Keywords
Interventional Nephrology, Nephrology
This article is included in the Health Services gateway.
Interventional Nephrology, Nephrology
Diagnostic and Interventional Nephrology (DIN) forms the core of a unified care service for patients with kidney disease. It is vital in providing rapid diagnoses and treatment for patients presenting to renal units. Diagnostic and interventional procedures are often undertaken exclusively by radiologists, vascular surgeons, or transplant surgeons in the UK. This can lead to fragmented service provision for renal patients, prolonging procedural waiting times, and has resulted in inconsistent and often inadequate exposure to procedural training for renal trainees in this field.
The development of DIN has been ongoing for the past three decades (Chan, 2013, O’Neill, 2000). We set out to change the regional institutional environment and establish a comprehensive interventional nephrology service at the South Tyneside and Sunderland Foundation Trust (STSFT) renal unit to serve as the test case for other units in the UK. In this paper, we present our service and training model, and data from the first full year of this service.
In the UK, access-related complications account for around 20% of nephrology bed days. Suboptimal vascular access has mortality and morbidity implications; patients who dialyse via a tunnelled dialysis catheter (TDC) have a seven times higher hospital admission rate with sepsis, and a four times higher mortality rate than patients receiving dialysis via an arteriovenous fistula (AVF) or graft (AVG) (Poinen et al., 2019, Pyart et al., 2020). Unfortunately, catheter-related bacteraemia tends to generate deep-seated infections, such as discitis and endocarditis, which require long hospital admissions and amount to a significant financial burden on the health service. Time spent without functional access puts the patient at risk of hyperkalaemia and pulmonary oedema, frequently necessitates hospital admission, and may require additional invasive procedures such as central venous catheter insertion and emergency dialysis (Niyyar and Chan, 2013).
Suitable vascular access sites are anatomically limited and, even with optimal care, are lost with increasing time spent on dialysis. Ultimately, patients have a higher mortality due to the inability to receive dialysis because of a lack of vascular access. Inevitably, where a service has suboptimal access to endovascular intervention, more patients are exposed to the risks and complications of dialysis via a TDC.
U.S. and Canadian observational literature demonstrate that dialysis patients undergo an average of 1-2 access-related procedures per year. In centres with an access surveillance programme, early intervention reduces the rate of vascular access thrombosis and ensures that dysfunctional access longevity is curtailed, while access without dysfunction is maintained (Salman et al., 2020, Robbin et al., 2018).
Vascular access planning, assessment, and intervention is complex and critically important for patients with end-stage renal disease. The nephrologist is in the optimal position to be able to assess the risks, benefits, and cardiovascular impact of each dialysis modality and access type. Balancing these factors with the expected longevity of access, life expectancy, and patient priorities enables the nephrologist to facilitate a shared decision-making process, delivering a truly patient-centred model of care utilising a multi-disciplinary team approach (Vachharajani et al., 2011).
Historically, nephrology has been a procedural specialty. Senior UK nephrologists were typically trained in peritoneal dialysis (PD) catheter insertion and AVF formation surgery and had experience in maintaining patency of AV shunts (a historic form of vascular access). It remains the case that nephrologists are well-positioned to undergo further training to competently perform the procedures required by our patients and emulate the successes of cardiologists and gastroenterologists in developing a subspecialist, interventional clinical field (Kalloo et al., 2016).
The current UK renal curriculum only mandates the ability to establish temporary vascular access for dialysis. However, most renal trainees have some experience in performing renal biopsies and tunnelled dialysis catheters. Some trainees also have access to non-surgically inserted PD catheter training, however the access to such training remains variable.
Internationally, DIN training framework has been established by several societies, including the American Society of Diagnostic and Interventional Nephrology (ASDIN), the Association of Vascular Access and InTerventionAl Renal physicians (AVATAR Foundation, India), and the Vascular Access Society of Britain and Ireland (VASBI), to name a few.
To further develop a rigorous pathway for DIN training in the UK, we have developed a training pyramid that details the proposed stages of progression — this is outlined in Figure 1. The trainees rotating through our unit are trained to stage 1, with those wishing to pursue further training progressing to stage 2 and 3.

