Keywords
Rubus glaucus Benth, probiotics, prebiotics, antioxidants, microencapsulation, physico-chemical properties
This article is included in the Agriculture, Food and Nutrition gateway.
Rubus glaucus Benth, probiotics, prebiotics, antioxidants, microencapsulation, physico-chemical properties
The Andean blackberry (Rubus glaucus Benth) is a native fruit of the high tropical Andean region of the Americas. This fruit belongs to the Rosaceae family (Schulz & Chim, 2019) and contains a diversity of active components as phenolic compounds, which include anthocyanins (mainly cyanidine-3-glucoside, and procyanidins) ellagitannins and flavonols (Yang et al., 2022; Schulz & Chim, 2019; Fernandes et al., 2018). Furthermore, blackberries have a significant content of vitamins (A, C, E, K, and B), and minerals (Ca, P, K, Mg, Fe) (Schulz & Chim, 2019; Oszmiański et al., 2015). This composition imparts relevant functional characteristics to blackberries, such as: reducing blood pressure, protecting against hepatic lesions, acting as an anti-inflammatory, being antimicrobial, and suppressing human cancer cells (Schulz & Chim, 2019).
Nowadays, consumers demand stable and functional foods that are easy to prepare and provide nutritional value that generate health benefits (Liu et al., 2022). Fresh fruits offer important antioxidant compounds, which, under appropriate processing conditions, can be turned into powders that meet current market demands. The incorporation of probiotics and prebiotics in formulations can further enhance their functional properties (Guergoletto et al., 2017; Huang et al., 2017).
According to the International Scientific Association for Probiotics and Prebiotics (ISAPP), probiotics are “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (Hill et al., 2014). They also have a positive impact on aspects such as maintaining intestinal microbiota, improving the immune system, and reducing blood pressure, among others (Das et al., 2022). Some studies have evaluated the probiotic encapsulation in fruit-based matrices using spray drying (SD): Lactobacillus rhamnosus in orange juice (Sengun et al., 2020), Bifidobacterium spp. in jussara juice (Paim et al., 2016), Lactobacillus casei in orange, pineapple, and raspberry juices (Olivares et al., 2019), Lactobacillus plantarum ATCC 14917 in fermented pomegranate juice (Mantzourani et al., 2019), and Lactobacillus casei LPAA 02 in acerola-siriguela juice (Souza et al., 2021), inter alia.
On the other hand, the ISAPP definition of prebiotics is “a substrate that is selectively utilized by host microorganisms conferring a health benefit” (Gibson et al., 2017). Inulin is considered a prebiotic fibre because it is a non-digestible oligosaccharide which is recognized as a safe food that has a positive impact on health (Carlson et al., 2018). Other research suggests that inulin promotes the growth of bifidobacteria and lactobacilli (Oluwatoyin et al., 2022, Luca & Oroian, 2019). Additionally, prebiotics have been shown to reduce blood pressure, glucose, cholesterol, triglycerides, and phospholipids in blood (Tawfick et al., 2022). Some authors report that adding inulin could improve cell survival during spray drying. Kingwatee et al., (2015) studied lychee juice with added Lactobacillus casei 01 and different mixtures of encapsulants and inulin, and identified that when inulin was added cell survival increased to 1 or 2 Log CFU/g.
Spray drying (SD) is a widely used encapsulation technology in both the food and pharmaceutical industry, which allows the active compounds, including: vitamins, phenols, anthocyanins, (Jafari, et al., 2023; Araujo-Díaz, et al., 2017), probiotic microorganisms, prebiotics (Wang et al., 2022) and colorants, to be encapsulated. One of the main advantages of this technology is the short residence time, making it ideal for heat sensitive products and resulting in successful retention of flavour, colour, and nutrients.
The quality of the powdered product obtained by SD depends on the physicochemical stability of the feed, which is typically a colloidal system such as an emulsion, suspension, or mixture where different forces are involved: Van der Waals, electrostatic, steric, hydration, hydrophobic and phase separation (Mateos & Palazzo, 2022). Likewise, feeding properties (total solids, viscosity, density, surface tension, particle sizes, zeta potential, and spectral absorption index) play an important role on their stability (Tontul & Topuz, 2017; Marulanda et al., 2018). In the same way, the type of encapsulant used affects the powder quality (Lee et al., 2018), as well as the design characteristics of the equipment (atomizing disc, number, height and length, nozzle or pressure nozzle, size and number, two fluid nozzles, type of flow co-current/countercurrent/mixed) (Tontul & Topuz, 2017) and operating conditions (air inlet temperature, air outlet temperature/feed flow, drying air flow/residence time/pressure of the drying chamber, atomizing disc speed/pressure at the nozzle or nozzle) (Shishir & Chen, 2017).
The blackberry has high potential in the food industry due to its nutritional and sensory qualities, but its perishable nature with high respiration rates and a short post-harvest life limit its fresh marketing (Horvitz et al., 2017). Spray drying offers an effective alternative to preserve and improve fruit characteristics by converting a fruit suspension into a stable powder with longer shelf life (Tontul & Topuz, 2017). In this context, the aim of this research was to assess the impact of formulation and the spray-drying process on blackberry powder and its effect on physicochemical properties, the viability of the microorganism Lacticaseibacillus casei, and the antioxidant capacity of the product.
