Keywords
Organ damage, inflammatory markers, sepsis mouse models, lipopolysaccharides
Sepsis is a life-threatening disease, and animal models of sepsis are minimal. This study aims to find the optimal dose of LPS to make a sepsis mouse model by examining the presence of target organ damage.
This study used 30 mice divided into four groups. The control group (3 mice) injected 0.5 cc NaCl 0.9% intraperitoneally (i.p.). Group A (9 mice) was injected with lipopolysaccharides (LPS) 0.125 mg/kg B.W. i.p. given on the first and second day, group B (9 mice) was injected with LPS 0.15 mg/kg B.W. i.p. given on the first and second days, and group C (9 mice) was injected by LPS 0.3 mg/kg B.W. single dose i.p. On the third, fourth, and fifth days, the termination of each group of three mice and examination of the NF-κB, tumor necrosis factor-α (TNF-α), C-reactive protein (CRP), alanine aminotransferase (ALT), the expression of NF-κB in the liver and kidneys, and histopathology in the liver and kidneys were conducted.
NF-κB, C-reactive protein (CRP), alanine aminotransferase (ALT), NF-κB examinations, and tumor necrosis factor-α (TNF-α) in all treatment groups increased when compared with the control. The highest degree of histopathological features of the kidneys and liver and the results of immunohistochemistry examinations on the liver and kidneys were shown in group C.
The optimal dose of LPS to make a sepsis mouse model was 0.3 mg/kgB.W with the most severe target organ damage dan significant increased of inflammatory markers.
Organ damage, inflammatory markers, sepsis mouse models, lipopolysaccharides
In this new manuscript, we have added and revised several things, including:
1. Added basic LPS dose selection for sepsis model mice in the introduction.
2. Several corrections regarding typing errors
3. Apart from carrying out statistical analysis again, we also display images of the results of statistical analysis again using boxplots in the hope that this can help readers more easily interpret the results of statistical analysis.
4. We added research details to the image captions such as explaining the name of the group and the number of mice in each group
5. We added a picture of the research implementation flow to clarify research techniques
6. We replaced the literature in the bibliography with newer literature.
7. We rearranged the conclusions according to the results of the research carried out, and adapted to the research objectives.
8. On the histology image we added the magnification scale that we used.
9. We combined images 7 & 8, images 9 & 10, images 11 & 12 because one is a histology image and the other is a quantification image according to the reviewer's suggestions.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
The incidence of sepsis in Indonesia and abroad is increasing, a concern in the medical world today. In Europe and America, the incidence of sepsis ranges from 0.4 to 1/1,000 of the population. Moreover, sepsis mortality is also high, reaching 30%, whereas sepsis shock gets 80%. Therefore, the early diagnosis of sepsis and rapid management can improve the prognosis of the patients. More than 30 million people are estimated to be exposed to sepsis each year globally, and it potentially causes 6 million deaths each year. As per data from the Surviving Sepsis Campaign in 2012, the mortality rate from sepsis is approximately 41% in Europe, which is higher than in the United States, that is, approximately 28.3%.1–3
Sepsis is a set of biological, pathological, and biochemical symptoms of the body in response to infection. In 1991, the criteria for sepsis were divided into sepsis, severe sepsis, and septic shock. The diagnosis of sepsis is established if it meets at least two of the four criteria of Systemic Inflammatory Response Syndromes, namely, body temperature of >38°C or <36°C, heart frequency of >90 x/min, breath rate of >20 x/min or PaCO2 of <32 mmHg, leukocyte count of >12000/mm3, or <4000/mm3. Severe sepsis is considered if the sepsis criteria are met and accompanied by organ malfunction.3
In 2017, Rhodes et al., in Survival Sepsis Campaign: International Guideline for Management of Sepsis and Septic Shock: 2016, presented the latest definitions for sepsis and septic shock. According to the guidelines, sepsis is the life-threatening functioning of organs caused by the failure of the body’s response to infection. Severe sepsis, as found in the previous guidelines, is eliminated and goes directly to the criteria for septic shock. Septic shock is defined as the continuation of sepsis with circulatory and cellular/metabolic malfunctions and is associated with high mortality risk.3 Furthermore, to establish the diagnosis of sepsis and septic shock, an assessment was carried out using SOFA (Sequential Organ Failure Assessment) scores. As for the criteria for septic shock, if sepsis is obtained, which requires vasopressor therapy to increase MAP ≥ 65 mmHg and increase lactate levels > 2 mmol/L (18 mg/dL), although adequate fluid resuscitation has been carried out. This change in the definition of sepsis has consequences for animal models for sepsis. Thus far, sepsis model animals still use inflammatory criteria to establish a diagnosis of sepsis.3
Previous studies used LPS injection to create animal models of sepsis. Giving LPS also causes MODS besides sepsis. LPS is the main component of the outer membrane of gram-negative bacteria. Gram-negative bacteria can cause 30-80% of sepsis. Sukamto et al. (2018) conducted a research using LPS injection at a dose of 0.25 mg/kgBW intraperitoneally to create a sepsis model mouse.4 Meanwhile, other study used larger LPS doses of up to 2 mg/kgBB, 5 mg/kgBB, and 10 mg/kgBB.5
In this study, we will make a sepsis model animal seen from the requirements of inflammation and organ damage as evidenced by histopathological features of the kidneys and liver. This study aimed to find the optimal lipopolysaccharides (LPS) dose to make a sepsis model in mice.
