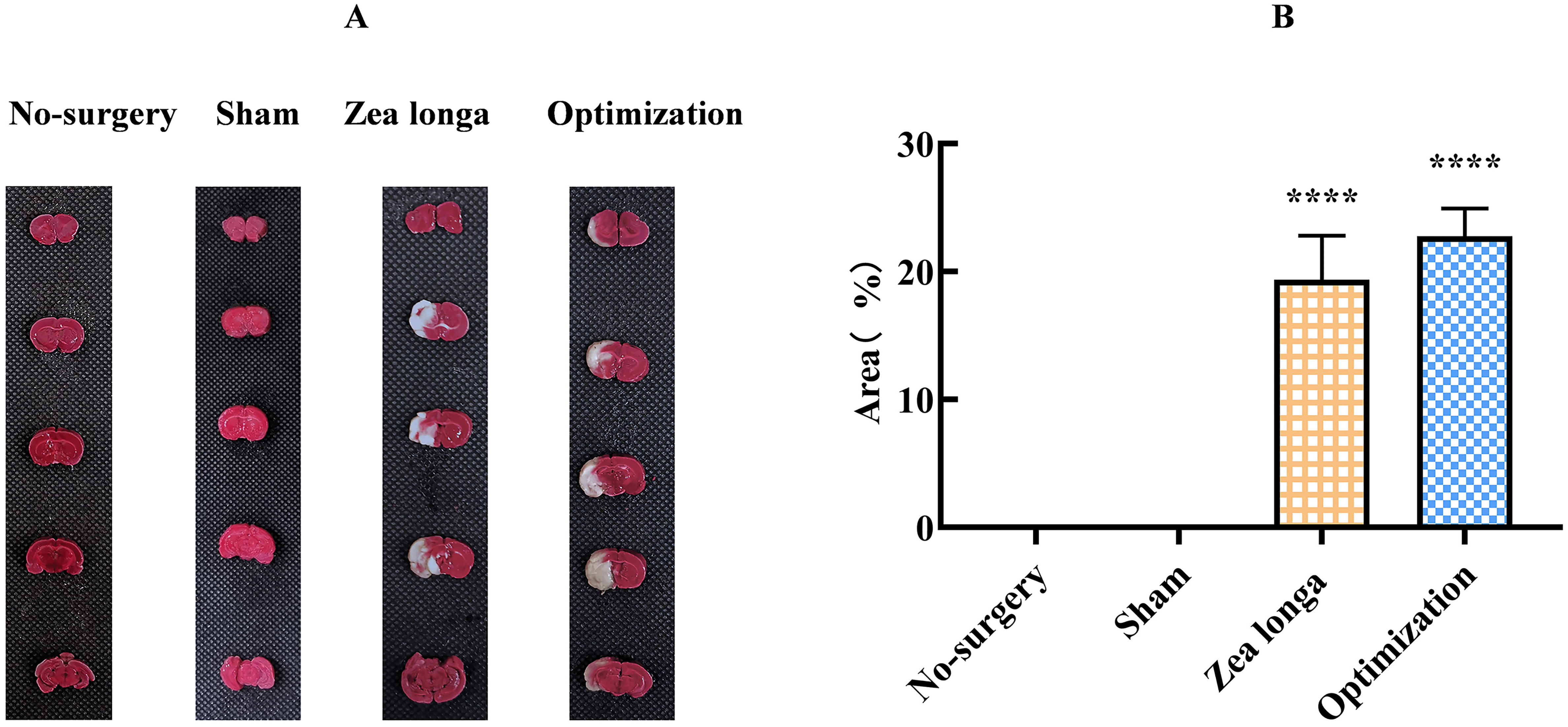
A. The results of TTC staining of brain tissues in each group, light red is normal brain tissue, white is infarcted tissue, and the infarcted area was measured to compare the statistical differences between groups. B. shows the comparison of the percentage of infarcted volume in each group for statistical difference comparison. **** indicates p <0.0001.