The materials used were Andean blackberry concentrate (Nutrium S.A.S, Medellín, Colombia, 13-15 °Brix, citric acid 3.1-5.5%, and pH 2.5-3.5), maltodextrin DE 18-20 (Ingredion Colombia S.A; Medellín, Colombia), inulin (Fibruline S20, Cosucra, Warcoing, Belgium), probiotic microorganism L. casei ATCC 393 (Microbiologics, St. Cloud, United States), broth, and agar MRS for bacteria (Man, Rogosa and Sharpe, Scharlau, Barcelona, Spain).
L. casei was activated in 10 mL of MRS broth at 37 °C for 72 h in anaerobiosis. Then, 90 mL of broth was added, and it was incubated at 37 °C for between 8-9 h (exponential phase). Later, it was added to 900 mL of culture medium and incubated for between 14-15 h (early stationary phase) at the same temperature. Finally, it was centrifuged at 440 g for 15 min at 25 °C, the supernatant was removed, and the biomass was obtained by washing the pellet with sterile water.
Batches of 2000 g were prepared; initially the blackberry concentrate was dissolved until a suspension with a solid content of 8.8% was obtained by using a Silverson L5M-A (Chesham, United Kingdom) homogenizer for 10 min at 10000 rpm. Also, a premix of maltodextrin (MD), inulin (3.06% w/w) and water (homogenized for 5 min at 10000 rpm) was prepared; the biomass was then added (≈ 8.58 x 1010 CFU/g) to this and the process continued at 2000 rpm for 1 min. This last premix was then incorporated into the blackberry suspension manually, and finally it was homogenized at 10000 rpm for 1 min.
A pilot SD equipment (Vibrasec PASALAB, Medellín, Colombia), 1.5 co-current flow operated at subatmospheric pressure (0.373 kPa) was used. The response surface methodology was used with a face-centered (α=1) central composite experimental design with 21 experiments (Table 1). The independent variables are the following: MD (4-8%), air inlet temperature (IAT) (120-140°C), air outlet temperature (OAT) (70-80°C), and atomizing disc speed (ADS) (20000-24000 rpm). The dependent variables are the following: viability L. casei (LogCFU/g), moisture (M), water activity (aw), total phenols (TP), antioxidant activity (DPPH, ABTS), anthocyanins, solubility (S), hygroscopicity (H), colour (L*: luminosity, a*: red/green chromaticity and b*: yellow/blue chromaticity), particle size (D10, D50, D90), and finally the process yield (Y). In order to minimize the effect of the temperature in the drying chamber, the dust was removed every 15 minutes. The dust collected in the jacketed cyclone container was cooled using water at 30 °C in order to favour the survival of the microorganism.
To determine the optimal operating conditions, an experimental optimization process was conducted. A criterion was established for each dependent variable to achieve the best quality attributes in the BPLI powder. The dependent variables were modelled using a second-order polynomial equation (Equation 1). Model fit was evaluated using the lack of fit method and the regression coefficient (R2). The experimental values at the optimum conditions were obtained from three replicates and compared to the theoretical value of the mathematical model to assess the accuracy of the model.
Where Y is the dependent variable, β0 is constant, βA, βB, and βC are the linear coefficients; βAB, βAC, and βBC are the coefficients of the interaction of the linear factors; and βA2, βB2, and βC2 are the quadratic coefficients.
10 g of suspension were homogenized with 90 mL of universal peptone (0.1% w/v), and successive dilutions up to 109 were made. These dilutions were prepared in order to attain greater accuracy in colony number counting (Marín-Arango et al., 2019). For the blackberry powder, 10 g was rehydrated in 90 mL of saline solution (0.85% w/v); this was stored for 30 min at room temperature to activate the microorganism, and then serial dilutions up to 106 were prepared. The samples were inoculated into the MRS agar by the deep inoculation method, and the cultures were incubated at 37 °C for 72 h for the suspension, and 240 h for the blackberry powder under anaerobic conditions.
The L. casei survival rate was determined according to dos Santos et al. (2019), employing Equation 2:
Where N is Log CFU/g after spray drying process, and N0 is Log CFU/g of feed suspension.
Physicochemical characteristics
Moisture (M) and water activity (aw)
The M was determined with a vacuum furnace at 60 °C to constant weight (AOAC, 2012), and the aw was determined with a dew point hygrometer Aqualab 4TE (Decagon Devices, Pullman, United States) at 25 °C (AOAC, 2012).
Solubility (S)
The S was carried out following Cano-Chauca et al.’s (2005) method with modifications: 100 mL of distilled water was homogenized with 1 g of blackberry powder for 3 min, the suspension was transferred to falcon tubes, and then centrifuged at 1060 g for 15 min. Subsequently, a 25 mL aliquot of the supernatant was taken in a Petri dish and placed in the oven at 110 °C for 1 h; thus, the weight of dry solids was obtained (WSS-25mL). S was calculated according to Equation 3, expressed in % dry base (db).
