Keywords
Fusobacterium necrophorum, Bacteroides thetaiotaomicron, Lemierre's Syndrome
Fusobacterium necrophorum, Bacteroides thetaiotaomicron, Lemierre's Syndrome
Fusobacterium infections have been described in the gastrointestinal, pulmonary, neurologic, musculoskeletal, soft tissue, and genitourinary systems.1–7 This case highlights a rare variant of a rare disease. However, it highlights a clinically important principle that variants of Lemierre’s syndrome exist and should be considered in the setting of Fusobacterium bacteremia. When Fusobacterium bacteremia is identified, investigation of venous patency local to the primary infection site is warranted. The foundation for the discovery of Lemierre’s syndrome was laid when Fusobacterium, a gram-negative rod-shaped anaerobe, was discovered in 1887 by Friedrich Loeffler.8 Bang and Schmorl further classified the bacteria in animal studies.9,10 Subsequently, Andre Lemierre discovered Fusobacterium in humans as described in his landmark dissertation on anaerobes in 1936.11 This set the stage for the discovery of a rare disease now which carries the eponym Lemierre’s syndrome. Lemierre’s syndrome is characterized by septic thrombophlebitis of the IJV due to an oropharyngeal primary infection with Fusobacterium in an otherwise young and healthy adult.11 Patients with Lemierre’s syndrome also present with fever, sore throat, exudative tonsillitis, and unilateral neck swelling and tenderness.3 Treatment generally includes anaerobic coverage with an antibiotic resistant to beta-lactamase.10 Anticoagulation for IJV thrombosis is controversial. Variants of Lemierre’s syndrome are rare and occur when the primary Fusobacterium infection develops in locations other than the oropharyngeal cavity.12 They are also characterized by septic thrombophlebitis local to the primary infection. Fusobacterium bacteremia is rare with an incidence of 0.99 in 100,000.12 For decades Fusobacteriae have been thought to be part of the normal oropharyngeal and appendiceal flora; however, a recent study published in 2007 suggests it is pathogenic.13 The appendix is the next most common location of a Fusobacterium infection.
A 38 year old healthy Hispanic male construction worker presented to the emergency department of Shelby Baptist Medical Center (SBMC) with one week of constant, sharp, right-sided flank pain, three days of fever and chills, and one day of a severe headache. He reported no chronic medical conditions, current medications, or supplements. He endorsed taking an unknown blood thinner for 60 days two years prior to his presentation for an unspecified reason. He had an appendectomy 10 months prior, recent travel to South America six months prior, and worked temporarily in a paper mill for multiple months within the past year. He denied allergies, tobacco use, alcohol use, or illicit drugs. He endorsed a balanced diet and regular exercise. The patient had two recent hospitalizations at other facilities for similar symptoms without resolution. The details of these hospitalizations were not accessible.
While at SBMC, the patient was diagnosed with two pyogenic hepatic abscesses, one of which was drained under computed tomography (CT) guidance and the other was not drained but treated with broad-spectrum IV antibiotics due to its proximity to significant anatomical structures. Blood cultures at SBMC grew Fusobacterium necrophorum (pathogenic rod-shaped gram-negative anaerobe), a hepatic abscess aspirate grew an unidentified gram-negative anaerobe, and urine and ascites cultures were negative. The patient was discharged from SBMC on oral amoxicillin-clavulanic acid and oral metronidazole.
However, one day later, the recurrence of his symptoms led him to the emergency department (ED) of Grandview Medical Center (GMC). On presentation, his temperature was 100.3° Fahrenheit, his heart rate was 131 beats per minute (bpm), his blood pressure was 131/88 millimeters of mercury, and his respiratory rate of 18 per minute. In the ED an initial history, review of systems, physical exam, labs, and imaging were performed as detailed below.