The cells in the hippocampal area of the blank group and sham-operated group were arranged regularly and neatly under light microscopy. The nucleus and nucleolus were clearly visible, with purple-blue granules or block-like structures in the cytoplasm of the nictitating bodies and naturally arranged neural protrusions. In the Zea longa group and the right hippocampal region of the optimization group, the cell arrangement was disorganized: some of the neuronal cells disappeared, the cytosol of the remaining neuronal cells was wrinkled and invaginated, the nerve fibers were abnormally curved, the intracytoplasmic Nissl disappeared and was blurred, the nucleus was solidified and homogenized, and the nucleolus was translocated (Figure 5).

A. Microscopic observation of morphology of hippocampal neurons for Nissl staining, the microscopic magnification of the upper and lower parts was 100x and 400x, respectively); B. The number of Nissl vesicles in hippocampal region of different subgroups were counted to compare the statistical differences. (**** indicates p <0.0001).
We recorded the model preparation time of the two different methods and found that the mean modeling time of the Zea longa group was 21.07 ± 6.11 min, while the mean modeling time of the optimization group was 17.22 ± 2.26 min. The mean modeling time of the optimization group was less than that of the Zea longa group, and the modeling time was shorter, and more importantly, the modeling time of the optimization group was more consistent and repeatable. The difference was statistically significant (Figure 6). Please see underlying data (Data file 5).14
The rats fasted for six hours before surgery and after surgery each rat was kept in a separate cage and the wound was disinfected with iodine twice a day to prevent infection. The rats’ food was softened with cow's milk and placed in the cage to facilitate feeding. The animals in the blank and sham-operated groups did not die during the experiment. The bodyweight of the animals in both groups increased gradually over time, but the sham-operated group showed a decrease in body weight on the first postoperative day, which was considered to be due to the physical injury to the rats due to the surgical trauma. The Zea longa group showed a significant weight loss in the first five days after surgery and a gradual increase in weight on the seventh day, while the Zea longa group showed a weight loss in the first three days after surgery and a gradual increase in weight on the fifth day. We speculate that the main reason for this trend is that the different modeling methods caused different damage to the rats. The number of survivors in both the Zea longa group and the optimization group decreased, and the number of survivors in the optimization group decreased less than that in the Zea longa group, and the difference was statistically significant (Figure 7). Please see underlying data (Data file 4 and 6).14
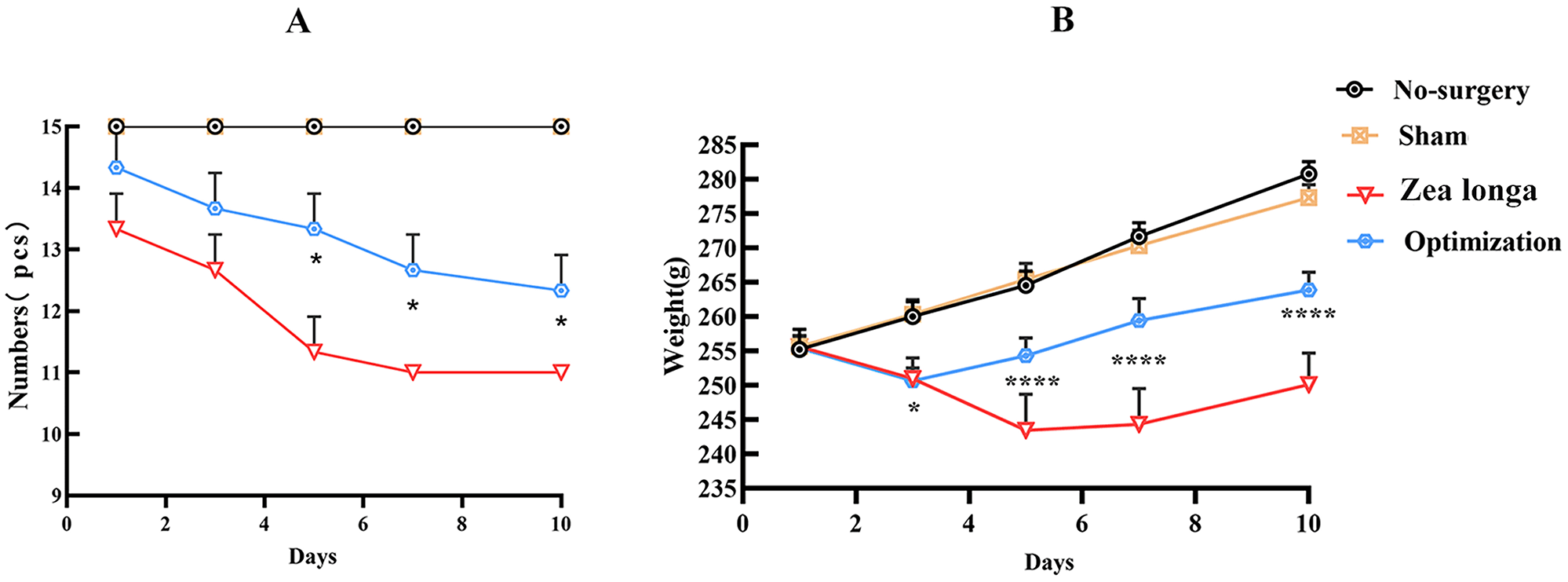
A. The statistics of the number of surviving rats at 10 days after surgery, comparing the differences in the number of surviving rats at different time points; B. The statistics of the body weight of rats at 10 days after surgery, comparing the changes in body weight of rats at different time points. (* indicates p <0.05, ** indicates p <0.01, **** indicates p <0.0001).