Where, WSS-25mL is weight of dry solids, W is sample weight, and M is moisture percentage.
Hygroscopicity (H)
The powder's hygroscopicity was determined according to the methodology described by Martínez-Navarrete et al. (1998). Approximately 2 g of the powder sample was added to a glass vessel and placed inside an airtight flask containing another vessel with a saturated potassium iodide (KI) solution (aw KI 25 °C = 0.689). The bottle was placed in a Memmert ICH-256-L chamber (Schwabach, Germany) at 25 °C, relative humidity (RH) 65% for 4 weeks, and the equilibrium humidity was determined at the end of this period.
Colour
The colour was determined according to Ferrari et al.’s (2012a) methodology using an X-Rite sphere spectrophotometer, model SP64 (Grand Rapids, United States), illuminant D65, observer 10°, and the specular component excluded. Colour on the surface of the samples was reported in the CIELAB colour space, where L* is luminosity, a* and b* are chromaticity parameters.
Particle size
The particle size was determined using the Mastersizer 3000 (Malvern Instrument Ltd, Malvern, United Kingdom) by laser diffraction, AERO S cell, and the MIE dispersion model with an absorption index of 0.5 and a refractive index of 1.45; particle size was expressed in percentiles (D10, D50, D90).
Yield
The Y of the product was determined as the ratio of the kg solids in the powder obtained and the kg solids in the SD feed (Tontul & Topuz, 2017).
Total phenols, anthocyanins, and antioxidant capacity
Extraction to determine TP and antioxidant activity was performed according to the Fang & Bhandari (2011) modified methodology. 0.1 g of blackberry powder was mixed with 40 mL of a methanol/water solution (70:30); the resulting mixture was centrifuged at 7080 g for 15 min at 20 °C and then passed through the qualitative filter paper (Boeco, pore between 8-12 μm). The supernatant was gauged at 50 mL with methanol/water.
Total phenols
The Folin-Ciocalteu colorimetric method was used for the quantification of TP, following the method described by Zahoor et al. (2018) with some modifications: 100 μL of the extract was mixed with 400 μL of sodium carbonate solution (Na2CO3) 0.07 M, shaken in a vortex, and left to stand for 5 min. Then 500 μL of the folin reagent (Merck, Rahway, United States) was diluted in distilled water (1:10 mL was added in a volumetric flask). After a 2 h reaction, absorbance was measured at 760 nm in a UV-vis spectrophotometer (Thermo Fisher, Waltham, United States). The results were expressed as mg eq gallic acid (GAE)/100g on a wet basis.
DPPH and ABTS
The antioxidant activity was determined according to Thaipong et al.’s (2006) methodology, using two reactions: DPPH radical (2,2-diphenyl-1-picrylhidrazyl, Merck, Rahway, United States) at 517 nm and ABTS+ [2,2´-azinobis- (3-ethylbenzothiazoline-6-sulphonate)], Merck, Rahway, United Stated) at 734 nm. 950 μL of a dissolution of each radical, and 50 μL of the extract were added and stored in the absence of light (30 min for DPPH) and (7 min for ABTS). Results were expressed as mg eq-Trolox/100g wet basis (mg eq-Trolox/100g).
Anthocyanins
Antocyanins extracts were obtained following the methodology described by Awika et al. (2005) with modifications. 0.5 g BPLI was extracted using 10 mL of acidified methanol (1% v/v HCL) in an ultrasonic bath for 20 min. The mixture obtained was centrifuged at 7000 g for 10 min, then the supernatant was decanted, and the residue was extracted consecutively with the solvent (2 x 7.5 mL). The supernatants were diluted in a 25 mL volumetric flask. The anthocyanin content was determined by the differential pH spectrophotometric method (AOAC, 2012) and expressed as cyanidine-3-glucoside (cyd- 3-glu). 2 mL of the sample were diluted to 10 mL with potassium chloride (0.025 M), and sodium acetate (0.4 M), which were used as buffer solutions at a pH of 1.0 and 4.5, respectively. To determine the anthocyanin content of BPLI, Santos et al.’s (2019) methodology was followed with modifications using Equations 4 and 5.
Where A520 and A700 (absorbances of each solution at 520 nm and 700 nm), MW (molecular weight of cyd-3-glu) = 449.2 g/mol, DF = dilution factor, ε (molar extinction coefficient for cyd-3-glu) = 26900 L mol-1 cm-1 and 103 = conversion factor from g to mg and from mL to L, W = sample weight (g), and v = dilution volume (mL).
Figure 1 displays the response volume graph of L. casei viability (Figure 1a), total phenol content (Figure 1b), antioxidant capacity (Figures 1c and 1d), anthocyanins content (Figure 1e), powder hygroscopicity (Figure 1f), solubility (Figure 1g) and colour parameters (Figure 1h and 1i) in biofortified blackberry powder. The above-mentioned characteristics show significant statistical differences (p < 0.05). In the same way, Table 2 shows the ANOVA (p-values) of the dependent variables with respect to the independent variables and their linear and quadratic interactions, where it was observed that variables M, aw, DPPH, b*, D10, D50, D90, and Y did not have significant differences (p < 0.05).
