Keywords
Cataract, Artificial intelligence, machine learning, deep learning, data optimization, image pre-processing, fundus images, slit-lamp images, hyperparameter tuning.
Background: Cataracts are common causes of visual impairment. Preventing blindness requires an early and accurate diagnosis. This review examines current cataract diagnosis strategies, explores data-driven machine learning algorithms for early detection, investigates the use of artificial intelligence (AI) approaches, assesses improvements in cataract detection accuracy, identifies research gaps, and provides recommendations for future studies.
Methods: We gathered labelled cataract and non-cataract fundus data from the Kaggle. Scholarly publications were sourced from reliable databases such as ProQuest, IEEE, ELSEVIER, Google Scholar, and PubMed. A detailed literature search with specific terms expanded the scope of this review. We included studies that used cataract and non-cataract fundus eye images from cross-sectional, retrospective, and prospective studies. The quality assessment used the AMSTAR tool, considering factors such as literature search comprehensiveness, study selection criteria, data extraction methodologies, and study validity (Table 1).
Results: This study encompassed 130 research publications, focusing on machine learning models and clinical-based diagnostic approaches for early-stage cataract identification. The performance of machine-learning models is influenced by factors such as dataset noise and limited reliable data. Barriers to the successful implementation of AI for cataract diagnosis were identified.
Conclusions: This review emphasises the obstacles hindering the broad application of AI in cataract diagnosis. Addressing these findings is vital for developing strategies to overcome these challenges and enhance cataract detection systems. To achieve improved accuracy and efficiency in cataract diagnosis, future research should prioritise efforts to enhance dataset availability and quality, reduce data noise, and refine machine-learning algorithms. Unlocking the full potential of AI and/or machine learning can lead to significant breakthroughs in cataract diagnosis, ultimately resulting in better patient outcomes and reduced visual impairments.
Cataract, Artificial intelligence, machine learning, deep learning, data optimization, image pre-processing, fundus images, slit-lamp images, hyperparameter tuning.
We have made significant adjustments to our paper in accordance with the advice provided in the peer review. We have now expressly stated that we have registered our protocol, in strict adherence to the PRISMA rules. In addition, we have enhanced the conversation regarding the use of the AMSTAR tool by offering comprehensive insights into the assessment criteria and the individual study results. Initially, our study solely emphasised accuracy as a performance indicator. However, we have now expanded our evaluation to include a comprehensive analysis of sensitivity(recall) and specificity(selectivity). This allows us to better assess the therapeutic use of the diagnostic models. In addition, we have ensured a fair and comprehensive discussion by considering the advantages and disadvantages of both fundus and slit-lamp images, rather than placing excessive emphasis on fundus images. In (Table 2) we also include other image modalities, their strength and weaknesses. we included several new figures to enhance the content. We added a figure on basic eye examinations (Figure 1. Basic eye examination), which details the eye diagnosis methods commonly used in clinical settings. Additionally, we incorporated a figure showcasing the proposed VIGG19 implementation architecture (Figure 3. Proposed VIGG19 implementation architecture), illustrating its exceptional performance in predicting image classes, such as identifying cataract or non-cataract in slit-lamp and fundus images. In the results section, a new figure (Figure 4. Results Evaluation), was included to evaluate the results, allowing us to assess the predictive capabilities of our machine learning and deep learning models using two dataset modalities: slit-lamp images and fundus images. These modifications guarantee a more thorough and exacting evaluation of the research incorporated in our review. We also added more references from 131 to 145 and now our reference list is 145 references.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
This review comprehensively evaluates existing cataract detection strategies by focusing on recent advances in data-driven machine learning (ML) and/or artificial intelligence (AI) methodologies. The purpose of this study was to address the constraints and challenges associated with traditional clinical assessment methods and investigate the feasibility of employing ML and/or AI to automate cataract diagnosis. Furthermore, this review identifies gaps in the current literature and suggests prospective directions for future research on cataract detection.
The aims and research questions of this review are as follows:
• To evaluate the shortcomings of current cataract detection approaches, particularly those related to early detection and standardisation.
• To investigate the use and advancement of ML and/or AI approaches in cataract diagnosis.
• To identify the difficulties and limitations of merging traditional methodologies with AI in cataract diagnosis, such as the availability of annotated datasets and grading system consensus.