This pyramid details the various stages of training in D&IN. Temporary vascular catheter insertion is the only mandatory skill in the UK training curriculum (below dotted line). Stages 1, 2 and 3 are separated by solid lines and have the associated skills detailed in the figure.
The renal unit at our hospital provides dialysis to 300 patients living in our catchment area in three dialysis centres. The renal ward is a tertiary referral ward, receiving patients from these areas for the diagnosis and management of acute kidney injury and other acute renal diseases. All access-related assessment and procedures are performed in Sunderland Royal Hospital.
Renal biopsies and tunnelled/temporary dialysis access procedures were previously performed after patients were admitted to the renal ward. This led to the recurrent cancellation of procedures due to non-availability of inpatient beds; a service audit previously demonstrated a 62% cancellation rate for renal biopsies due to bed unavailability. AVF and AVG formation procedures and most PD catheter insertions were performed by surgical teams.
The aim for DIN at our renal unit is a high-quality service that is responsive to patients’ needs and complements the interventional radiology and vascular surgery departments. We have developed a day-case service, providing all the work already undertaken by the nephrology team, whilst expanding our remit to also include work that has previously been performed by these other departments — this model is outlined in Figure 2. The skillset of four nephrologists and two senior nurses at STSFT meant that we were uniquely positioned in the UK to rapidly develop a sustainable multi-professional service.
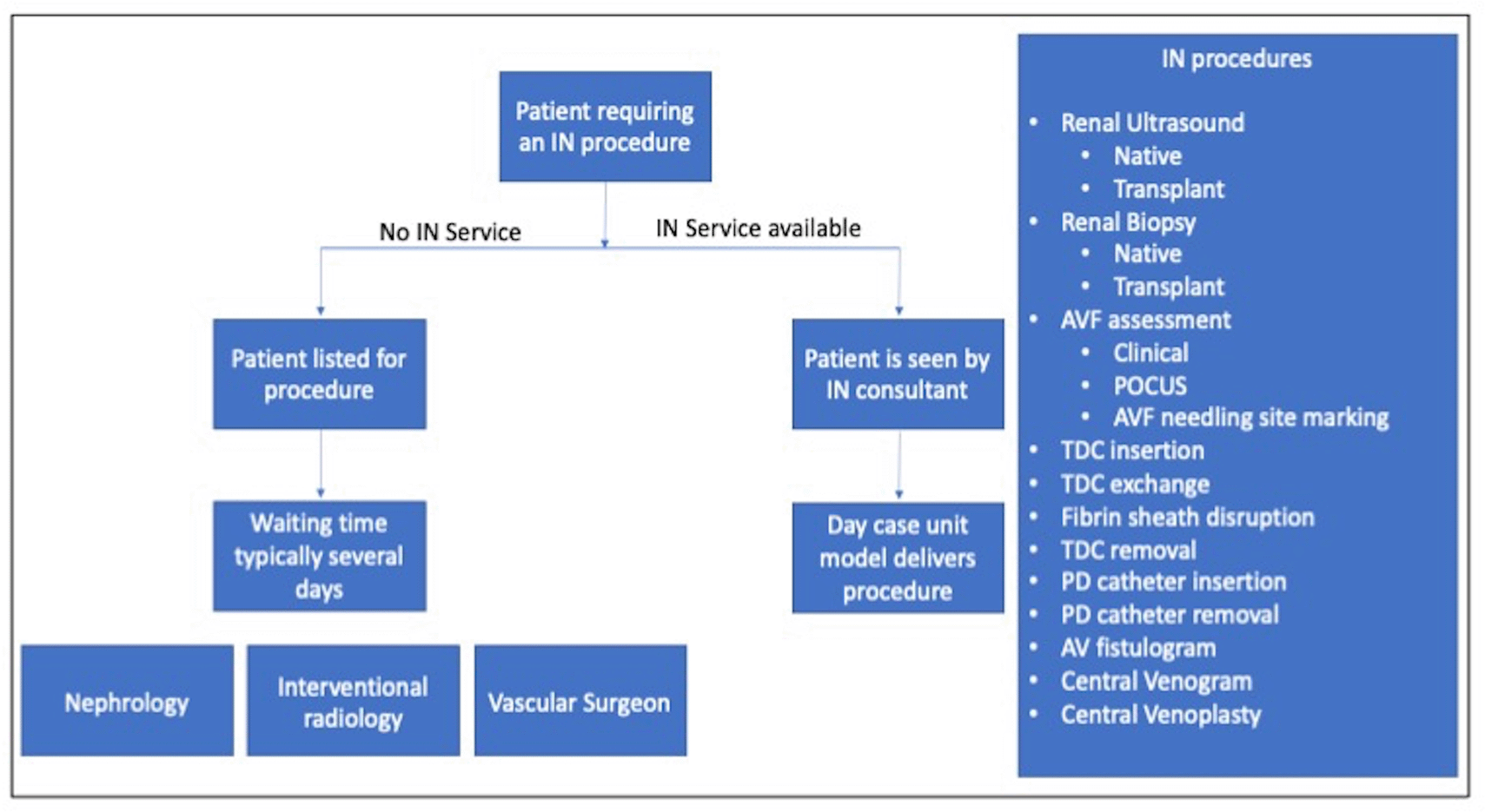
Currently, nephrologists have three fluoroscopy lists per week, with capacity to perform up to nine tunnelled haemodialysis or peritoneal dialysis catheter procedures. Nephrologists place almost all PD catheters, with surgeons only doing so in patients with coexistent surgical issues (such as the presence of abdominal hernias). Two days per week are dedicated to non-fluoroscopy work such as renal biopsies, AVF scans and Point of Care Ultrasound (POCUS). These lists operate on a day-case basis with pre-procedural assessment and post-procedural recovery being performed in the day-case IN unit.
Prior to the development of our dedicated DIN unit, a small proportion of vascular ultrasound was also performed by nephrologists, with the majority being performed by the radiology department. In 2017-2018, 280 renal access scans were performed by a vascular ultrasonographer. This includes vein mapping (performed before AVF/AVG surgery), fistula maturation scans, and diagnostic scans in the event of symptoms or dialysis complications, such as poor flow rates or needling difficulty.
