Keywords
Rattus norvegicus, chronic kidney disease, osteoporosis, histopathology, unilateral ureteral obstruction method
Rattus norvegicus, chronic kidney disease, osteoporosis, histopathology, unilateral ureteral obstruction method
Chronic kidney disease (CKD) is a disease that is widely known throughout the world. Its prevalence continues to increase every year. About 1 in 10 of the global population has CKD at some stage. The prevalence of CKD stages 1 to 5 is 13.4% of the global population, of which 10.6% are in stages 3 to 5 (Hill et al., 2016; Elias et al., 2018). In Indonesia, the number of new patients and active patients with end-stage CKD continues to increase from year to year, and the number of patients undergoing hemodialysis increases from year to year. In 2016, there were 25,446 new patients and 52,835 active patients with end-stage CKD. These numbers continued to increase until 2018, with the number of new patients increasing to 66,433 and the number of active patients reaching 132,142 patients (Indonesia Renal Registry, 2018).
One of the most frequent complications due to the progression of CKD is the occurrence of disturbances in mineral metabolism and bone pathology. Metabolism disorders in bones are caused by chronic inflammatory processes and increased oxidative stress, which causes increased bone destruction, as well as disturbances in the process of vitamin D metabolism in damaged kidneys (Babayev and Nickolas, 2015; Cafiero et al., 2018; Damasiewicz and Nickolas, 2018). This can result in hypersecretion of parathyroid hormone (PTH) by the parathyroid glands, which is known as secondary hyperparathyroidism. Excessive PTH circulating in the blood will cause hypersecretion of the receptor activator of nuclear factor kappa-Β ligand (RANKL) from osteoblasts, causing the increased osteoclastogenesis process (Friedl and Zitt, 2017). Chronic inflammatory processes are associated with increased rates of bone remodeling and osteoclastogenesis through stimulation of PTH excretion, production of proinflammatory cytokines (tumor necrosis factor-α/TNF-α), expression of the RANKL, and production of reactive oxygen species (ROS) (Callaway and Jiang, 2015; Iwasaki et al., 2017; Kazama, 2017; Neale Weitzmann and Pacifici, 2017; Walsh et al., 2018; Mosbah, 2019). The increased bone remodeling process, where the rate of bone resorption exceeds the rate of bone formation, will result in clinical conditions of osteopenia, which can progressively lead to osteoporosis (Hruska et al., 2017). Disturbances in the quantity and quality of bone lead to an increased risk of fracture in patients with CKD, which increases with CKD-stage progression (McNerny and Nickolas, 2017; Evenepoel et al., 2019; Asadipooya et al., 2021). The risk of fracture of the femur is known to be high in patients on hemodialysis in various countries, where the risk is increased four to 13 times compared with the general population (Kazama, 2017).
Bone biopsy and histopathological examination are still the gold standards for assessing bone quality in patients with CKD and are a consideration in administering therapy, based on the analysis of the speed of bone formation and its mineralization characteristics (Damasiewicz and Nickolas, 2018; McNerny and Nickolas, 2017; Mosbah, 2019). The increase in bone remodeling does increase the volume of bone trabeculae, but the new trabecular bone formed will be irregular and have less connective tissue, which affects its strength. Examination with histopathology generally only assesses bone trabeculation, while secondary hyperparathyroid conditions can also cause thinning of the bone cortex (Drüeke and Massy, 2016).
Albino rats of the Wistar strain (Rattus norvegicus) are one of the most well-known and easy-to-obtain laboratory test animals. The rat kidney has one papilla and one calyx that opens directly into the ureter. The nephrons are also superficially located for easy access microscopically (Lossi et al., 2016). Unilateral ureteral obstruction (UUO) is a model that is widely used to study obstructive nephropathy. This method is often performed using rodents and is most commonly used to study acute kidney injury (AKI) and CKD because it is believed to mimic the process of chronic obstructive nephropathy in humans. This model has advantages in reproducibility, where there is little variation between animals, a shorter manufacture time, and a relatively easy surgical technique. The intervention of acute obstruction will result in the formation of the AKI model, and persistent obstruction for 1–2 weeks will provide a histopathological picture of CKD kidneys (Ucero et al., 2014; Martínez-Klimova et al., 2019).
The development of CKD conditions until the emergence of pathological conditions in the bones is an important thing to know, related to the choice of therapy given. A previous study analyzing bone histopathology in a Sprague Dawley rat model that was given adenine for 4 weeks to induce CKD and a high-phosphorus diet for 38 weeks showed changes in trabeculation by loose and thin-walled bones, which characterizes osteoporosis (Ni et al., 2018). Another study using the Institute of Cancer Research (ICR) model of CKD mice induced by the UUO method for 7 days showed a decrease in trabecular bone mass in the trochanteric part of the mice femur, indicating sensitivity to intervention using the UUO method (Gu et al., 2013). Data regarding the onset progression of bone pathological conditions in experimental animal models with CKD, particularly in Wistar rats induced by the UUO method, are still limited. Therefore researchers are interested in obtaining scientific evidence, which can be used as the basis for further research with the same test animal models. In this study, we monitor the duration until the histopathological appearance of osteoporosis is discovered in Wistar strain rats with the UUO model of CKD.