• Propose tactics and future initiatives for enhancing cataract diagnosis, such as extensive dataset collection, uniform grading criteria, and hybrid ML model exploration.
This review focuses on people with cataracts and underlines the use of machine learning and/or artificial intelligence (AI) approaches in cataract detection and diagnosis. It examines the global prevalence of cataracts, the limitations of traditional diagnostic methods, and the potential benefits of data-driven machine learning and/or artificial intelligence technologies.
A scoping study was conducted to provide a detailed overview of the existing literature on cataract detection. It identifies gaps and limitations in the literature and proposes potential future study topics. This review guarantees that the assessment is based on trustworthy evidence, thus contributing to the improvement of knowledge in cataract diagnosis. This study investigates the possible application of artificial intelligence (AI) techniques for early detection and addresses the limits and constraints of current cataract diagnosis methods. By assessing hurdles and adopting AI in cataract diagnosis, this study aimed to increase the efficiency, accuracy, and accessibility of cataract diagnostic services.
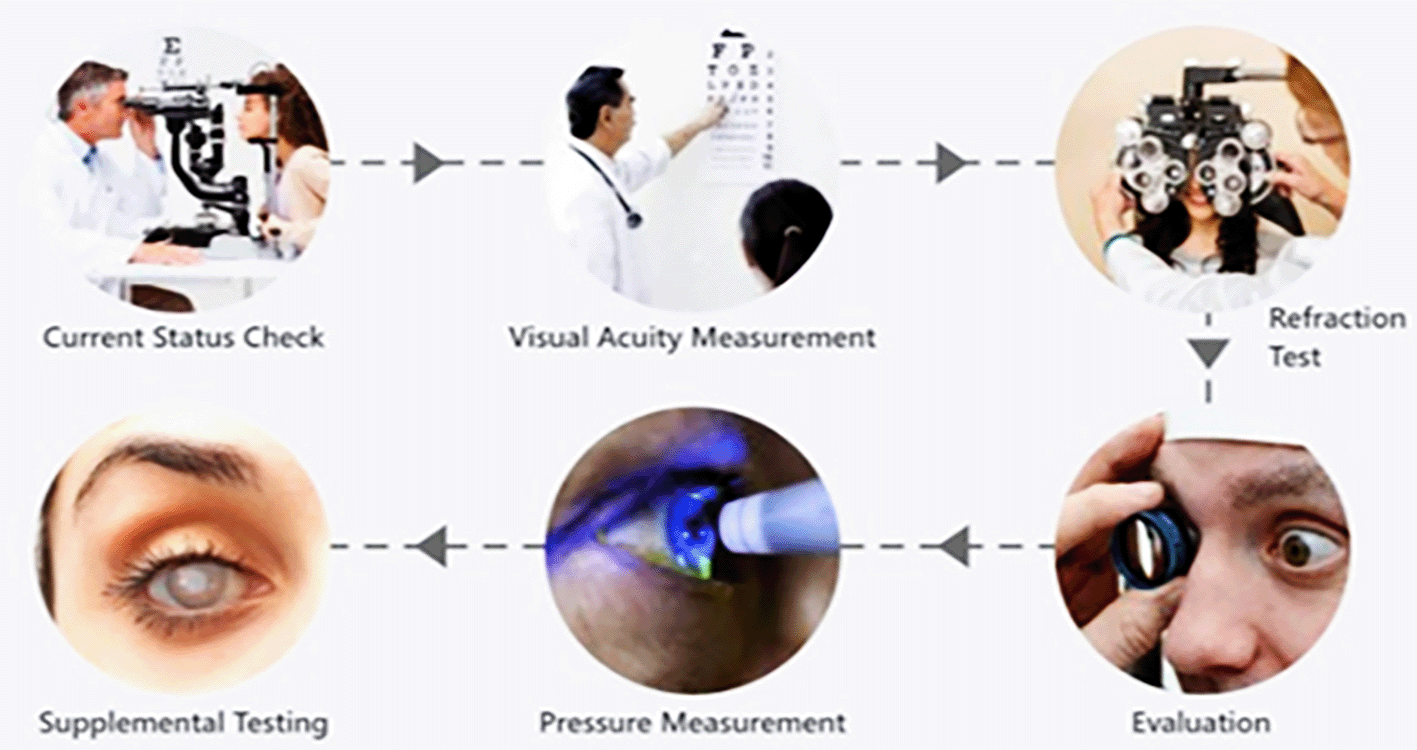
The first stage of the current eye examination process is the current status check, followed by visual acuity measurement, refraction test, evaluation, and pressure measurement. The screening process is completed by conducting supplemental testing, as illustrated in (e.g., Figure 1).