However, the previously established ‘conventional’ pathway for patients with symptomatic or dysfunctional vascular access was inefficient. Patients typically underwent two stages of assessment: an outpatient vascular ultrasound (wait time two-four weeks), and then fistulography ± fistula angioplasty, performed by an interventional radiologist. Scans also needed to be reported by a radiologist prior to a referral for intervention being made, further lengthening the process. On this conventional pathway, patients with potentially dysfunctional vascular access were subject to waiting times of up to several weeks before a diagnostic scan and radiological intervention were performed. This is a significant time during which stenoses and thromboses can progress, and vascular access may be lost altogether, necessitating the establishment of alternative, temporary access with its associated risks.
Since the implementation of the IN model, most ultrasound scans are now performed by our four interventional nephrologists at the point of care. On this lean pathway, outlined in Figure 3, patients in whom issues have been identified during dialysis sessions can be referred directly from the dialysis unit to the IN department for a same-day scan. Equally, patients can be referred for fistula maturation scans, and assessments of those which have become symptomatic. Patients with positive sonographic findings, such as the presence of a stenosis, are then referred on the same day for intervention. In the case of stenosis, this takes the form of fistulography ± angioplasty, procedures which are also increasingly being performed by interventional nephrologists within the unit.
Since the mid-1990s, Interventional Nephrology has been a recognised subspecialty in the US, and latterly in Canada. Two models exist: a hospital-based service, and a free-standing vascular access clinic. The development of the subspecialty was in response to precisely the same challenges that we experience in the UK (Niyyar and Work, 2009).
Whereas historically a domain of interventional radiology, 40-45% of dialysis angioplasty and thrombectomy procedures performed in the US are now undertaken by Interventional Nephrologists. Unless mandated by co-existing systemic pathology, all procedures are performed on a day-case basis. Patients have access to procedures within 24 hours of referral.
Since the development of Interventional Nephrology, US vascular access-related bed days fell from 1.8 to 0.3 days/patient/year and missed dialysis treatments fell from 0.8 to 0.2 treatments/patient/year (Mishler et al., 2006).