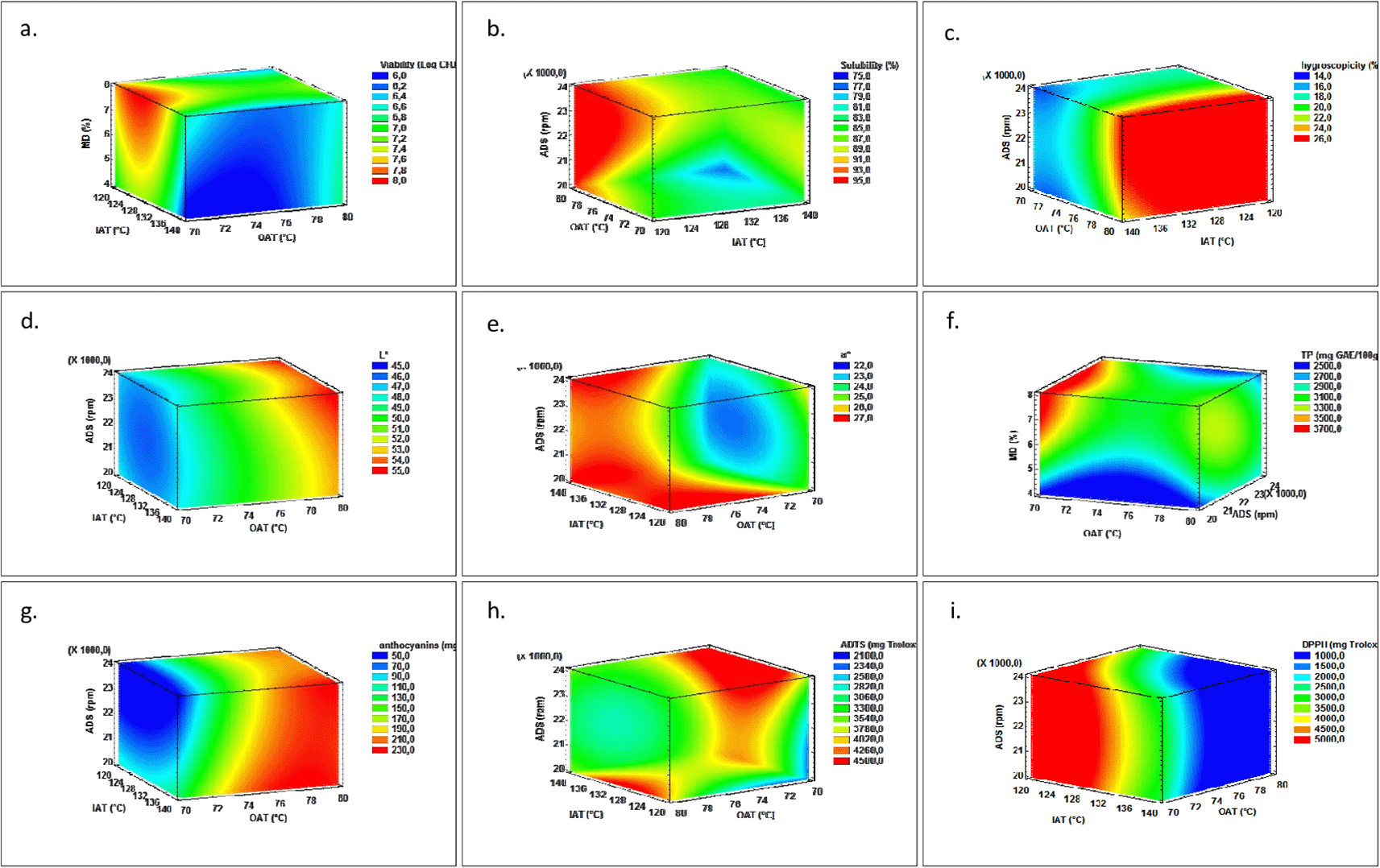
1a. L. casei viability, 1b. Solubility, 1c. Hygroscopicity, 1d. Luminosity (L*), 1e. Chromatic variable a*, 1f. Total phenol (TP), 1g. Anthocyanins, 1h. ABTS+, 1i. DPPH.
The viability of the microorganism was evaluated through cell counts and reported as log CFU/g. The suspension containing blackberry concentrate, probiotic, and inulin showed an average microbial count of 10.4 ± 0.6 Log CFU/g. After SD, a lethal effect was observed, where the count fluctuated between 6.1 and 7.9 Log CFU/g, corresponding to a survival rate of 58.6 and 76.0%, respectively. These results adhered to both Colombian and international regulations (> 106 CFU/g) (Minsalud, 2011; Liao et al., 2017). The microorganism survival rate in the BPLI was affected by SD, with a decrease of 2 logarithmic units. Dos Santos et al. (2019) reported that in free cells of Lactobacillus acidophilus La-5 microencapsulated with inulin by spray drying, there was a reduction between 3.8 and 1.5 Log CFU/g, equivalent to a survival rate in the range of 62.2 and 86.5%.