Ophthalmologists employ a range of specialised tools and instruments to perform thorough eye examinations. These instruments, such as the slit lamp and ophthalmoscope, are used to thoroughly examine the structures of the eye. Additionally, the Snellen chart and near vision card are employed to measure visual acuity. Instruments such as the phoropter and retinoscope are used to ascertain the accurate prescription for glasses or contact lenses. The slit lamp, indirect ophthalmoscope, and fundus camera enable thorough assessments. Various types of tonometers, like as applanation and non-contact variants, are used to measure intraocular pressure. Additional diagnostic tests, such as visual field analysis, optical coherence tomography, fundus fluorescein angiography, electroretinography, and ultrasound scans (A-Scan and B-Scan), offer more information about the eye’s condition, ensuring a comprehensive and precise evaluation for effective diagnosis and treatment.2
Cataract screening techniques are crucial for identifying and managing this common eye condition, especially in the elderly population. Traditional methods like the Slit Lamp, Ophthalmoscope, and various diagnostic tests are commonly used by ophthalmologists to assess eye health and function.3 While these methods have been effective in diagnosing cataracts and other eye conditions, they have limitations that can impact their efficacy in certain situations.3
One limitation of current cataract screening techniques is the reliance on manual assessments, which can introduce subjectivity and human error.4 Variability in interpreting visual acuity results among practitioners can lead to inconsistent diagnoses. Additionally, the need for specialised equipment like the Slit Lamp and Fundus Camera may restrict access to comprehensive eye evaluations, particularly in remote or underserved areas where such tools may be scarce.5 Moreover, the high cost of certain diagnostic equipment, such as the EPIC-5100 system and LENSTAR LS 900, can create financial barriers for patients seeking eye examinations.4
On the other hand, machine learning methods driven by data present a promising alternative for cataract detection and screening. By utilising advanced algorithms and extensive datasets, machine learning models can improve the accuracy, efficiency, and accessibility of cataract diagnosis.6 These techniques can address the limitations of manual assessments by offering objective and consistent evaluations based on data-driven insights.6 Machine learning algorithms can analyse high-resolution images from tools like the Pentacam HR and OCT, facilitating automated detection and classification of cataracts with enhanced precision.6
Furthermore, machine learning approaches tackle the issue of generalisability by adapting to various datasets and imaging techniques, ensuring robust performance across diverse patient populations and clinical environments.6 The capability of machine learning models to learn from different data sources, including clinical and imaging data, enhances the accuracy and reliability of cataract diagnoses (e.g., Table 1).6 Additionally, incorporating explainable artificial intelligence (XAI) techniques in machine learning algorithms can enhances transparency and interpretability, allowing clinicians to comprehend and trust the decisions made by these models.6
Despite the technical challenges associated with data-driven methodologies, such as the requirement for high-quality data and precise ground truth information, the potential advantages of employing machine learning for cataract screening outweigh these challenges.6 By integrating machine learning into cataract detection processes, healthcare providers can streamline screenings, enhance diagnostic accuracy, and optimize patient outcomes. The shift towards data-driven approaches in cataract screening signifies a significant advancement in contemporary ophthalmic practice, offering a route to more efficient, reliable, and accessible eye care for individuals globally.6
Diverse machine learning methods have been successfully employed in the identification and diagnosis of cataracts to improve early detection procedures. Support Vector Machines (SVM) have demonstrated potential in assessing the severity of cataracts and forecasting their progression through the analysis of fundus and slit-lamp images, while also taking into account environmental and lifestyle factors.7 Support Vector Machines (SVMs) provide an automated and impartial approach that outperforms conventional examination methods, assisting in accurate cataract diagnosis. In addition, the K-nearest neighbour (K-NN) approach has been used to identify cataracts, demonstrating its capacity to accurately distinguish between normal and cataractous eye conditions.8
PCA has played a crucial role in reducing the dimensionality of data and uncovering important features of eye images that are related to cataract identification. This has contributed to the creation of precise diagnostic tools.9 The Random Forest algorithm, a method of combining multiple learning models, has been used to detect cataracts in retinal imaging data. It successfully deals with noisy and correlated input information to accurately identify important diagnostic traits.10 In addition, the Naive Bayes Classifier, which employs a probabilistic methodology, has effectively categorised patients with accuracy using different attributes, hence assisting in the precise identification of cataracts.