Interventional nephrology has been demonstrated to be safe: a series of 14,000 patients had the highest success rate and lowest complication rate in the medical literature, and a series of 6000 patients also had safe levels of radiation exposure (Beathard, 2015).
The service provision at our unit was planned after a review of the parameters measured by ongoing, prospective performance measures collected by our institution’s performance audit tool. We submit our data annually, on haemodialysis access, peritoneal dialysis access, and AVF/AVG use to the national UK Renal Registry. The UK Renal Registry is a national registry that receives performance data from all UK renal units.
Patient outcomes were evaluated for an array of diagnostic and therapeutic interventional procedures performed at the IN unit, SDIN (the Sunderland Diagnostic and Intervention Unit), within the first year of its establishment. The data collection was registered with the South Tyneside and Sunderland clinical effectiveness team with the following reference code: CA9545. This was a retrospective collection of data and did not involve diversion from standard care as a result no ethical approval was required. This was confirmed by the research department at the South Tyneside and Sunderland NHS Foundation Trust. Data was retrospectively collected for the period beginning 1st October 2019 to 1st October 2020 for patients who underwent the following procedures: ultrasound guided renal biopsy, TDC insertion and exchange, PD catheter insertion/exchange, and AVF POCUS. These figures were compared to the cohort from the one-year period pre-SDIN, between the 1st of October 2018 and the 31st September 2019. All results are expressed as mean and percentages unless otherwise specified. There were no p value calculations as retrospective observational nature of the study meant that data collection was not powered to calculate significance.
We evaluated the incidence of the following post-procedure complications before and after Sunderland Diagnostic and Interventional Nephrology (SDIN) service was established: pain, peri-nephric haematoma, macroscopic haematuria, bleeding requiring transfusion, bleeding requiring surgical or radiological intervention, and death. These were chosen to enable comparison with a large systematic review and meta-analysis involving 118,000 native kidney biopsies published by Poggio et al. in 2020 (Poggio et al., 2020). We also evaluated biopsy waiting times and biopsy specimen quality. Biopsy specimens with 10 or more glomeruli were deemed adequate, as per the Banff criteria (Roufosse et al., 2018).
104 day-case renal biopsies were performed in the first year of the SDIN service (75 native, 29 transplant) with 18 of those considered urgent (rapidly progressive kidney disease). This was fewer than in the pre-SDIN period, during which 144 biopsies were performed (106 native, 38 transplant), 54 of which were urgent. However, the median waiting time from referral to non-urgent biopsy fell from 12 working days in the pre-SDIN period to seven working days after the implementation of SDIN. The median waiting time for urgent biopsies also fell from three working days to just one working day.
Our data also demonstrates that the overall quality of biopsy specimens improved since the establishment of SDIN, with specimens comprising a greater number of glomeruli on average, and a higher proportion of specimens being adequate and histopathologically diagnostic. This data is outlined in Table 1.
Table 1 lists the post-biopsy complication rates identified at SDIN, and compares these to the pre-SDIN period and those published by Poggio et al. This data demonstrates a higher proportion of patients reporting post-biopsy pain and macroscopic haematuria in the SDIN cohort (11.5% and 13.5% respectively) compared to pre-SDIN (7% and 9.1%) and the published data (4.3% and 11%), an observation which may be partially explained by more intensive patient monitoring and improved documentation implemented during SDIN. However, ongoing prospective data collection is being performed at our unit to ensure these complications are minimised.
Conversely, we report a lesser incidence of post-procedure haematomas in SDIN (3.9%) compared to Poggio et al. (11%). Reassuringly, we also report similar incidences at SDIN (difference of <1%) of the three most serious post-biopsy complications — bleeding requiring blood transfusion, bleeding requiring surgical/radiological and intervention, and death from biopsy. In both of our cohorts, there was one patient who required arterial embolization to stem bleeding. There were no deaths within either of our cohorts, and none of the patients required nephrectomy.