The response volume graph (Figure 1a) shows a higher viability of L. casei when SD operates at low IATs and OATs (120 and 70 °C) when the MD proportion varies between 6 and 8%. No significant differences were observed in relation to the ADS range (20000-24000 rpm), (Table 2). This behaviour could be attributed to the action of MD and inulin as thermoprotective agents and the growth stimulants of L. casei, which minimize different types of stress associated with loss of viability: oxidative, osmotic and mechanical, and desiccation (Kalita et al., 2018, Huang et al., 2017). However, thermal stress caused by high IAT and OAT in SD has been reported as one of the main factors influencing the survival of the microorganism (Huang et al., 2017; Zhang et al., 2016). Since it modifies the cell wall, there is a possible loss of the integrity of the lipid bilayer and the deterioration of cell membrane functions, which could contribute to breaking DNA chains (Perdana et al., 2013).
Another critical factor is the early stationary phase in which the microorganism was inoculated, which allows greater viability during drying because physiological states are induced that trigger survival response to stress (Huang et al., 2017).
L. casei type microorganisms exhibit different responses in survival levels in SD processes depending on probiotic, wall material, food matrix, and process condition employed: 81% with L. casei, L. rhamnousus, and L. plantarum in acerola-siriguela juice with 5% MD (IAT: 140 °C) (Souza et al., 2021); 72.5% with L. casei ATCC 393 in skimmed milk (IAT: 170 °C, OAT: 80/85 °C) (Dimitrellou et al., 2016); 40% with L. casei BL 23 in sweet whey (IAT: 170 °C and OAT: 63 °C) (Huang et al., 2016); 70% with L. casei NRRL B-442 in cashew apple juice (IAT: 120 °C and OAT: 75 °C, encapsulant MD and MD+gum arabic (GA)) (Pereira et al., 2014); 52.4% with L. casei in orange powder (IAT: 140 °C, OAT: 85-95 °C, MD encapsulant) (Alves et al., 2016); and finally, 5.7 and 6.8 Log CFU L. casei/mL in lychee powder (IAT: 150-170 °C and OAT: 60-90 °C, MD encapsulants, inulin, GA) (Kingwatee et al., 2015).
To identify the prebiotic characteristic of BPLI, the content of MD and inulin present in 26.12 g of the powder (10% solids) reconstituted in 250 mL of water was theoretically calculated. In this portion, 7.65 g of MD and 4.73 g of inulin were obtained. According to ISAPP (Gibson et al., 2017) the amount required to designate a product as a prebiotic is 3 g per day; therefore, in this case, the BPLI could be considered as a powder with prebiotic characteristics.
The mean values of M and aw of BPLI ranged between 2.1-5.6% and 0.202-0.317, respectively; no significant differences were observed (Table 2). Similar results were obtained by Souza et al. (2021) in juice powders with probiotics. In the same way, these values are within the ranges obtained by SD in blackberry-derived powders employing different encapsulants (Da Fonseca Machado et al., 2018; Franceschinis et al., 2014). In addition, low M and aw values guarantee good microbiological stability (Souza et al., 2021; Pereira et al., 2015; Dos santos et al., 2014), the long-term survival of L. casei in aw < 0.3 matrices due to the low molecular mobility of water (Dos santos et al., 2014), and, in general, have an influence on fluidity, tackiness, and stability during storage (Santhalakshmy et al., 2015).
S is a property of great interest in the powder industry and is much appreciated by the modern consumer. It depends on multiple factors such as those proper to the composition of the raw material, the surface area of particles, and the type of encapsulant used (Tontul & Topuz, 2017). Figure 1b shows the response surface plot for the S of BPLI. There were significant differences in the quadratic interaction of the ADS for solubility (p<0.05) as shown in Table 2, which is represented by the curvature and maximizes S at 22000 rpm. Mean values between 76.9±2.3-94.8±1.3% were observed in biofortified blackberry powder. In general, the BPLI presented a good S attributed to the affinity of its components with the aqueous phase, which allows easy diffusion of the water through the interior of its structure which is enhanced by the MD. The S of the biofortified powder is enhanced when the system operates under conditions of IAT (120-128 °C), and OAT (72-80 °C), in the whole range of ADS and MD. The lowest S values were found when the ADS was 20000 rpm, IAT: 136-140 °C, and OAT: 76-80 °C. This last behaviour is contrary to what was reported by some authors who associate the increase of the S with the increase of the IAT (Tontul & Topuz, 2017) when MD and OAT are high.
Some researchers have reported S values in several food matrices: Ávila et al. (2015) reports high values in sugar cane (97.87-98.1%) due to high sugar content and hydroxyl (OH) groups in molecules. On the other hand, Gagneten et al. (2019) reported S values of 95.4, 92.5, and 93.2% in raspberry powder, blackcurrant, and elderberry respectively. Franceschinis et al. (2014) reported values of S in blackberry powder with MD and MD-Trehalose (98.63-100%) that had a MD of 40%.