Machine learning approaches have greatly progressed the field of cataract research by providing automated and efficient solutions for detecting and grading cataracts. These techniques have utilised algorithms including Support Vector Machines (SVMs), K-Nearest Neighbours (K-NN), Principal Component Analysis (PCA), Random Forest, and Naive Bayes to increase the precision and efficiency of cataract diagnosis, ultimately leading to better patient outcomes and treatment approaches.
The field of cataract diagnosis has made great progress because to recent developments in state-of-the-art deep learning models, including ResNet50, DenseNet201, InceptionV3, CSDNet, and EfficientNetB0.11–13 These models have exhibited remarkable advancements in diagnosing cataracts and have also been effectively utilised in multiple medical fields, displaying a high level of accuracy in recognising conditions such as otitis media, skin lesions, and pneumonia.11–13 ResNet50, DenseNet201, and InceptionV3 have shown exceptional accuracy in identifying skin lesions and otitis media. They have even surpassed human specialists in several cases, as evidenced by research.12 By utilising deep learning architectures, these models demonstrate exceptional proficiency in extracting distinctive characteristics and classifying images, hence significantly improving diagnostic skills in many medical applications.11–13
The VGG19 model is a cutting-edge artificial intelligence model that demonstrates outstanding accuracy in cataract identification. It is a transfer learning model. Nevertheless, the prediction performance of the VGG19 model and similar machine learning models in real-world settings may be hindered by challenges such as methodological transparency, external validation, and data representativeness. Therefore, it is necessary to address these issues in order to improve the reliability and applicability of the VGG19 model and other similar machine learning models. Furthermore, the CSDNet model has exhibited exceptional precision in detecting cataract situations, emphasising the promise of deep learning techniques in enhancing diagnostic results.14 Obtaining a high-quality dataset for training the model can be challenging, as more than 80% of the available dataset is unstructured.15
Traditional machine learning techniques such as Support Vector Machine (SVM), K-Nearest Neighbours (K-NN), Principal Component Analysis (PCA), Random Forest, and Naive Bayes are useful for detecting cataracts. However, advanced deep learning models have recently emerged as more accurate and efficient tools for diagnosing various medical conditions, including cataracts.15 The utilisation of advanced deep learning models like ResNet50, DenseNet201, InceptionV3, CSDNet, and EfficientNetB0 can greatly enhance the accuracy and dependability of cataract detection, hence resulting in more efficient healthcare solutions.15 Overall, the incorporation of advanced deep learning models in cataract diagnosis is a substantial advancement in the area. It provides improved accuracy and effectiveness in identifying and classifying different medical problems, such as cataracts.
This paper examines the VGG19 model as a case study, focusing on its architecture and comparing its training performance accuracy, model loss, generalizability, and other metrics with other state-of-the-art AI models. The experiment will utilise two imaging modalities: slit lamp and fundus pictures. Nevertheless, it is crucial to mention that this review does not encompass other types of picture data. The paper examines the performance of the VGG19 model, including its architecture and the equations that represent each layer of the model. The focus is on understanding the advantages and disadvantages of these layers in enhancing the categorization of ocular images, whether they have cataracts or not. The study conducted by Gill, Anand, and Gupta at Chitkara University focuses on cataract detection. The study highlights the use of the VGG19 model, which is a variant of the Visual Geometry Group (VGG) network. The VGG19 model is notable for its deep convolutional design, consisting of 19 layers. The transfer learning VGG19 model was used to classify photos into cataract and non-cataract categories. The VGG19 model utilises parameter equations, such as Convolutional Layer Parameters, to calculate the Output Feature Map. The formulas include weight matrices, trainable parameters, ReLU activation functions, and convolution processes. The Pooling Layer Parameters and the fully linked layer are essential components in the classification process. The formula for the convolution layers is , where is the weight matrix of learnable parameters updated during training, σ is the ReLU activation function, and * denotes the convolution operation. After the convolutional layer, the Pooling Layer Parameters consist of no learnable parameters and a max-pooling operation, which is determined using the formula , where R represents the pooling area. The other crucial layer is the completely connected layer, which comprises the following parameters: Weights is a matrix of learnable parameters, whereas Biases is the scalar/single value applied to the output. The equation for this linear transformation parameter is . The study centres on a binary classification experiment, utilising a sigmoid function to ascertain the likelihood of cataract occurrence. The output layer employs binary cross-entropy as the loss function, which is a frequently used option in similar situations.