This experimental study with a posttest-only control group design was conducted on mice with septic AKI. The study’s sample size was calculated using an Institutional Animal Care and Use Committee formula. Inclusion criteria were Male mice, BALB/c strain, aged 8–10 weeks, body weight (BW) 26–27.5 g, no physical disability, and normal activity. Mice that died during the treatment period was the exclusion criteria.6
The study protocol has received ethical approval from the Health Research Ethics Committee of Moewardi Hospital (approved number: 7686/VIII/HREC/2021).
The study subjects were male mice subspecies of Mus musculus strain Balb/C aged 3–4 months, body weight 20–30 g, obtained from the Faculty of Veterinary Medicine, Gajah Mada University. This experiment used 30 mice, which were randomized and divided into four groups. The sampling method used in this study was purposive sampling. The control group (3 mice) injected with 0.5 cc NaCl 0.9% i.p. Group A (9 mice) was injected with LPS 0.125 mg/kg B.W. i.p. administered on the first and second days, group B (9 mice) was injected with LPS 0.15 mg/kg B.W. i.p. administered on the first and second days, and group C (9 mice) was injected with LPS 0.3 mg/kg B.W. single dose i.p. On the third, fourth, and fifth days, the termination of each group of three mice and examination of the NF-κB, tumor necrosis factor-α (TNF-α), C-reactive protein (CRP), alanine aminotransferase (ALT), the expression of NF-κB in the liver and kidneys, and histopathology in the liver and kidneys were conducted.
Induction of sepsis using LPS from Escherichia coli 0111:B4-purified by phenol extraction production Sigma-Aldrich USA, Product Number: L2630. NF-κB examination used the enzyme-linked immunosorbent assay (ELISA) method with mouse NF-κB (Nuclear factor kappa B) ELISA kit reagent (Product Number: NFκB p65 (F-6):sc-8008) from Santa Cruz Biotech. Alanine aminotransferase (ALT) examination using reagents from DiaSys response ®920 with optimized UV-test method according to International Federation of Clinical Chemistry and Laboratory Medicine. Assessment of TNF-α via the ELISA method using mouse TNF-α ELISA kit reagent (BZ-087661F-AM) from Bioenzy. CRP examination via the ELISA method using mouse CRP ELISA kit reagent (A19003) from Abclonal.Inc.
Mice were kept in four cages made of plastic tubs covered with wire at the top. The conditions during acclimatization and treatment were controlled in a fixed environmental range, with a room temperature ranging from 23-26°C with the aim that the test animals could adapt according to the animal’s biological time and the conditions to be occupied during the experiment. Temperature, water supply, the number of mice in the cage, and the change of husks were all done the same for all groups of mice. Adaptation to mice with care in cages with a size of 28 × 30 × 12 cm so that they can move freely and not be stressed. After all the research processes were completed, the experimental animals were terminated by anesthetizing them with inhalation of chloroform and then decapitation was carried out.
Interventions of BALB/c mice, the examination of NF-κB, TNF-α, ALT, and CRP, histopathological preparations and immunohistochemistry examinations on the liver and kidneys were conducted at the Experimental Animal Care Center (PAU UGM, Yogyakarta), Histology and Cell Biology Laboratory (Faculty of Medicine, UGM, Yogyakarta), and Anatomical Pathology and Histology Laboratory (Faculty of Medicine, UNS Surakarta), respectively. The authors were unaware of the allocation group so that all the mice were handled, monitored and treated in the same way while conducting the experiment.
The kidneys and livers of all mice were embedded using paraffin and sliced at 0.5 mm thickness. The slides were incubated with 1:1,200 antimouse NF-κB antibodies (from Santa Cruz Biotech) overnight. After washing with buffer solution, the slides were incubated with an IgG Rabbit Probe HRP secondary antibody (from Biocare Medical) for 30–45 min. The slides were washed with buffer solution and stained with DAB solution for 10 min to remove an excessive amount of the secondary antibody. Finally, the slides were counterstained with hematoxylin solution for several minutes. The stained slides were observed under a light microscope (Model: Olympus CX21, manufacturer: Ningbo Huasheng Precision Technology Co.Ltd) 400× magnification, and positive staining of NF-κB was scored 0% = 0, <25% = 1, 26%–50% = 2, 51%–75% = 3, and >75% = 4.7
Once the mice’s kidneys and liver had been embedded using paraffin, they were cut into 0.5 πm thickness and stained with hematoxylin–eosin. Degeneration, necrosis, inflammation, and bleeding were assessed under a light microscope (Model: Olympus CX21, manufacturer: Ningbo Huasheng Precision Technology Co. Ltd) with 40 and 100 times magnification. Two independent pathologists carried out the histopathological examination. The results of renal histopathology were graded into 0 = none, 1 = mild, 2 = moderate, 3 = strong, and 4 = severe.8 The results of liver histopathological were graded to 0 = histological features of liver cells in the normal centrilobular region, 1 = the histological features of liver cells undergoing necrosis is limited in the centrilobular region (necrosis is limited to zone III of the liver), 2 = the histological features of liver cells undergoing an expansion of necrosis covers the centrilobular region 22 until it reaches zone II in the liver, and 3 = the histological picture of liver cells undergoing an expansion of necrosis covers the centrilobular region to the middle of the trial portal area (necrosis reaches zone I in the liver).9
The statistical analysis used was IBM® SPSS® Statistics 25 for windows. The data of NF-κB, TNF-α, CRP, and ALT were presented as mean ± S.D., whereas the data of NF-κB and matrix metalloproteinase (MMP)-9 were presented as percentages of positive staining cells. Numerical data were assessed for normality and homogeneity using Shapiro–Wilk and Levene’s tests, respectively. Comparative analysis of TNF-α among groups was analyzed statistically using a one-way analysis of variance, followed by the least significant difference (LSD) post hoc test at a significance level of p < 0.05. Meanwhile, categorical data of NF-κB and numerical data that did not pass the normality and homogeneity test (NF-κB, TNF-α, CRP, and ALT) were analyzed statistically using the Kruskal–Wallis test followed by the Mann–Whitney test.