This research was a descriptive observational study, with a prospective cohort approach. Treatment and observation of the rat model were carried out at the Laboratory of the Center for Food and Nutrition Studies, Gadjah Mada University's Inter-University Center. Preparation of femoral bone histopathology samples was carried out at the Histology and Cell Biology Laboratory, Faculty of Medicine, Gadjah Mada University, while femoral bone histopathological samples were read at the Pathology Anatomy Laboratory and Histology Laboratory, Faculty of Medicine, Sebelas Maret University, Surakarta. All experimental procedures involving animals were carried out in keeping with guidelines from the National Institutes of Health Guide for the Care and Use of Laboratory Animals to ameliorate any suffering of animals (Tan, 2004). Expected and unexpected adverse events were recorded to identify deficiencies in procedures or study design.
The population for this study was albino rats of the Wistar strain (Rattus norvegicus). A total of 13 male white rats were used in this study. The rats were aged 3–4 months, and weighed between 150 and 300 grams. Adaptation of the rats was carried out for 7 days, with treatment in standard cages, so they could move freely and not be stressed. Before the intervention, the experimental animals were kept for a week for acclimatization at a temperature of 21–23°C with controlled humidity (50±5%) in a 12-hour artificial light cycle (08:00 h to 20:00 h) to help them to adapt to the same conditions as their various origins. All rats were located individually in polycarbonate cages (0.90×0.60×0.60 m). Every animal model was fed with a standard pellet, and water was provided ad libitum with the husk replaced every 3 days. All animal models were routinely inspected and observed regarding their food consumption and fecal characteristics.
The Rattus novergicus rat model was chosen because it has been widely used in previous studies so genetic data are easy to obtain and the model can provide research results with a high level of validity. In addition, the bone physiology of rats resembles that of humans even though bone growth is twice as fast. There is also a genetic similarity as the lamellar bone architecture of rats resembles humans, and they have a relatively short life with a fast bone turnover rate, making it easier to carry out multigenerational research. Care and maintenance are relatively inexpensive, and rats have the ability to adapt to a laboratory environment. Rats are also considered more suitable than other four-legged animal species to study morphology of the bone.
After being anesthetized by administering ketamine (Dexa Medica, Tangerang) 35 mg/kg body weight (BW) and xylazine (Inter Chemie, Holland) 5 mg/kg BW intramuscularly, the animals were then subjected to the experimental animal model of CKD in this study used the UUO method by ligating the left ureter. Within 24 hours, this model can reduce the blood flow rate in the kidney and the glomerular filtration rate (GFR). The next response was interstitial inflammation (2–3 days peak), tubular dilatation, tubular atrophy, and fibrosis which occurs starting at 7 days and after 10 days shows oxidative activity and chronic inflammatory processes (Hai-Chun et al., 2010).
The research was divided into three stages. The first stage lasted for 10 days, forming the rats into a CKD model through left ureteral ligation. The CKD rat models were then randomized and put into four containers, each containing three rats. In the second stage, each group of rats was given the same treatment for a predetermined duration (7th day, 14th day, 21st day, and 28th day after CKD modeling). Then, in the third stage, the research was completed, and data were collected from the sample. Male white rats from each group were sacrificed according to the time specified in each group, and femur bone tissue was taken for histopathological examination. General observations for signs of pain or suffering in the animal were conducted daily as needed. The moribund condition was used as a humane endpoint (Tan, 2004).
After fixing the femoral bone tissue for 72 hours, the tissue samples were incubated in a 6% nitric acid solution for decalcification for a week. The solution was added back daily and the decalcification was assessed with a needle in the last few days. After, the tissue was decalcified, rehydrated in alcohol, embedded in paraffin wax, and sectioned buccolingually with a microtome. The obtained sections were stained with hematoxylin and eosin, and photos taken using a light microscope with a camera attachment (Arabacı et al., 2015; Kızıldağ et al., 2020).
Histopathological readings were performed by a pathologist. Analysis of the research data was carried out qualitatively by comparing the histopathological picture of the femoral bone tissue in each test group.