MCAO is the most commonly used experimental stroke model, which can simulate the process of ischemic stroke, but limitations such as time-consumption, high mortality, and unstable neurological deficit scores have not been addressed. The commonly used methods for the preparation of MCAO models include the wire embolization method15 and the thrombus method.16 The filament method has been continuously improved to establish a stable and reproducible MCAO model and is the closest animal MCAO model to human stroke, so most MCAO models are prepared by the filament method. However, the classical method of MCAO modeling of transient Zea longa can have a mortality rate of up to 50%,17,18 which becomes a key factor limiting the study of animal models of MCAO. The long preparation time of the model, the trauma caused by the surgery itself, the different animal strains, the way the model was prepared, and the ischemia time all contribute to the high mortality rate of the model. Some groups have reported mortality rates as high as 50% in C57BL6 mice17 and 71% in Balb/C mice,19 which may be the result of cerebral vascular variability.20 Therefore, adult SD male rats were used in this study because the cerebral arteries of SD rats are very similar to human cerebral arteries3,4,21 whereas SD rats have less intraoperative hemorrhage and male SD rats may avoid the neuroprotective effect of estrogens.22–24
Transient ischemia of the brain can be achieved by inserting a filament into the MCA either via the ECA or CCA,25,26 but the traditional method of preparing the Zea longa model is prone to accidental insertion of the filament into the PPA either way due to the physiological curvature and alignment of the vessel,3 which is the main difficulty in the preparation of this model. Some investigators have suggested that this problem can be avoided by ligating the proximal end of the PPA or bending the wire peg to bring it closer to the medial alignment of the vessel.8 However, the location of the PPA is closer to the base of the brain and ligating the PPA would increase the difficulty of the procedure while causing more damage to the muscles and vessels and altering the original blood supply to the rats, which is not conducive to their survival. Although ligature of the PPA can be avoided by bending the pegs more medially, its stability and reproducibility are low, which is not conducive to the preparation of the model. We used the method of pulling the blood vessel to change the alignment of the filament by pulling the a and b wires to the upper left to change the angle between CCA and PPA, so that the CCA and ICA tend to form a straight line at the same level, and the head of the wire pins can be inserted into the ICA more easily in a short time (Figure 2). This avoids ligating the ECA and PPA and can significantly shorten the modeling time and improve the modeling efficiency. At the same time, the reduced deep muscle damage in the rat’s neck is also beneficial to postoperative recovery and improved survival rate.