E-coli LPS administration induced the septic AKI, leading to increased NF-κB levels. Figure 2 shows that levels of NF-κB increased in all mice groups treated with LPS. The results of the Kruskal–Wallis NF-κB test showed statistically significant differences (p < 0.001). Statistical analysis continued with the post hoc test with the Mann–Whitney test to determine which groups had statistical differences.
The results of the Mann–Whitney Test showed significant differences between the control group and all treatment groups (p = 0.05). NF-κB levels increased in the entire treatment group starting on day 3. NF-κB levels increased the most in group B (average day 3 729.57 ng/mL, day 4 735.49 ng/mL, and day 5 742.86 ng/mL). Mann–Whitney test results showed no difference between the treatment groups (p > 0.05). Based on these results, giving a dose of LPS 0.3 mg/kg B.W. in divided doses (group B) can increase the highest NF-κB levels when compared with the other groups.
High levels of NF-κB stimulated TNF-α secretion, as shown in Figure 3. TNF-α levels increased statistically significantly (p < 0.01) in comparison with the control group. The results of the LSD test showed that there were significant differences between the control group and all treatment groups (p < 0.05). TNF-α levels increased in the entire treatment group starting on day 3. TNF-α levels increased the highest on day 5, which can be seen in groups B (16.99 ± 28 pg/mL) and group C (16.74 ± 30 pg/mL). The statistical analysis results in the two groups showed no statistical difference (p = 0.44). On the third day of comparison between groups A and B, there was a statistically significant difference (p = 0.001), this was also found in the comparison between groups A and C (p = 0.000), but there was no significant difference in groups B and C (p = 0.918). Based on these results, giving a dose of LPS 0.3 mg/kg B.W. in a divided dose can provide the highest increase in TNF-α levels in comparison with the other groups.
An increase in TNF-α levels was followed by an increase in CRP levels, as shown in Figure 4. The results of statistical analysis using the Kruskal–Wallis test showed a significant difference with a p-value of <0.01.
The results of the Mann–Whitney Test showed significant differences between the control group and all treatment groups (p = 0.05). CRP levels increased in the entire treatment group starting on day 3. CRP levels increased the most in group B4 (18.22 ± 0.17 ng/mL). The results of the Mann–Whitney test showed no difference between the treatment groups (p > 0.05). The average in group B (17.88 ± 0.61 ng/mL) was greater than group C (16.73 ± 0.17 ng/mL). Between groups B3, B4, B5, C3, C4, and C5, there was no significant difference (p > 0.05). Based on these results, giving a dose of LPS 0.3 mg/kg B.W. in divided doses (B) can provide the highest increase in CRP levels in comparison with the other groups.
Increased levels of NF-κB, TNF-α, and CRP indicate the activation of the inflammatory cascade. In this study, one of the markers of inflammation at the tissue level was marked by increased expression of NF-κB in the liver and kidneys. NF-κB expression in the liver obtained a statistically significant difference (p = 0.004).
Figure 5.1 shows that NF-κB expression on the liver significantly differed between the control group and all treatment groups with p-values of <0.05. The NF-κB expression on the liver began to increase on day 3 in all groups. The highest increase was in group C. Statistical analysis in group C with group A showed a statistically significant difference (p <0.05). NF-κB expression in group B did not have significant differences. Based on these results, giving an LPS dose of 0.3 mg/kg B.W. in single doses can provide the highest increase in NF-κB expression in other groups. Figure 5.2 shows an immunohistochemistry examination of NF-κB expression on the cytoplasm of liver tissue hepatocyte cells (yellow arrows).