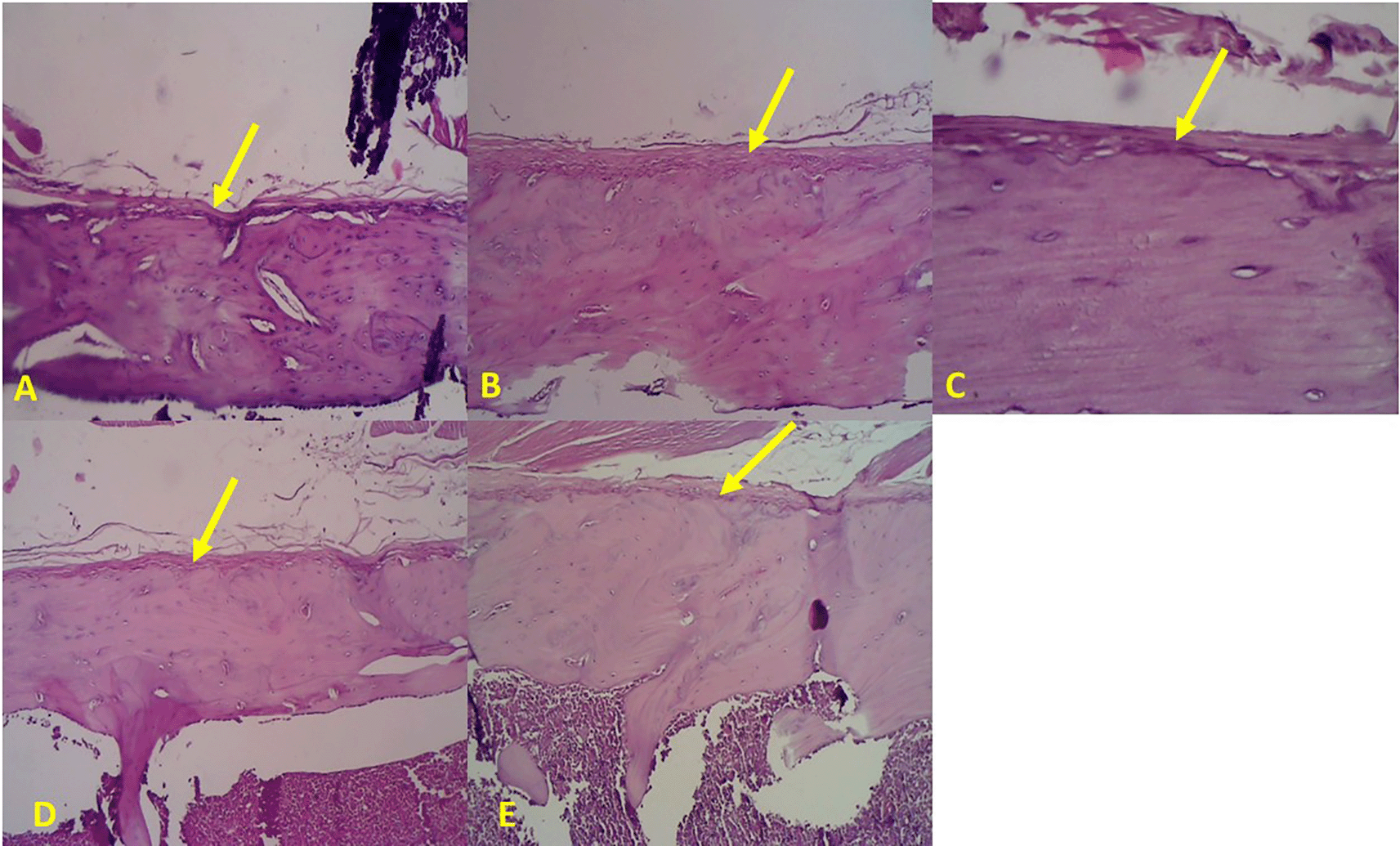
Bone histopathology showed an increase in the thickness of the outer fibrous layer of the periosteum in the metaphyseal area of the bone (yellow arrows) in line with the duration of induction: A. Control; B. 7th day after CKD modeling; C. 14th day after CKD modeling; D. 21st day after CKD modeling; E 28th day after CKD modeling (Figure 1).

Bone histopathology showed an increase in the thickness of the outer fibrous layer of the periosteum in the diaphyseal area of the bone (yellow arrows) in line with the duration of induction: A. Control; B. 7th day after CKD modeling; C. 14th day after CKD modeling; D. 21st day after CKD modeling; E 28th day after CKD modeling (Figure 2).
Bone histopathology showed an increase in the gap between the trabecular bones in the metaphyseal area (yellow arrows) in line with the duration of induction: A. Control; B. 7th day after CKD modeling; C. 14th day after CKD modeling; D. 21st day after CKD modeling; E 28th day after CKD modeling (Figure 3).
One of the most frequent complications due to the progression of CKD is the occurrence of disturbances in mineral metabolism and bone pathology. Chronic inflammatory processes are associated with increased rates of bone remodeling and osteoclastogenesis. Increased bone remodeling, where the rate of bone resorption exceeds the rate of bone formation, will result in clinical conditions of osteopenia, which can progressively lead to osteoporosis (Hruska et al., 2017).