Furthermore, the study investigates the intricate mechanisms of the VGG19 model, which is a complex convolutional neural network consisting of 19 layers. The research focuses on the application of transfer learning techniques to identify cataract photos. The architecture of the model includes scaling fundus/slit-lamp images, employing various convolutional layers with different specifications, and utilising fully connected layers with ReLU activation functions. The output layer employs a sigmoid layer to transform logits into probabilities, facilitating precise categorization. The convolutional layers utilise 3x3 filters to capture crucial information, while the ReLU activation function incorporates nonlinearity to account for intricate data interactions. Pooling layers decrease the spatial dimensions to prevent overfitting, whereas fully linked layers combine features for classification, with the sigmoid parameter improving the accuracy of class probability predictions. The system’s feature extraction procedure begins by identifying basic properties in order to detect tiny changes in the retina. It then progresses to more complex features to differentiate between different cataract scenarios. The study highlights the importance of fine-tuning model parameters, such as filter weights, bias terms, learning rate, batch size, and epochs. These parameters can be adjusted based on the specific task at hand to improve the performance of the VGG19 model in detecting cataracts using fundus/slit-lamp images. Techniques such as hyperparameter tuning, namely through grid search, random search, and Bayesian optimisation, can be utilised to improve the model and accurately classify cataract and non-cataract pictures.
Cataracts play a substantial role in the global prevalence of visual impairment.16–18 The identification of ocular diseases at an early stage is critical for preventing vision loss and improving patient outcomes.18,19 Despite the potential of artificial intelligence (AI) in cataract screening, its widespread usage is limited in many healthcare settings, particularly in areas with limited access to eyecare services and economic constraints.17,18,20–22 The use of AI technologies has the potential to increase the chance of an early diagnosis of cataracts,20,21,23,24 reduce undetected instances or features linked to cataracts (e.g., change in retinal veins and artery size and structure, shape of cataract formation on the lens, colour change of the lens disc, uneven corneal surface, leukocoria, or red reflex appearance in the pupil), and allow time for treatment before the cataract is at an irreversible severe stage, thereby improving community health.24–32 Numerous AI approaches have been developed to address the challenges associated with cataracts, such as limited-service availability, mistakes, and misdiagnoses. Both clinical and AI-based techniques have been proposed for computerised cataract diagnosis and treatment. Currently, the most common method for identifying cataracts is through manual eye examinations performed by ophthalmologists, which frequently include slit-lamp examinations, ophthalmoscopes, etc.33–36 However, this strategy has drawbacks owing to variances in physician experience, which result in variable diagnostic outcomes.36–38 The application of human vision as a standard detection technique used by eye specialists has a limited ability to detect tiny changes in lens opacity using different types of image modalities, which may result in missed early-stage cataracts or misdiagnosis of ocular illnesses, particularly those genetically linked to cataracts.20,21,23,37,39–42 However, these limitations prevent effective use of eye screening tools in cataract detection. The availability of correctly labelled, high-quality data is important for the efficient training of AI systems.34,43 To ensure patient trust, ethical factors such as patient privacy, data security, and informed consent must be addressed.44 Building trust in eye care services, whether through clinical examinations or AI-powered solutions, encourages more people to undergo regular eye examinations. Collaboration and coordination among technology specialists, healthcare professionals, and politicians are required for the successful integration of AI into existing eyecare infrastructure.45 A validation method is required to ensure the dependability and authenticity of the AI systems.46 The WEKA tool can be used to determine the effectiveness of AI models in the accurate and early detection of cataracts by assessing different algorithmic models.47–49 The simulation results can aid in the selection of an optimal algorithmic model that accomplishes the intended objectives and provides cutting-edge solutions. Improving the algorithm’s performance entails meticulous adjustments through hyperparameter tuning and careful exploration of the hyperparameter space using approaches such as grid search, random search, and Bayesian optimisation.50,51 Data collection and preparation, designing an appropriate model architecture, separating data for training and validation, optimising hyperparameters, and conducting model training and validation are all critical aspects for developing improved cataract detection models.50,52 These elements are critical for improving the effectiveness of machine-learning models.
Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines53,54 were followed in this work. However, the study is currently available online, and there is a registration information, https://www.crd.york.ac.uk/prospero/#myprospero including registration numbers.
Among the eligible sources of evidence, this review includes numerous crucial characteristics. First, the studies were limited to those published in the previous past 15 years, allowing us to capture the most recent developments in cataract detection methodologies, such as advances in AI and/or machine learning, while also taking into account considering traditional diagnostic methods. We aimed to investigate the issues associated with early-stage cataract diagnosis and highlight the importance of the data provided by focusing on current publications. Second, in order to be considered, the publications must be written in English. Because English is widely utilised in scientific research, this decision was made to ensure that writers fully comprehend and evaluate the findings, as well as consistency in data extraction and interpretation.
The inclusion criteria were fully published journal articles to highlight the quality and trustworthiness of the evidence.55 This technique ensured that the review contained only papers that had completed a rigorous evaluation procedure, including peer review. This study examined studies that used fundus and slit-lamp datasets, which are both extensively used in. This technique ensured that the review contained only papers that had completed a rigorous evaluation procedure, including peer review. This study focuses on two types of image modalities, namely slit-lamp and fundus images and slit-lamp, for specific reasons. Slit-lamp images provides high-resolution images of the eye’s anterior segment, enabling detailed examination of the lens and other structures.56–61 It allows the precise localisation and characterisation of different types of cataracts based on their position within the lens. Additionally, the dynamic adjustment of light and slit orientation permits various perspectives and lighting conditions for viewing cataracts in real-time.55,62 In contrast, fundus images indirectly evaluate lens clarity by assessing the appearance of the red reflex.63–65 They enable the examination of not only the lens but also other structures at the back of the eye, aiding in the identification of associated pathologies. Fundus imaging is non-invasive and well tolerated by patients, facilitating easy monitoring. Digital documentation of fundus photos (images) allows for convenient tracking of cataract progression and selecting the appropriate time for surgical intervention.27,28,31,58,66,67 Slit-lamp and fundus imaging are critical for the early detection and evaluation of cataracts. Slit-lamp images are useful in a variety of investigations owing to their ease of use and accessibility.68–74 However, fundus imaging can reveal important information regarding posterior segment involvement and its impact on retinal health.66,75–77 It is critical to recognise that these imaging modalities should be used in conjunction with other diagnostic tools and that a full eye examination performed by a skilled ophthalmologist is still the gold standard in cataract diagnosis.78–82 Furthermore, the incorporation of AI and machine learning algorithms shows promise in terms of increasing cataract detection; however, further study and validation are needed to ensure clinical application and efficacy.49,83–85
In terms of patient characteristics, there were no age or sex restrictions on the inclusion criteria. It is crucial to note, however, that most studies have concentrated on people aged 50 years and older, potentially overlooking other age groups. This is in contrast to the research direction of this study, in which people of various ages and sexes were deemed eligible for inclusion, ensuring a comprehensive assessment of various cataract diagnostic methodologies across diverse patient populations and advancing our understanding of the subject.
Overall, the inclusion criteria for evidence sources were carefully selected to include recent breakthroughs, be understandable and reliable, and include a wide range of diagnostic procedures and patient characteristics.
This review used several databases and direct interactions with the authors to acquire a diverse range of credible materials. The databases chosen, including ProQuest, IEEE, Elsevier, and PubMed, are well known for their vast coverage of scientific literature across a wide range of subjects, including cataract research.86 These databases provide access to peer-reviewed journals, conference papers, and scholarly publications, allowing a comprehensive search of relevant materials. Furthermore, email interactions among authors were used to evaluate individual journal articles and identify other sources, thereby boosting the thoroughness of the literature review. To ensure that the review included the most recent studies, the most recent search was undertaken in June and July 2023. The period from 2008 to 2023 was chosen with care to include the most recent research findings on cataracts and related diagnostic tools. This review provides an up-to-date understanding of cataract diagnosis and incorporates the most recent advancements in this field by including recent publications published during this designated period. A thorough and extensive collection of information resources for this literature review was compiled using a combination of database searches and direct communications with the authors. This method ensures that various published works are included and has the ability to unearth pertinent supplemental studies, resulting in a full and reliable review of the issue.