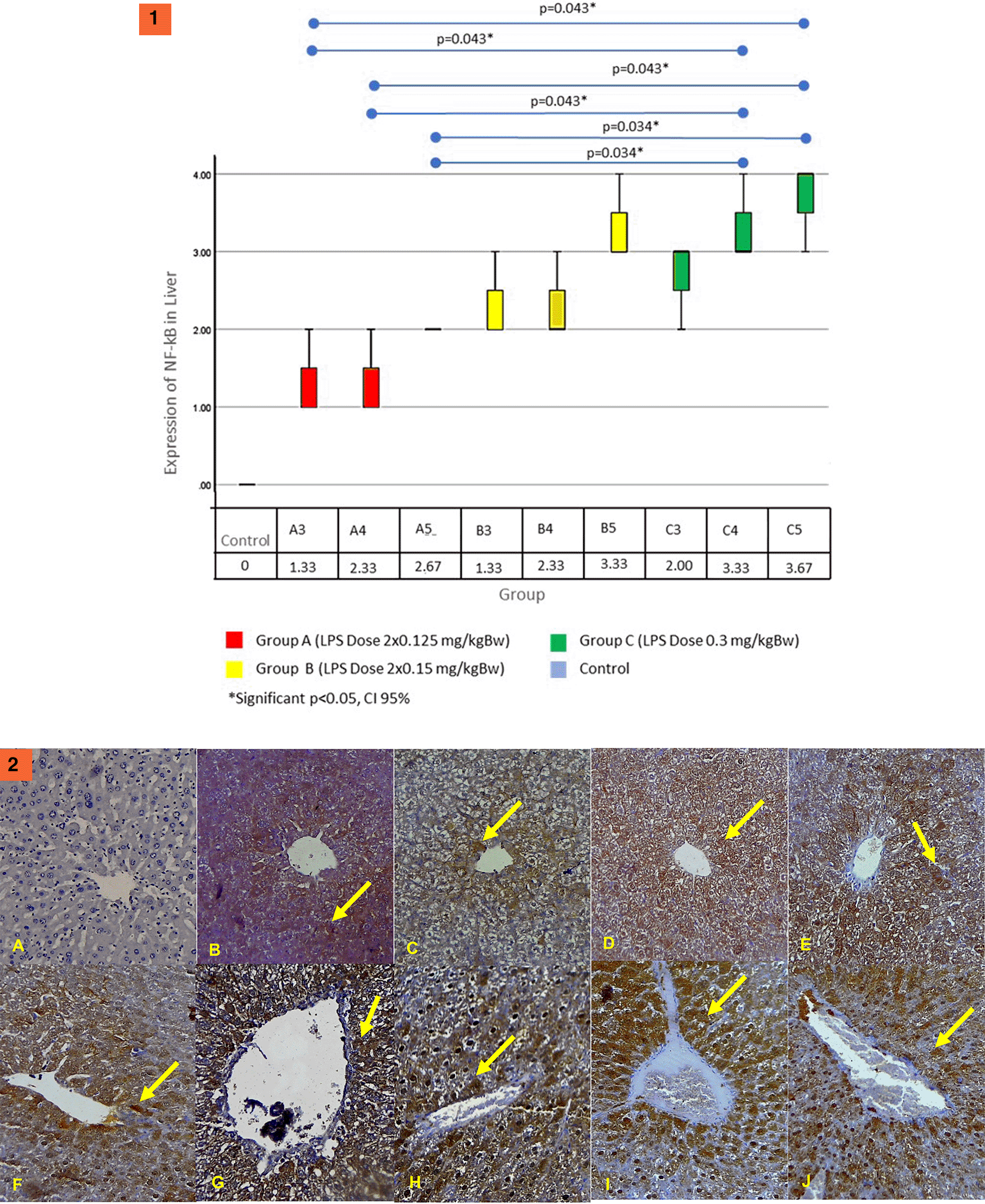
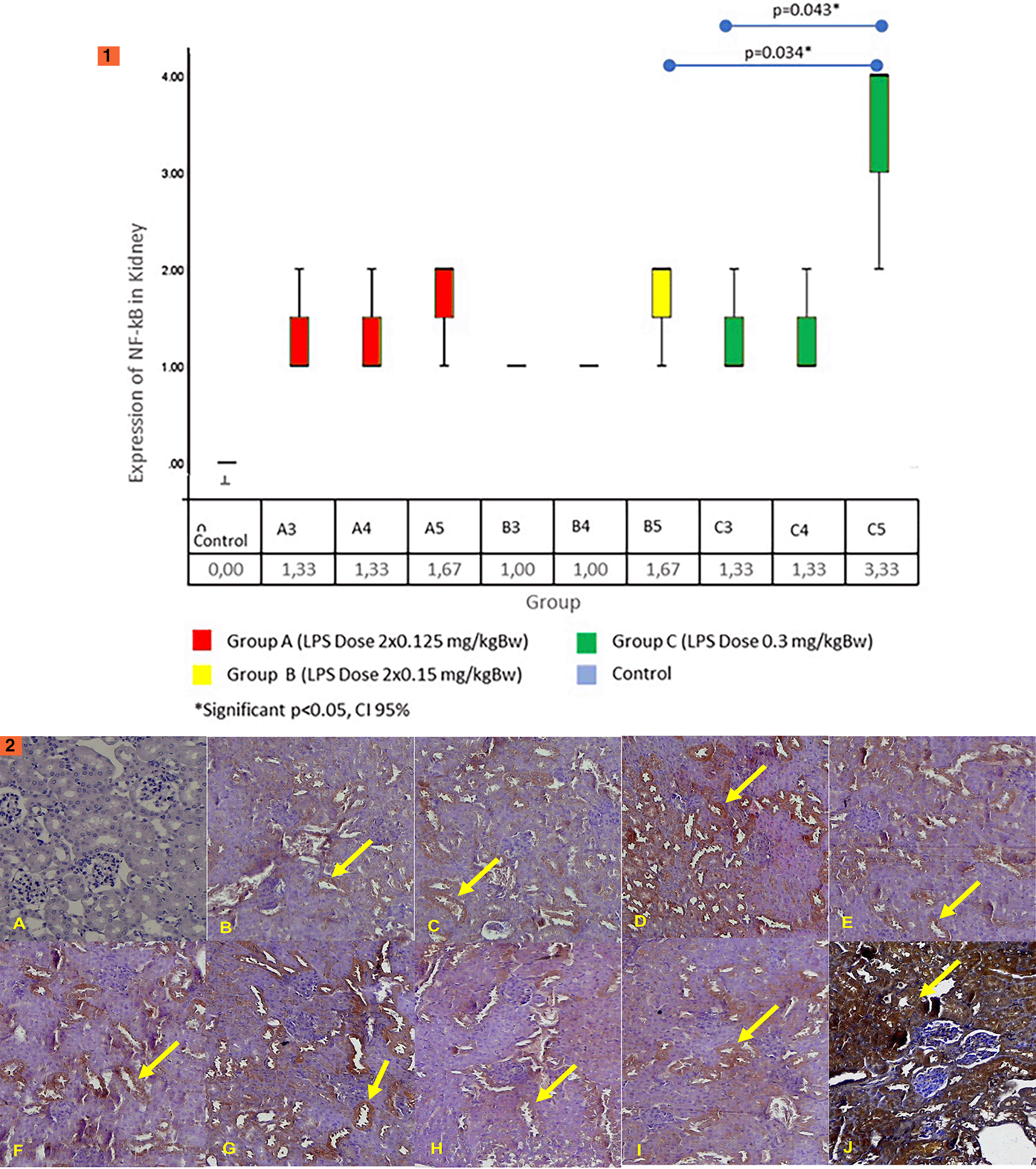
The results of the Kruskal–Wallis test on the renal NF-κB expression obtained a statistically significant difference (p = 0.026). Figure 6.1 shows the results of the renal NF-κB expression. The results of the Mann–Whitney test showed significant differences between the control group and all treatment groups (p < 0.05). In all groups, the renal expression of NF-κB begins to increase on the third day. The highest increase was in group C5. Group C5 had significant differences with group B3 (p = 0.034) and B4 (p = 0.034). However, it has no significant differences between groups A (all day) and B5. These results show that LPS dose administration of 0.3 mg/kg B.W. in single doses (group C) can increase the highest NF-κB expression in comparison with the other groups. In Figure 6.2 we can see an immunohistochemistry examination showing NF-κB expression on the cytoplasm of epithelial cells of proximal tubules of the renal tissue (yellow arrows).