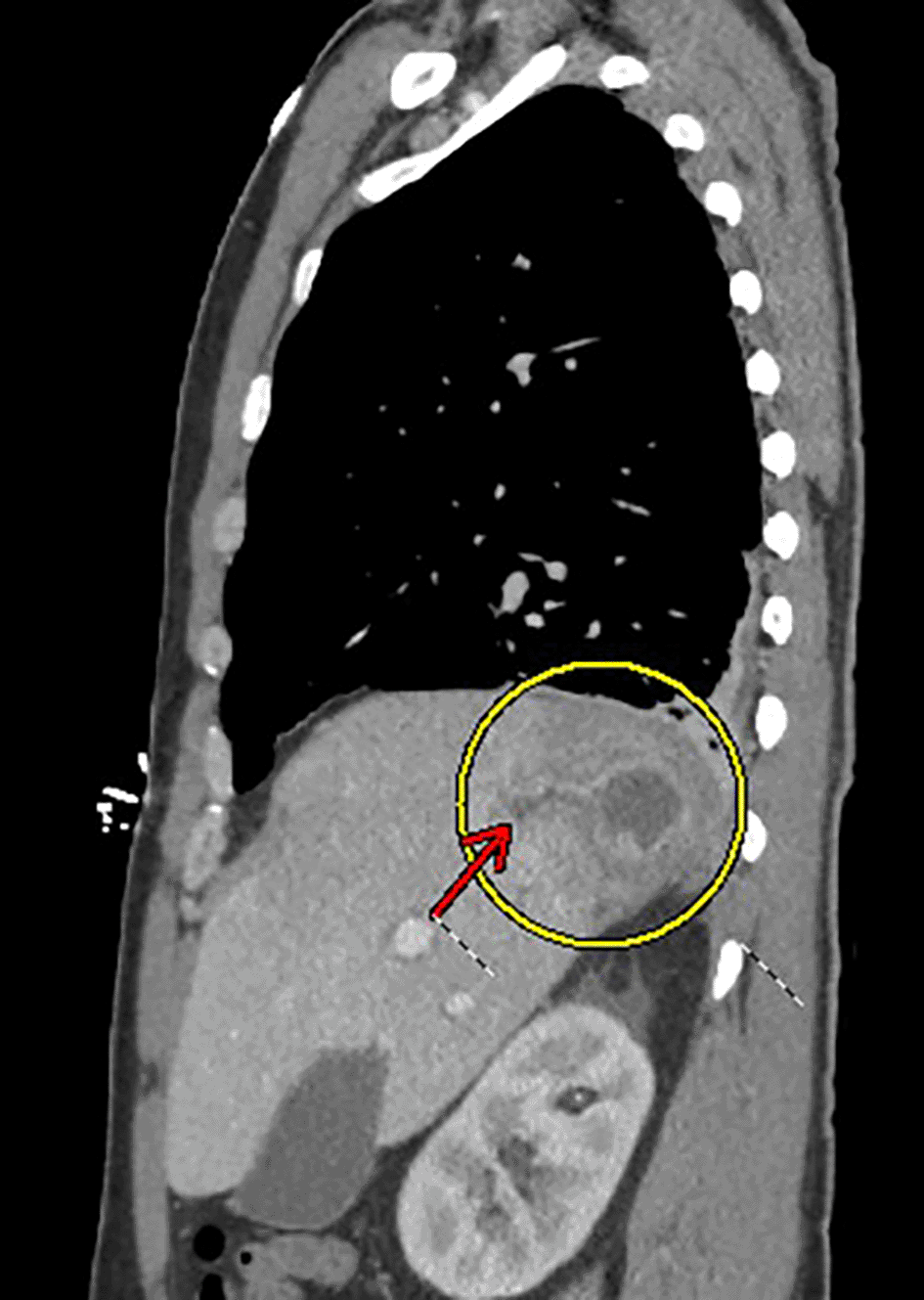
Physical examination at the time of admission revealed a well-appearing male in his 30s in no acute distress. His head and neck were normocephalic, atraumatic, and supple without lymphadenopathy. His oropharynx appeared non-erythematous with moist mucous membranes, and his trachea was midline without palpable masses. He was able to phonate in full sentences. He had normal chest wall expansion with clear breath sounds at both lung fields without wheezes, rhonchi, or crackles. Cardiac auscultation and pulses in his bilateral upper and lower extremities were within normal limits. His abdomen had laparoscopic surgical scars, but was soft, non-tender, and non-distended with normal bowel sounds. The neurological exam including cranial nerves, strength, sensation, and reflexes was normal. A complete blood count (CBC) showed an elevated white count of 26.1 with neutrophilic predominance. His hemoglobin, hematocrit, and platelet counts were within normal limits. A comprehensive metabolic panel showed creatinine 1.44, glucose 199, sodium 133, potassium 3.8, calcium 8.1, alkaline phosphatase 164, aspartate aminotransferase (AST) 35, alanine aminotransferase (ALT) 84, and total bilirubin 0.8. Additional labs showed lactic acid 5.2 and lipase 871. One sample of stool ova and parasite studies was negative (guidelines recommend sending stool samples on three consecutive days, and daily stool samples for three days were ordered, but only one day was collected). Stool culture was pan-negative (including Salmonella, Shigella, Campylobacter, E. coli 0157, Aeromonas, Yersinia, and Shiga Toxin 1 or 2). Hepatitis panel was pan-negative (including Hepatitis A Ab IgM, Hepatitis B Core Ab IgM, Hepatitis B Surface Ag, and Hepatitis C Ab), and HIV Ag/Ab was negative. A CT with contrast of his chest, abdomen, and pelvis was obtained that showed a 2.8 cm × 3.3 cm × 2.8 cm hepatic abscess at the junction of segments 7 and 8 of the liver complicated by local hepatic venous thrombosis (Figures 1 and 2). Blood cultures drawn at GMC were negative. Interventional Radiology (IR) was consulted for drainage of hepatic abscess. The sample from hepatic abscess grew Bacteroides thetaiotaomicron (commensal rod-shaped gram-negative anaerobe)–though it is important to note that he had recently received inpatient antibiotic coverage from SBMC with outpatient antibiotic coverage at discharge.