We evaluated the 90-day procedural complication rates from TDC insertion and exchange at our unit, as well as the 30 and 90-day catheter-associated infection (CAI) rates and procedural waiting times. TDC removal reasons at one-year post-insertion were also evaluated for each group. CAI was defined as either a positive catheter line culture, or a positive peripheral blood culture with no other identified source of infection. Catheter-associated sepsis was defined as CAI resulting in sepsis (infection plus systemic inflammatory response syndrome). This data is displayed in Table 1.
99 tunnelled dialysis catheter insertion and exchange procedures were undertaken in the first year of SDIN, as compared to 132 in the pre-SDIN period, with a median waiting time of three days for both cohorts. Catheters inserted during the SDIN period remained in place for a mean duration of 156 days, as compared with 101 days in the pre-SDIN cohort, with no procedural failures within SDIN.
90-day complication rates remained broadly similar for both cohorts, with 66.7% of TDCs having no complications in the SDIN cohort. The most common non-infective complication among both cohorts was minor bleeding during insertion (13.1% SDIN, 14.4% pre-SDIN), all of which were managed successfully during the procedure. 5.1% of catheters developed primary failure within 90 days in the SDIN cohort and required replacement. There were no patients in either cohort who experienced major bleeding and no deaths due to the procedure.
The one-year TDC removal reasons are also outlined in Table 1. 13.1% of TDCs remained in place after one year in the SDIN cohort, a decrease from 26.5% pre-SDIN. The two most common reasons for removal were infection (21.2%) and definitive alternative access gained (20.1%). We note positively that the proportion of patients whose TDC was removed due to successful renal transplantation increased from 0.8% pre-SDIN to 3% at SDIN, and that 9.5% more patients were offered alternative access modalities, such as AVF/AVG, in line with best practice.
Finally, our 30 and 90-day CAI rates are reported. Within SDIN, 15.2% of patients developed a CAI within 90 days, of which 6.1% developed within 30 days after insertion. 9.1% of patients went on to develop line sepsis within 90 days, however all were successfully treated with antimicrobial therapy, and no patient deaths resulted from this. Further data comparing the pre-SDIN and SDIN cohorts is outlined in Table 1. Infection frequency was also standardised and expressed as number of infections per 1000 catheter days — both cohorts had an incidence of 2 infections per 1000 catheter days within the 90-day observation periods. Published data on CAI rates varies widely between units, but generally ranges between 0.2 and 6.5 infections per 1,000 catheter days for TDCs (Winnicki et al., 2018); our unit therefore reports similar infection rates to those published.
A total of 16 fluoroscopically guided PD catheters were inserted by interventional nephrologists during SDIN, as compared with 12 catheters in the pre-SDIN period.
Catheters remained in place for an average of 153 days. Complication rates were evaluated over a 28-day period between both cohorts. Due to the small sample size, further detailed analysis of outcomes was not undertaken. Overall, a general reduction in complication rates was observed since SDIN was established. 11 (69%) catheters had no complications within the 28-day observation period, 3 (19%) catheters required manipulation by an interventional nephrologist following insertion to reposition in the pelvis due to poor function, and two (12%) catheters required removal; one catheter due to non-function and the other due to intra-abdominal infection. Further comparisons between both cohorts can be found in Table 1.
Figure 3 details the differences between the previously outlined conventional and novel diagnostic imaging pathways for ultrasound assessment of dysfunctional AVFs within our unit. We evaluated the difference in patient waiting times at each stage on both pathways, along with the overall waiting time from imaging being indicated to intervention taking place. We also evaluated the quality of the POCUS scans performed by nephrologists at the unit by comparing the findings from these scans to findings at fistulography — the gold standard imaging modality.
279 AVF POCUS scans were performed during the SDIN period. The waiting time from referral to scan was reduced from a mean of 35 days on the conventional pathway to 2 days on the novel pathway. The mean waiting time from scan to intervention was also reduced from 21 days to 14.8 days. Therefore in total, the time between diagnostic imaging being indicated and intervention occurring was reduced from an average of 56 days on the conventional pathway to 16.8 days after implementation of SDIN, an overall reduction of 39.2 days. When compared to fistulographic findings, the overall sensitivity of the POCUS scans for detecting fistula pathology was 98.4%, with a specificity of 95.8%.