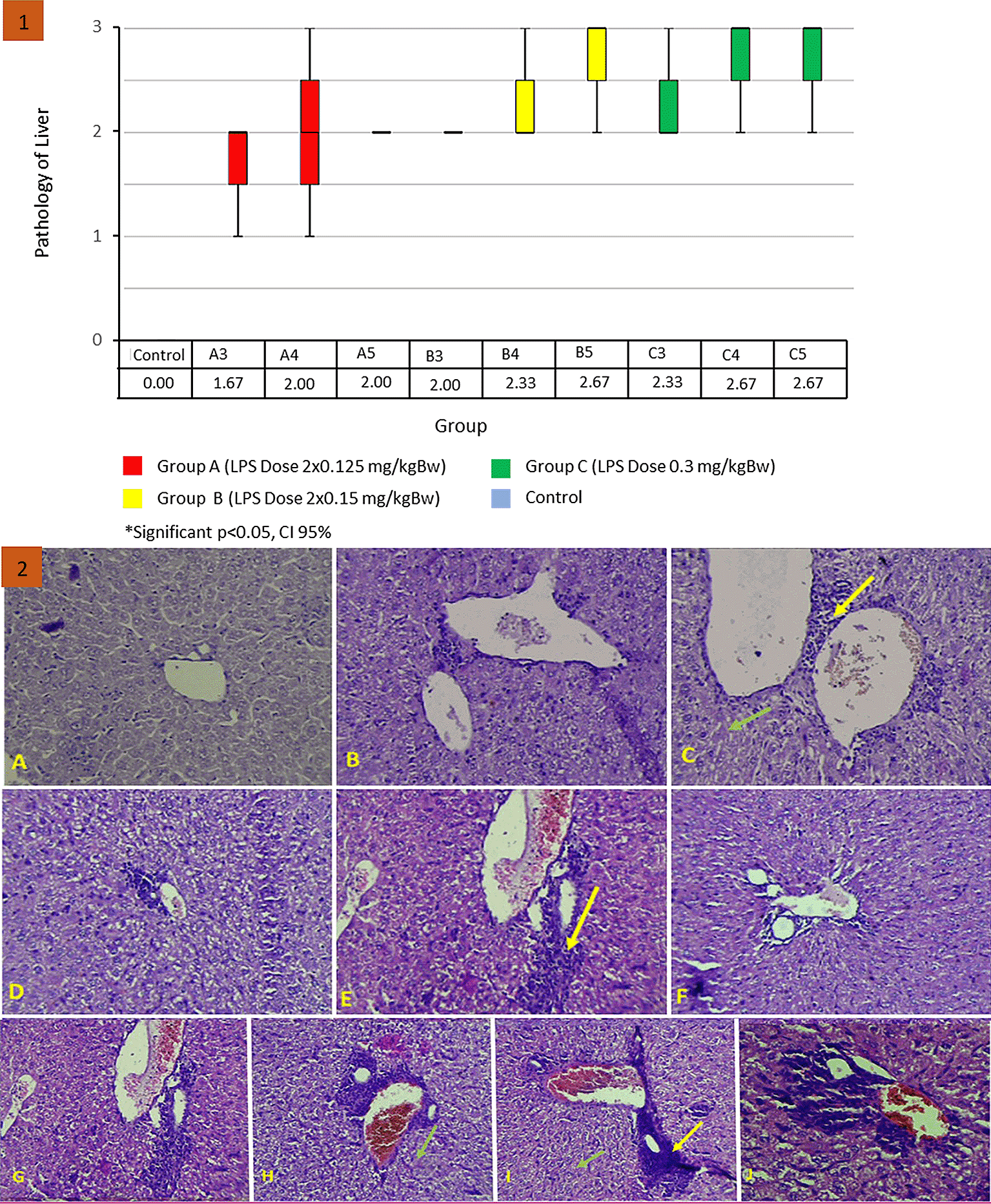
Uncontrolled inflammation due to LPS induction can result in tissue and cell damage. The results of the Kruskal–Wallis test on the liver histopathological score found no statistically significant difference (p = 0.061). The results of the renal histopathology score can be seen in Figure 7.1 . Although there was no statistically significant difference, the treatment group had a greater mean rank than the control group. The largest mean rank was found in group C with LPS levels of 0.3 mg/kg B.W. Liver histopathology scores began to increase on day 3 after LPS injection in all treatment groups. Figure 7.2 shows the histopathological depiction of hepar, showing inflammatory images (yellow arrows) and necrosis (green arrows) in hepar tissue. A. Normal control group score 0. B. Group A3 score 2. C. Treatment group A4 score 2. D. Treatment group A5 score 2. E. Treatment group B3 score 2. F. Treatment group B4 score 2. G. Treatment group B5 score 3. H. Treatment group C3 score 3. I. Treatment group C4 score 3. J. Treatment group C5 score 3.