The patient was admitted to the intensive care unit and his treatment plan involved broad-spectrum coverage with administration of IV vancomycin, cefepime, and metronidazole. Further, he received aggressive IV fluid resuscitation according to sepsis guidelines. Once stabilized, his hepatic abscess was drained under CT guidance by IR. We consulted infectious disease who recommended four weeks of antibiotic coverage with IV ceftriaxone 2 grams daily at an infusion clinic and four weeks of oral metronidazole 500 milligrams (mg) four times daily with close outpatient follow up at an infectious disease clinic. A peripherally inserted center catheter (PICC) was placed at discharge for antibiotic infusions.
For hepatic venous thrombosis local to the patient's hepatic abscesses, he was started on a therapeutic heparin drip at admission. This was held before the hepatic abscess was drained to avoid bleeding complications. Subsequently, he received therapeutic anticoagulation with apixaban. We planned to keep the patient on a six-month course of anticoagulation (apixaban 10 mg) oral twice daily for seven days followed by apixaban 5 mg oral twice daily for six months with reevaluation of the need for continuing anticoagulation after six months.
The patient's lactic acidosis, elevated lipase, and acute kidney injury resolved with IV fluids over his six-day hospital course.
We propose the most likely source of the patient’s infection was his recent appendectomy. Kanellopoulou, et al. found that appendicitis is the most common cause of pylephlebitis, or infectious suppurative thrombophlebitis of the portal venous system,14 and abdominal surgeries have been shown to lead to new cases of the abdominal variant of Lemierre’s syndrome.2,15
In 2017, Jayasimhan et al.16 conducted a systematic review finding 48 cases of hepatic abscesses associated with Fusobacterium. Ten of these cases were either confirmed or suspected to be due to a lower gastrointestinal infection. Treatment was highly successful with complete resolution in 47 of 48 cases. Treatment consisted of hepatic abscess drainage plus extended-length medical therapy with beta-lactams in combination with metronidazole, monotherapy with metronidazole, carbapenems, or fluoroquinolones. In the sole case with a poor outcome, the patient’s Fusobacterium bacteremia was not treated because it was not discovered until post-mortem.16 Fusobacterium is a rare cause of pylephlebitis, an inflamed thrombosis of the portal vein. Pylephlebitis is more frequently caused by: Streptococcus viridans, Escherichia coli, and Bacteroides fragilis.17 Historically, death rates of pylephlebitis have ranged from 50 to 80 percent but have improved to 25 percent with earlier detection and more aggressive antibiotic therapy.18 One theory for the significantly better outcomes in Fusobacterium infections is the absence of antibiotic resistance because the infections are so rare. However, due to the difficulty of making the diagnosis of abdominal variant of Lemierre’s syndrome, it is reasonable to assume that multiple cases are likely missed, and selection bias might result in an overly optimistic prognosis.
There is some controversy about anticoagulation in pylephlebitis. A systematic review found similar mortality rates in the “antibiotic alone” and “antibiotic plus anticoagulation” groups.14 The same review found the mortality rate to be zero for the “anticoagulation alone” group.14 However, the number of cases in this review is too small to make definitive recommendations. Considering these mixed results and due to the historical benefit of anticoagulation in the setting of thrombosis, most authors favor early anticoagulation.19
Strengths of our diagnosis include a rational explanation of the most likely etiology for the patient’s presentation and course considering the specificity of a Fusobacterium infection. A confirmed Fusobacterium bacteremia in the setting of two pyogenic hepatic abscesses with localized hepatic venous thrombophlebitis after a recent appendectomy supports a diagnosis of abdominal variant of Lemierre’s syndrome.
Weaknesses in our case include the lack of Fusobacterium found in the patient’s hepatic abscess aspirates and the possibility of alternative diagnoses (though thought to be highly unlikely). At SBMC we confirmed the presence of Fusobacterium bacteremia and a gram-negative rod from the patient’s hepatic abscess aspirate. This matches the profile of Fusobacterium, but no final microbiological identification was determined. At GMC the hepatic abscess aspirate grew Bacteroides thetaiotaomicron, a commensal gram-negative anaerobe, with a negative blood culture. However, these cultures were drawn after the administration of IV and oral antibiotics from SBMC. Additionally, the timing of the appendectomy to presentation (nine months) could suggest another etiology. Fusobacteria infections may take weeks-to-months to develop into abscesses partly because anaerobic bacteria grow slower than aerobic bacteria. However, nine months is a relatively long course for an abscess to form and become symptomatic. Lastly, only one of the three stool samples ordered was collected. Based on guidelines, parasites like Entamoeba histolytica which often cause liver abscesses in the setting of recent travel can be missed with just one stool sample.
This case supports the need for clinicians to consider additional anatomical locations (including the liver and portal veins) for infectious thrombophlebitis in the setting of Fusobacterium bacteremia in addition to Lemierre’s syndrome’s traditional locations affecting the oropharynx and IJV. When Fusobacterium bacteremia is identified, clinicians should evaluate the vasculature local to the primary infection for thrombophlebitis. It is important to recognize and treat Fusobacterium bacteremia early because antibiotic resistance has not developed, and current treatments have shown universal resolution16 while the mortality rate for pylephlebitis is 25%.19
Written informed consent for publication of clinical details and images was obtained from the patient.
Data availability is not applicable to this article as no new data were created or analyzed in this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Gut microbiota and critical care
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 20 Jul 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)