In this paper we report the establishment of a novel diagnostic and interventional nephrology service at a large teaching hospital in the UK. This is a unique service with no similar provision currently available anywhere else in the country. We outline our service model and a selection of patient outcomes after the first full year of this service.
Diagnostic and interventional nephrology is a continually evolving subspecialty of nephrology and remains an exciting area of development. It provides fast and safe access to procedures associated with establishing and maintaining dialysis provision. A day-case model also reduces the reliance on inpatient beds, thereby improving time to access services.
Our data suggests that the delivery of a day-case DIN service is both safe and effective in reducing waiting time for crucial diagnostic and interventional procedures for renal patients. Although fewer renal biopsies and TDC procedures were performed at SDIN, this observation is likely related to the significant impact and service disruption that resulted from the emergence of the COVID-19 pandemic. Though, none of the procedure lists in the SDIN unit were cancelled due to the pandemic. The reduction in numbers is more likely to be reflective of the reduction in patients coming for regular follow up due to the pandemic. Despite this, waiting times for TDCs remained constant, while those for renal biopsies were globally improved. Most encouragingly, we demonstrate that implementing a novel diagnostic POCUS imaging pathway for dysfunctional AVFs resulted in significantly shorter times to diagnosis and intervention, thereby facilitating the preservation of dialysis access. Reassuringly, our data supports the conclusion that the implementation of a dedicated DIN service remains safe, with our unit reporting broadly similar complication rates to those reported pre-SDIN and in the wider medical literature.
DIN reduces the reliance on other specialties, thereby streamlining care for patients. This model also creates wider training opportunities for renal trainees that were hitherto not consistently accessible (Selvaskandan et al., 2022). Data from a recent study in South and Southeast Asia highlighted training as a major barrier for nephrologists performing these procedures (Ramachandran et al., 2021). Training initiatives by the International Society of Nephrology (ISN) play a key part in sharing the learning and experience between centres across the world. Lopez et al. recently published their work in establishing a modular approach to training in DIN with the help of the ISN in Nicaragua (Lopez et al., 2021), and this represents an important example of the value of collaboration in progressing training in this emerging field. Vachharajani et al., have discussed successful models of DIN training in academic medical centres across the USA. This paper discusses a successful collaborative approach between private practice, academic centres and other interventional specialties such as cardiology (Vachharajani et al., 2010).
In line with the vision of the ISN for developing training in IN, we hosted our first ISN funded fellow in IN from Pakistan. The fellow has since successfully completed his training and returned to Pakistan to establish an interventional nephrology programme there. We aim to continue building our collaborations and have recently started the development of a sister unit at the Kamanga Medics Hospital in Mwanza, Tanzania.
Research and innovation in DIN remain underfunded and underperformed. The development of IN as a subspecialty interest with robust curricula, well defined training pathways and post graduate training should lend itself to further research in DIN (Roy-Chaudhury et al., 2012). We have lead the development of DIN training in the UK utilizing online webinars at international conferences, and have recently done hands on simulation training in IN at the recently concluded UK Kidney Week conference. We are in the process of launching a post graduate certificate course in association with Newcastle University. The ambition is to develop it as a master’s programme that would include research in DIN as an integral part of training.
We are acutely aware of the limitations of this data with its small sample size and observational nature. However, we have demonstrated unequivocally that implementing a dedicated DIN unit within our hospital has reduced procedural waiting times, enhanced independence from other interventional specialties, and facilitated the maintenance of definitive vascular dialysis access. We believe that this service serves as a springboard for future innovation and research in developing new service models, improving training, and fostering novel research in interventional nephrology in the UK.
Open Science Framework: Outcomes from the first dedicated diagnostic and interventional nephrology (IN) service in a UK renal unit, https://doi.org/10.17605/OSF.IO/DY2AN (Srivastava, 2023).
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Moura-Neto JA, Ronco C: Finerenone and Chronic Kidney Disease Outcomes in Type 2 Diabetes.N Engl J Med. 2021; 384 (11): e42 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Vascular access
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 23 Jun 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)