Renal histopathology scores increased in all treatment groups. The increase started on day 3 and peaked on day 5 after the LPS injection. The Kruskal–Wallis test on the renal histopathology score obtained a significant difference statistically (p = 0.001). The results of the renal histopathology score can be seen in Figure 8.1.
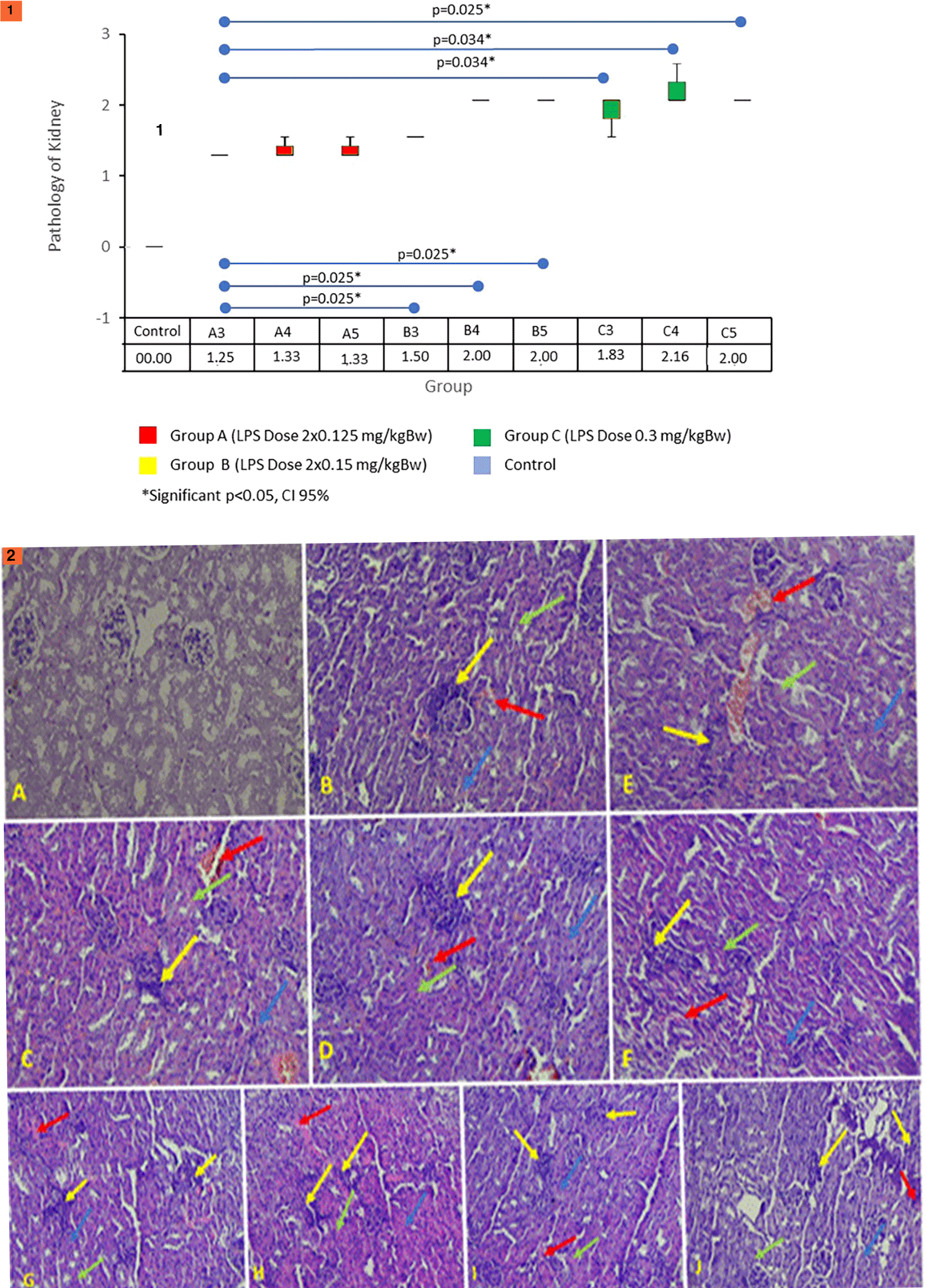
The test between the control and all treatment groups has a significant difference (p < 0.05). Statistically, group A3 has significant differences when compared with groups B and C (p < 0.05). Group B4 had no significant difference when compared with groups B5, C3, C4, and C5 (p > 0.05). Group C3 had significant differences when compared with group A3 (p = 0.034) but did not differ significantly from groups B3, B4, B5, C4, and C5 (p > 0.05). Based on these results, it can be concluded that starting from day 3, there has been kidney damage, with the most severe damage in group C (LPS dose 0.3 mg/kg B.W.). Figure 8.2 shows histopathological features of the kidneys showing inflammatory markers (yellow arrows), necrosis (green arrows), hemorrhage (red arrows), and degeneration (blue arrows) in renal tissue. A. Normal control group score 0. B. Group A3 score 1.25. C. Treatment group A4 score 1.25. D. Treatment group A5 score 1.25. E. Treatment group B3 score 1.5. F. Treatment group B4 score 2. G. Treatment group B5 score 2. H. Treatment group C3 score 2. I. Treatment group C4 score 2. J. Treatment group C5 score 2.