During our experiments, we found that the size of the filament’s head was crucial to the success of the MCAO model, and the size and length of the bolus head should be adjusted according to the body weight of the rat.27 Some researchers compared the effect of different sizes of filament with the success rate of MCAO models, and the results showed that filaments with silicone coated heads had a higher success rate and the infarct volume was more consistent. This may be because silicone coated plugs can fully block the MCA and block blood circulation.28 The depth of the plug also affects the success rate of the model, and in our experience, a depth of 18–20 mm is appropriate for the insertion of the plug, which is just sufficient to occlude the MCA.29 Therefore, a silicone coated filament with a 2 cm marker point in the middle section (3400A Western Thickening) was used to easily determine the depth of filament insertion and improve the success rate of modeling. It is worth noting that the time of ischemia-reperfusion also plays an important role in the success of the MCAO model, which is usually 60, 90, or 120 minutes.4 However, some researchers30 have pointed out that when the blockage time is greater than 90 minutes it triggers ischemic injury in the hypothalamus leading to spontaneous hyperthermia in the MCAO model, and the increase in body temperature leads to a larger infarct volume and increased mortality.31–33 We chose 1 hr of ischemia, which can reduce mortality but obtain good infarct results. Meanwhile, a thermostatic heating pad was used to maintain the body temperature of rats at 36.5–37.0 °C during the modeling procedure, and a rectal thermometer was used to detect the body temperature, which avoided the brain infarct volume enlargement caused by high body temperature in rats.
In addition, increased pre- and post-operative care of MCAO had a positive effect on the survival rate of the rats. The high blood glucose levels exacerbate ischemic injury in model rats,34,35 so to avoid physiological postprandial blood glucose elevation, rats were fasted six hours before surgery to maintain blood glucose in the normal range. At the same time, preoperative fasting can avoid the obstruction of the esophagus by food residues after anesthesia and avoid accidental death during the preparation of the model. Routine postoperative wound disinfection and anti-infection facilitated wound healing. At the same time, because of the temporary damage to the masticatory muscles due to surgical trauma,36 softening the food with milk reduces the difficulty of free feeding in rats and ensures the nutrition required by the organism. These improvements are conducive to improving the postoperative survival rate (Figure 7). Also, the longer-term model weight changes and survival rates were not reported by other investigators and are important for studying the cycle of pharmacological intervention after MCAO modeling.
In conclusion, this study proposes that by pulling the vessel to change the course of the thread embolus optimizes the long preparation time and high mortality rate of the traditional Zea longa modeling method. This makes the preparation of the MCAO model easier, it is time saving, there is a lower mortality rate, a higher successful modeling rate and better repeatability.
Harvard Dataverse: Underlying data for ‘Pulling wire to avoid misinsertion of filament and vascular damage and improve ischemic stroke models' success rate and reproducibility’. https://doi.org/10.7910/DVN/6PHB1D. 14
This project contains the following underlying data:
• Data file 1: Infarct volume.xlsx
• Data file 2: Neurological function score.xlsx
• Data file 3: Nissl data.xlsx
• Data file 4: Numbers.xlsx
• Data file 5: Operation time.xlsx
• Data file 6: Time and weight.xlsx
• Video file: Operation.mp4
• Figure 1: Schematic diagram of the main arteries of the head and neck of the rat during the modelling of the different groups middle cerebral artery occlusion (MCAO) and the position of the filament.
• Figure 2. Schematic diagrams of the embolization paths of different methods.
• Figure 3. Neurological function scores and success rates of rats in different groups.
• Figure 4. Results of the volume and morphological changes of brain infarcts in rats of different groups (MCAO).
• Figure 5a. Nissl staining in hippocampal region of rats in different subgroups (MCAO).
• Figure 5b. Nissl staining in hippocampal region of rats in different subgroups (MCAO).
• Figure 6. Modeling time of different methods.
• Figure 7. The number of surviving rats and body weight changes after surgery.
Harvard Dataverse: ARRIVE checklist for ‘Pulling wire to avoid misinsertion of filament and vascular damage and improve ischemic stroke models' success rate and reproducibility’. https://doi.org/10.7910/DVN/6PHB1D. 14
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for developing the new method (or application) clearly explained?
Partly
Is the description of the method technically sound?
Partly
Are sufficient details provided to allow replication of the method development and its use by others?
Yes
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Ischemic stroke
Is the rationale for developing the new method (or application) clearly explained?
Partly
Is the description of the method technically sound?
Partly
Are sufficient details provided to allow replication of the method development and its use by others?
Partly
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
Partly
References
1. Percie du Sert N, Alfieri A, Allan SM, Carswell HV, et al.: The IMPROVE Guidelines (Ischaemia Models: Procedural Refinements Of in Vivo Experiments).J Cereb Blood Flow Metab. 2017; 37 (11): 3488-3517 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Cerebral ischemic stroke
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 17 Apr 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)