H is a physical property that reflects the level of a material of water adsorption in a known RH medium. In powdered food products, lower H values help to avoid stickiness problems (Tontul & Topuz, 2017). Figure 1c presents a hygroscopicity response surface plot. The mean H values for BPLI varied between 14.5±0.5-24.4±0.2% and showed significant differences (p < 0.05) with respect to OAT. These values were higher as the OAT increased. This behaviour was consistent with the lower values of aw and humidity in the BPLI at a high OAT, which increases the adsorption force and the motive force to the transfer of water mass from the controlled environment to the food. Similar values have been reported in blackberry powder obtained by SD (18.8-27.3 g/100 g bs) (Ferrari et al., 2012a); while Gagneten et al. (2019) reported H < 20% in raspberry powder, blackcurrant, and elderberry (13.1±0.9; 11.8±0.3, and 12.7±1.5% respectively) using MD 20% as an encapsulant. The least hygroscopic BPLI was obtained at high IAT (140 °C) and low OAT (70-74 °C), MD (4-6%), highlighting that the MD had a contrary effect to what Tontul & Topuz, (2017) reported. It could possibly form a complex MD, inulin, and sugars with lower adsorption strength.
The BPLI colour is one of the most attractive features of the product. It is described as having the classic pink colour, attributed to the anthocyanins content and the air occupied in the interior space of its structure, which generates greater homogeneity of the refraction index that makes the product clear (Cortés-Rodríguez, 2004). This is intensified by the presence of the MD due to the dilution effect. The L* and a* showed significant differences (p < 0.05) with respect to OAT (Figure 1d and 1e, respectively). Additionally, a* exhibited these differences regarding the linear interaction IAT-MD and the IAT, OAT, and ADS quadratic interactions. However, it stands out that despite the statistical variations (Table 2), the colour of the BPLI was not a critical variable since the changes found were small (L*: 46.0-52.8, a*: 22.5-27.2 and b*: 3.0-4.5) and unnoticeable to the human eye. Several authors reported this situation (avocado powder: Marulanda et al., 2018; coconut powder added with active components during storage: Lucas-Aguirre et al., 2019). The BPLI colour parameters are located in the I quadrant of the a*b* chromatic plane, in the grey zone that gives it a matte appearance, a predominance of reddish pigmentation tones, and low intensity of yellow tones. Some research on blackberry powder colour with MD and MD-Trehalose has reported higher L* and b* values in products obtained by SD rather than lyophilization; however, chromaticity a* was not affected (Franceschinis et al., 2014). On the other hand, Ferrari et al. (2012a) reported an increase in L* (clearer) with the increase in MD.
The D10, D50, and D90 percentiles did not differ significantly from the independent variables (Table 2); their mean values fluctuated as did their standard deviation for D10 (10.0±0.2-18.8 ±0.2 μm), D50 (24.2±0.3-34.0±0.4 μm), and D90 (44.1±0.9-254±9.7 μm). In general, low particle sizes were observed, which increases the surface area of the particles and favours a greater degradation of sensitive compounds (Tontul & Topuz, 2017). Some authors have reported particle size values in berries: Gagneten et al. (2019) for raspberry powder [D10 (4.22±0.19 μm), D50 (7.48 ± 0.26 μm), and D90 (13.41 ± 0.49 μm)] and for blackcurrant [D10 (4.64±0.15 μm), D50 (8.3±0.0 μm) and D90 (14.4±0.4 μm)]. Some differences were observed between the results of this study and those reported in previous research, which may be due to factors such as the type of fruit, processing, and spray drying conditions that impact the final particle size.
Y had values between 47.7 to 73.4%, which could be attributed to the loss of solids that adhered to the walls of the interior drying chamber due to the adhesiveness of the particles with the metallic material and the cohesiveness between the same particles. Other causes not associated with the SD process must also be considered such as the loss of solids due to the material that adhered to the walls of the feed preparation tank and the pipes that adhered to the atomizing disc. The total sum of these losses represents a high percentage value due to the fact that the batches for each experiment were 2 kg. A tendency to improve the Y with an increase in MD is highlighted, which could be attributable to a synergistic effect with the prebiotic fibre. Furthermore, to improve the yield process, one potential solution could be to dry larger batches exceeding 2 kg, or to implement a continuous feeding system for the suspension. Some authors have reported a similar situation in cactus pear powder with MD and inulin (Saenz et al., 2009) and in powder of sugar cane with MD (Ávila et al., 2015). Other authors have reported a higher yield of jussara powder with starch than in a mixture of inulin and MD (Paim et al., 2016); however, the authors conclude that the latter are essential for the conservation of anthocyanins.
Figure 1 shows the results obtained for TP, anthocyanins and antioxidant capacity determined by DPPH, and ABTS+ in BLPI, where an effect due to the independent variables associated with the SD process was observed: TP (3699.1±15.1-2467.5±87.9 mg GAE/100g), anthocyanins (228.6±5.0 and 52.6±6.3 mg eq-cyd-3-glu/100g), and the antioxidant capacity ABTS (2165.3±27.1-4463.7±120.0 mg eq-Trolox/100g) and DPPH (1011.7±21.6-5519.9±14.9 mg eq-Trolox/100g); all of these values are lower compared to those of the blackberry concentrate and the suspension (Table 3).