One of the earliest indicators of liver damage increase, when liver damage occurs, is ALT levels. In this study, ALT levels increased in all treatment groups. This increase significantly differs from the control group (p = 0.002). Figure 9 shows the results of ALT levels.
The results of the Mann–Whitney test showed a significant difference between the control group and all treatment groups (p = 0.05). ALT levels increased in the entire treatment group starting on day 3. ALT levels increased the most in group B5 (38.84 ± 0. 48 U/L). The results of the Mann–Whitney test showed no difference between the treatment groups (p > 0.05). The average in group C (36.79 ± 0.73 U/L) was greater than that in group B (36.46 ± 1.11 U/L) between groups B3, B4, B5, C3, C4, and C5; there was significant difference (p > 0.05). Based on these results, administering a dose of LPS 0.3 mg/kg B.W. in a single dose (C) can provide the highest increase in ALT levels when compared with the other groups.
NF-κB, CRP, ALT, and TNF-α levels in all groups increased on day 3 after treatment. There were significant differences when compared with the control group. NF-κB and CRP levels increased the most in the B group. Histopathological features of the kidneys and liver and the results of immunohistochemistry examinations on the liver and kidneys showed the highest increase in C group.
The host’s response to the invasion of pathogens can cause excessive inflammation, inhibit the immune system, and disappear the homeostasis of the immune system. In the early phases of sepsis, the innate immune system mediated by pattern recognition receptors (PRRs) will interact with pathogen-associated molecular patterns (PAMPs) that activate NF-κB and translocate into the nucleus. Ultimately, such stimulation increases the secretion of proinflammatory cytokines, including TNFα, interleukin-1β (IL-1β), IL-6, IL-12, and IL-18 involved in the pathogenesis of sepsis.10,11 In this study, at the beginning of sepsis, there was an increase in inflammatory markers of NF-κB in plasma and expression in the liver and kidneys. In this study, an assessment of NF-κB was carried out not only in plasma but also in the target organs, namely, the liver and kidneys. This is because all acute inflammatory conditions anywhere will also increase NF-κB in plasma. Research conducted by Liu et al. showed an increase in NF-κB expression in renal tubular cells in the mice in the LPS-induced sepsis mouse model.12 This is also in line with research by Ren et al., which proves that in mouse kidneys induced with LPS, there is a higher level of phosphorylation of NF-κB and IκB.13 LPS will bind to toll-like receptor 4, which is PRR found in kidney cells, resulting in the activation of several signaling pathways, one of which is NF-κB. Activation of the signaling pathway increases the transcription of proinflammatory cytokines, such as TNF-α, IL-6, and IL-1β, which then bind to several transmembrane receptors and strengthens the inflammatory response that occurs. Excessive inflammation can impact the renal parenchyma directly and cause tubule cell apoptosis.13
In this study, higher NF-kB expression in the sepsis mouse model follows a study conducted by Li et al., which showed a higher NF-kB expression in the liver of the mice in the sepsis mouse model.14 Another study by Li et al. also showed that in the liver of the mice in a sepsis mouse model, an increase in phosphorylated NF-kB was obtained.15 Kupfer cells will respond to the presence of LPS by secreting proinflammatory cytokines, such as IL-6, IL-8, and TNF-α and other proinflammatory mediators, such as IL-1β, IL-12, reactive oxygen species, and nitric oxide, which together cause damage to endothelial cells and hepatocytes. Additionally, neutrophils were recruited to the liver due to the release of TNF-α and leukotriene B4, which inflicted further damage to hepatocytes. Liver sinusoid endothelial cells also respond to the presence of LPS by releasing endothelin-1, which activates proinflammatory cytokine transcription factors, such as NF-κB.16
The inflammatory markers assessed in this study were CRP and TNF-α. In this study, CRP and TNF-α in plasma also increased. This is similar to a study by Li et al. in 2018, which showed an increase in CRP and TNF-α levels in a sepsis mouse model.17 Research on patients with sepsis conducted by Patel et al. also showed similar results, namely, higher levels of TNF-α in patients with sepsis than in healthy controls.18 The liver response to LPS is the secretion of a wide variety of proinflammatory cytokines by Kupfer cells, mainly IL-6 and IL-18. The secretion of IL-6 causes the synthesis of acute-phase proteins, one of which is CRP.