Osteoporosis is a disorder of bone metabolism, in which the balance between bone formation and resorption shifts, causing the rate of bone resorption to exceed the rate of bone formation, resulting in decreased total bone mass, thinning of trabecular and cortical tissue, and bone fragility (Fan et al., 2010). Cortical bone thickness, which is an important factor determining the flexural strength of bone, is determined by the processes of bone formation and resorption in both periosteal and endosteal tissues (Fan et al., 2010).
In the normal mouse model (control), femoral bone tissue can generally be divided into two main bone architecture components, which are periosteum and endosteum (trabeculae). The periosteum is a layer of dense connective tissue that covers the outer surface of bones. The periosteum has an important role in the bone remodeling process. Osteoclasts and resorption pit cells are more abundant in fibrous tissue in the periosteum in osteoporosis than in normal conditions (Fan et al., 2010).
Wistar strain male albino rats (Rattus norvegicus) whose left ureter has been ligated produce a rat model with CKD. Within 24 hours, this model can reduce the blood flow rate in the kidney and the GFR. The next response is interstitial inflammation (2–3 days peak), tubular dilatation, tubular atrophy, and fibrosis, which occurs starting at 7 days and after 10 days will show oxidative activity and chronic inflammatory processes (Hai-Chun et al., 2010). In this study, rats inducted with UUO were treated with standard feed and drink for 10 days to form a CKD model. The chronic inflammatory process then leads to an increase in the speed of bone remodeling and osteoclastogenesis through stimulation of PTH excretion, production of proinflammatory cytokines (TNF-α), expression of the RANKL, and production of ROS.
Based on a comparison of the sample readings for each rat in each test group, on the 7th day after CKD modeling, thickening of the periosteal fibrous tissue was seen, but not yet accompanied by an increased gap of the trabecular tissue. On the 14th and 21st day after CKD modeling, there was an increase in the thickness of the periosteal fibrous tissue, both in the metaphyseal and diaphyseal areas. This thickening was in line with the length of treatment time, where periosteal fibrous tissue was thicker and more cells were found on the 21st day after CKD modeling. In addition, on the 21st day after CKD modeling, an increase in the amount of space between the trabecular networks began to be found.
On the 28th day after CKD modeling, the histopathological picture of the femoral bone tissue showed the most severe osteoporosis appearance. This was characterized by thinning of the trabecular bone tissue and the most distant gap between the trabeculae, indicating a characteristic of osteoporosis. The periosteal fibrous tissue was also the thickest, indicating an increased number of osteogenic cells and resorption pits.
The findings in this study follow other studies that have been done previously, where it was found that osteoporosis rat models had lower trabecular bone volume, thickness, number, and connective tissue density, and a more distant gap between trabecular bones when compared with normal mice (Fan et al., 2010). In the same study, it was also found that, in the periosteal tissue in the metaphysis, the osteoporosis mice model had a thicker and more abundant cellular layer compared with normal mice. Although there was no significant difference in the thickness of the fibrous layer, the number of cells in the fibrous layer of osteoporotic rats in the metaphysis area was greater than that of normal mice (Fan et al., 2010).
A previous study analyzing bone histopathology in a Sprague Dawley rat model that was given adenine for 4 weeks to induce CKD and a high-phosphorus diet for 38 weeks showed changes in trabeculation of loose and thin-walled bones, which characterizes osteoporosis (Ni et al., 2018). Another study using the ICR model of CKD mice induced by the UUO method for 7 days showed a decrease in trabecular bone mass in the trochanteric part of the mouse femur (Gu et al., 2013). The current study using male white rats of the Wistar strain (Rattus norvegicus) induced by the UUO method to produce a CKD model showed that the most distant gap between trabecular bone tissue, demonstrating the osteoporosis process, was on the 28th day after CKD modeling. The onset difference between the findings of this study and previous studies may be influenced by several factors, including the type of test animals and the CKD induction method. The limitation of this study is qualitative comparative analysis only using the histopathological method.
Based on the research that has been done, the histopathological onset of osteoporosis in the Wistar strain rat model of CKD appears most clearly on the 28th day after CKD modeling. This was characterized by thinning of the trabecular bone tissue and the most distant spaces between trabeculae.
This study has been approved by Universitas Sebelas Maret Ethical Committee (Number: 882/IX/HREC/2021).
- Zenodo. Histological Dataset [Data set]. https://doi.org/10.5281/zenodo.7397288 (Ermawan et al., 2022a).
This project contains the following underlying data:
Histological picture and definition of histological results
- Zenodo. ARRIVE author checklist [Data set]. https://doi.org/10.5281/zenodo.7472000 (Ermawan et al., 2022b).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We gratefully thank the Orthopedic and Traumatology Institution of Universitas Sebelas Maret for its help and support.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)