TP were statistically affected by linear ADS-MD interaction and quadratic OAT interaction (Table 2). The most favourable operating conditions were low OAT (70 °C), MD between 6 and 8%, ADS in the range 20000-24000 rpm (Figure 1b), and IAT 120-128 °C (data not shown). This behaviour may be attributed to the lower thermal stress and the protection offered by MD-inulin on the active components. Greater values have been reported in this investigation than those in Singh et al. (2019) (162.6-209.9 mg GAE/100g) for Jamun powder (Indian blackberry) where the IAT>185 °C had a significant effect; or in Franceschinis et al. (2014) for blackberry powder encapsulated with MD and MD+Trealosa (340±39 and 294±43 mg GAE/100g respectively). Furthermore, Colín-Cruz et al. (2019) reported the retention of TP in blackberry+L. acidophilus powder encapsulated with different wall materials: gum arabic (GA) (95.1%), MD (81.1%), whey protein concentrate (WPC) (75.7%), GA-MD (98.4%), GA-WPC (90.1%), and MD-WPC (87.1%).
Specifically, a diverse group of phenolic compounds comprising anthocyanins, monomeric phenols, and polymeric phenols have been reported for the Andean blackberry (Schulz & Chim, 2019). The cyd-3-glu and cyanidine 3-rutoside are the anthocyanins most often found. Phenols are a complex group represented by flavonoids, gallic acids and derivatives, phenolic acids and derivatives, and ellagitannins. These compounds can suffer thermal damage in different ways that lead to their reduction during the SD process. The thermal deterioration of anthocyanins glucosidase is associated with the hydrolytic opening of the pyrylium ring to form chalcone glucosidase (Fernandes et al., 2018). On the other hand, phenols display variability based on their chemical structure. For instance, the presence of a methoxyl group in hydroxybenzoic acids results in the loss of CO2 and CH3. Meanwhile, the loss of H2O, CO2, and C3H2O2 was observed in hydroxycinnamic acid esters. Also, flavonoids and condensed tannins are labile in thermal processes; where procyanidins are the most labile phenolic compounds and are prone to generate deprotonations and multicharged ions, especially in tetramers and major oligomers (De Paepe et al., 2014).
Anthocyanins are active compounds sensitive to deterioration due to several reasons: oxygen, light, metal ions, high pH, high temperature, and residence time (Da Fonseca-Machado et al., 2018). It is highlighted that the anthocyanin contents obtained fluctuated between 52.6±6.3 and 228.6±5.0 mg eq-cyd-3-glu/100g; however, these values were lower than those reported by Ferrari et al. (2012b) in blackberry powder obtained by SD (642.7; 628.2 and 639.0 mg/100g db), using the encapsulants MD (7%), GA (7%) and mixture MD (3.5%), and GA (3.5%), respectively. This difference could be mainly attributed to the use of blackberry concentrate in this research, which is subjected to thermal stress during the vacuum evaporation process. This could have contributed to the preliminary degradation through cyd-3-glu polymerization reactions (Wu et al., 2010) that are anthocyanin predominant in blackberry pulp (Schulz & Chim, 2019; Ferrari et al., 2012b).
When SD anthocyanins degrade, they show significant differences (p < 0.05) mainly because of the effect of the OAT factor. Unexpected behaviour was observed as there was an increasing tendency when OAT values were higher (76-80 °C). The higher anthocyanin content in BPLI is potentiated at IAT conditions (136-140 °C) and in all ADS and MD ranges. This situation could be attributed to several uncontrolled factors such as the variation of its composition in the raw material as well as to the protective effect of MD. MD is a glucose polysaccharide that can protect anthocyanins by non-covalent inclusions through hydrogen bridges and van der Waals interactions. Additionally, some authors indicate that the flavylium cation of anthocyanins with dextrins forms a complex that prevents the transformation of anthocyanins to other stability species (Colín-Cruz et al., 2019). Once the anthocyanins have been included in the maltodextrin matrix, they can withstand high thermal conditions. These results are consistent with those previously obtained by other authors who indicate that dextrins adequately protect anthocyanins and phenolic compounds during spray drying processes (Colín-Cruz et al., 2019; Fernandes et al., 2018).
Variations of anthocyanins can also be attributed to the anthocyanin extraction method since it has been reported that they are sensitive to temperatures higher than 60 °C in acid environments. Also, there are losses between 20-50% (Araujo-Díaz et al., 2017) and are considered stable below that temperature (Wang et al., 2017). Da Fonseca- Machado et al. (2018) reported 1.36±0.08 mg eq-cyd-3-glu/g bs in anthocyanin-rich extracts of blackberry residues obtained by SD, which were in a 5:2 ratio encapsulant:extract; these indicated that high temperatures decrease the concentration of encapsulated bioactive compounds, especially when they are thermosensitive. Franceschinis et al. (2014) obtained the monomeric contents of anthocyanins in blackberry powder with MD and an MD-Trealosa of 70±3 and 61±2 mg of cyd-3-glu/100g, and associated losses with high process temperatures. Colín-Cruz et al. (2019) reported the percentage of anthocyanins retention in blackberry powder + L. acidophilus that were encapsulated with GA (88.4%), MD (90.4%), WPC (80.2%), GA-MD (99.0%), GA-WPC (85.9%), and MD-WPC (87.3%).