Meanwhile, IL-8 plays a role in hepatocyte apoptosis and an increase in TNF-α concentrations.16 Inflammatory markers began to increase on day three after intraperitoneal LPS administration. LPS doses of 0.3 mg/kg B.W. single dose gave the highest results compared with other groups. This study follows the criteria for early sepsis that has been adhered to, which is 3 days.19 In this study, inflammatory markers also experience the highest increase in the group given an LPS dose of 0.3 mg/kg B.W. at a single dose. This is in accordance with research conducted by Hijma et al., which shows that the higher the amount of LPS given, the higher the inflammatory markers.20
The assessed organs damaged due to sepsis were the brain, lungs, heart, liver, and kidneys; coagulation function was also assessed. In this study, evidence of organ damage due to LPS administration was carried out in the liver and kidneys. The results of the ALT examination in plasma increased significantly starting day 3 with the administration of a single dose of LPS 0.3 mg/kg, which had the highest value when compared with the other groups. Organ damage in the liver and kidneys was also evaluated as histopathological. The most severe liver and kidney damage occurred in administering a single dose of LPS 0.3 mg/kg. Kidney damage experienced by the sepsis mouse model in this study follows research conducted by Huang et al. and Sun et al., which proved that there was an increase in tubule damage scores in the kidneys of sepsis mice.21,22 Sepsis can interfere with the functioning of endothelial cells through inhibition of endothelial regeneration, induce leakage of fluids and cells, cause inadequate perfusion of organs, cause local ischemic and tubular injury, and even pose a risk of organ failure and shock.23 The bond between inflammatory mediators, such as damage-associated molecular patterns and PAMPs, and membrane receptors in renal tubule epithelial cells will increase oxidative stress and mitochondrial damage.
Meanwhile, the coagulation cascade and signaling of the activated autonomic nervous system will also lead to capillary occlusion by leukocytes and platelets and endothelial damage accompanied by vasodilation and endothelial leakage. This will result in peritubular edema and a decrease in oxygen supply to the renal tubules.24 Additionally, the expression of MMPs that degrade extracellular matrix proteins has also increased, such as MMP-9. This protein is involved in the degradation of type IV collagen, a major component of the kidneys’ basal membrane, for remodeling the basal membrane during embryonic growth.25 Endothelial cells in the kidneys also undergo necrosis that will produce neutrophil gelatinase-associated lipocalin (NGAL) as a form of self-defense and biomarker of growth and differentiation of kidney tubular cells so that changes in NGAL levels can be used to predict kidney injury due to bacterial infections and sepsis.26–28
In this study, an increase in ALT characterized liver damage in the sepsis mouse model. The degree of histopathological damage was supported by research by Zhang et al., which proved that in the LPS-induced mice in the sepsis mouse model, there was an increase in ALT serum and an increase in pathology scores in liver histopathological features.29 Other studies by Dai et al. also showed that in the sepsis mouse model, there was hepatocyte damage characterized by a vague nucleus picture and vacuole degeneration, and an increased ALT serum was obtained.30 In clinical settings, increased ALT describes acute damage to liver cells.17 Besides necrosis in kidney cells, sepsis causes inflammation of hepatocytes through the proinflammatory cytokine IL-6. It will stimulate the synthesis of acute-phase proteins such as CRP, antitrypsin α-1, fibrinogen, prothrombin, and haptoglobin. The increased concentration of such acute phase proteins can lead to inhibition of the protein pathway C. Thus, the disorder is responsible for the increased activity of coagulation factors.16 LPS will also stimulate Kupfer cells to secrete TNF-α, IL-1β, IL-12, and IL-18, where Il-18 plays a significant role in the process of liver damage induced by LPS. Interleukin-18 will cause interferon-γ secretion responsible for hepatocyte apoptosis, increased TNF-α concentrations, and increased CD14 expression. CD14 is a monocyte/macrophage surface receptor that plays a role in the binding of the lipopolysaccharide-binding protein complex.16
This study observed differential NF-KB expression levels in the liver and kidney between group B and group C, with higher expression observed in group B. However, it was found that group C exhibited the most severe histopathological damage. The occurrence of this condition can be attributed to the administration of the highest dose to group 3. When the highest dose is administered at once, it results in more pronounced organ damage compared to the same dose administered in divided doses at separate intervals. The implementation of separate administration has the potential to enhance inflammation, albeit with reduced magnitude of damage. Furthermore, there exists the possibility that organ or tissue damage can be repaired during the interval of administration.
The change of sepsis definition, which was once caused by inflammation due to infection, became organ dysfunction caused by the dysregulated host response to infection, making an impact on making experimental sepsis animal model. This study advantage is to make a sepsis animal model based on both definitions, either inflammation parameter (CRP, NFKB, TNF-α) and organ damage (NGAL, ALT, and Histopathology) caused by LPS induction. In addition, this study previewed the onset of sepsis occurs from time to time.
The disadvantage of this study is that it is only done in male animals, while sepsis can occur in both males and females. This study also didn’t assess the onset when the organ damage caused the death. This study only measured until the fifth day after the induction of LPS. At the end of this study, there were no mice who died after the induction of LPS.
The optimal dose of LPS to make a sepsis mouse model was 0.3 mg/kgB.W with the most severe target organ damage dan significant increased of inflammatory markers.
Figshare: Arifin (2022): degree of organ damage and inflammatory markers in sepsis mice models data and histopathology.xlsx. figshare. Dataset. https://doi.org/10.6084/m9.figshare.21710438.v1.31
This project contains the following underlying data:
Figshare: ARRIVE Guidelines for ‘Degree of organ damage and inflammatory markers in sepsis mice models inducted by various doses of lipopolysaccharides’, https://doi.org/10.6084/m9.figshare.21710618.v1.32
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
We acknowledge research assistants, assistant laboratories in histology, and biomedical laboratories of the Faculty of Medicine of Sebelas Maret University and the Research and Community Service Institute of Sebelas Maret University.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Basic and Applied immunology, Sepsis, Stem cells
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neuroscience
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Basic and Applied immunology, Sepsis, Stem cells
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 12 Dec 23 |
read | |
|
Version 1 03 Jan 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)