The BPLI’s antioxidant activity as determined by the ABTS method did not show any defined behaviour that was statistically affected by the ADS, the linear interactions OAT-ADS and OAT-MD, and the quadratic interactions IAT and OAT for which the lower value of ABTS is shown with the reduction of the ADS. In addition, the higher values are reached with the increase of the ADS and in variable operating conditions: high OAT (78-80 °C), ADS ranging between (20000-24000 rpm); MD between 4 and 6% at low OAT (70 °C); MD between 4 and 8% at high OAT (78-80 °C); and IAT (128-132 °C). The antioxidant activity of Andean blackberry may be mainly associated with its phenol content and anthocyanins; the concentration of these compounds was affected differently by the variables associated with the SD process. Phenols were more affected by thermal stress than anthocyanins; however, MD offered protection for both compounds. Horvitz et al. (2017) found that there was no significant correlation between total phenols and antioxidant activity in blackberry extracts. Antioxidant activity, in addition to the anthocyanins, could be related to the Andean blackberry’s other phenolic compounds such as ellagitannins and ellagic acid derivatives, followed by minority phenols such as flavonols and phenolic acids (gallic, caffeic, coumaric, and ferulic) (Schulz & Chim, 2019).
Several authors have investigated the effect of spray drying on the activity of the ABTS radical: Franceschinis et al. (2014) reported 38.4 and 33.3% anti-radical activity in blackberry powder encapsulated with MD and MD+Trealosa, respectively at 175 °C IAT. Pereira et al. (2019) reported ABTS antioxidant capacity in juçara powder (859±44 μmol Trolox/g bs) at 160°C IAT and 86 °C OAT. For this, protein and carbohydrates had a protective effect against the degradation of the active components.
Although the antioxidant activity of BPLI measured by the DPPH method did not show significant statistical differences (Table 2), it showed a tendency to increase mainly due to the decrease in OAT that increases the values of antioxidant activity (Figure 1d). Compared to other research, the DPPH results obtained for BPLI were similar to values reported by Da Fonseca-Machado et al. (2018) (109.5±3.82 μmol Trolox/g bs) who encapsulated extracts rich in anthocyanins from blackberry residues through SD. Moreover, Ferrari et al. (2013) reported DPPH values in blackberry powder that had different levels of encapsulants: 7% MD, 7% GA, and 3.5% MD+3.5% GA, that were 210; 250; 240 μmol Trolox/g bs, respectively, which, established that the protein fraction of the GA contributed to Maillard's reaction and therefore to the increase of the antioxidant activity.
The experimental optimization process was carried out considering all the dependent variables as objective functions. Those variables that had statistically significant differences in the process were considered to have greater weight and impact: viability, S, H, L*, a*, TP, ABTS, and anthocyanins (Table 2).
The result of the optimization of multiple responses was a 71.9% desirability, and the independent variables of the SD process were set as such: IAT: 121 °C, OAT: 71.6 °C, ADS: 24000 rpm, and MD: 5%. Table 3 illustrates the criteria, weights, and impacts considered, as well as the experimental values obtained from 3 replicas of the optimal condition and the values predicted by the 2° order polynomial models that were adjusted to the response surface. Residual errors were considered acceptable with values ≤ 20% (viability, M, TP, S, H, L*, a*, b*, D10, D50, and D90). Moreover, Table 4 shows the regression coefficients obtained from the optimization process in this work. However, the variables associated with antioxidant capacity (DPPH, ABTS, and anthocyanins) were the ones that showed the greatest deviation from the predicted values, mainly due to greater instability and variability with respect to process temperatures. On the other hand, the large number of dependent variables considered does not favour all the established criteria being fulfilled. Experimental optimization in SD processes has been effectively applied in Jamun powder (Syzygium cumini L.) (Singh et al., 2019) and in the microencapsulation of probiotics in raspberry (Olivares et al., 2019).
This study successfully established the optimal drying conditions for obtaining a powdered biofortified blackberry concentrate, which were as follows: IAT 121.1 °C, OAT 71.6 °C, maltodextrine proportion 5% (w/w), and ADS 24000 rpm. Results indicated that low OAT had the most significant impact on the response variables analyzed. Furthermore, spray drying allowed the production of a food product with probiotic properties obtaining 5.60 x 107 CFU/g that meets the standards set by both the USA and Colombia (106 CFU/g). This product, known as BPLI, not only possesses antioxidant activity and desirable physicochemical properties for stable powdered products, but also offers the modern consumer the benefits of probiotics and antioxidants. Additionally, the powder can also be considered a prebiotic product thanks to its content of both inulin and MD.
figshare: Results of biofortified blackberry powder. https://doi.org/10.6084/m9.figshare.22332553.v3 (Marin Arango et al., 2023)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)