As part of the search strategy, a complete literature review was conducted using various academic databases. This link will lead you to an example of a PubMed database search strategy: https://pubmed.ncbi.nlm.nih.gov/. Relevant terms were linked in the search box using Boolean operators (AND and OR) to perform a thorough search. The primary purpose of this study was to identify and assess cataract journal articles, specifically techniques of diagnosis or detection, and to investigate the potential applications of artificial intelligence and machine learning. To boost search efficacy, constraints and filters such as specifying a publication date range and language limits were applied. The chosen database(s) was used to conduct the search and collect articles that met the present criteria. The titles and abstracts were evaluated for relevance, and publications that satisfied the qualifying criteria were selected for further research.
The ability to read the entire text of the selected papers was essential for gaining thorough knowledge of the methodology, findings, and consequences. To maintain the most recent scientific results, the search method must be regularly adjusted. Other databases, such as Scopus, Web of Science, and Embase, were considered in addition to PubMed to locate additional papers. Manual searches of relevant journals, conference proceedings, and reference lists have also been suggested as viable techniques to identify additional sources. In addition, grey literature sources, such as reports, theses, and dissertations, were recognised for their capacity to provide helpful insights and diverse perspectives.
Adapting the search technique to the specific needs of each database and changing the search terms and combinations are key components in providing effective and comprehensive results.
In this scoping investigation, a two-stage screening and eligibility technique was employed to select the appropriate sources of evidence. First, the papers were assessed by examining their titles and abstracts to determine their relevance to the research topic. This screening technique aided in the identification and removal of articles that were extraneous to or outside the scope of the study. Following the filtering procedure, the remaining articles were thoroughly assessed to determine whether they met the criteria. The admission criteria emphasised research on the application of AI and/or machine learning (ML) techniques in the diagnosis of cataracts. In the investigations, both cataract and non-cataract fundus eye images were preferred. Furthermore, these papers had to be completely available or fully published in English. A total of 130 studies met the inclusion criteria and satisfactorily addressed the review objectives. These studies provide important information on the application of AI and/or ML in cataract diagnosis. Articles that did not meet the inclusion criteria were excluded. Articles that were excluded had not been published in English or were unrelated to machine learning and traditional cataract diagnosis. A total of 270 items were deleted from the final analysis.
The following suggestions were made to improve the selection process:
Conduct a pilot screening phase: A small number of articles should be pilot screened prior to the entire screening operation.87 This simplifies the eligibility conditions while guaranteeing reviewer uniformity. To boost dependability and minimise prejudice, several reviewers should be included in the screening and eligibility process. Each reviewer independently reviewed the papers, and there were group discussions through Microsoft Teams to settle any concerns and reach an agreement.
Consider employing screening software or platforms: Using tools or software platforms such as Scholarcy, Zotero and AMSTAR. The utilisation of the AMSTAR tool for evaluating research papers on early cataract diagnosis by machine learning techniques entails a series of crucial measures to guarantee a methodical, uniform, and clear-cut procedure. Firstly, it is essential to establish explicit objectives that specifically aim to evaluate the quality of pertinent research. The inclusion criteria should encompass a range of study formats, including randomised controlled trials, cohort studies, and systematic reviews. The focus should be on human subjects who have or are at risk of developing early cataract. The studies should utilise machine learning techniques and provide performance metrics such as accuracy and sensitivity. The exclusion criteria should exclude papers that have not undergone peer review, studies that do not focus on early cataract diagnosis, articles that do not utilise machine learning, and animal research. The criteria should be recorded in a protocol that is easily available to all reviewers in order to guarantee clarity. Reviewers receive training sessions to comprehend the eligibility criteria and learn how to use the AMSTAR tool. This is followed by a calibration exercise, which involves screening a small number of articles to guarantee consistency. The screening procedure entails the independent evaluation of titles and abstracts, facilitated by tools such as EndNote or Covidence. This is followed by the retrieval and individual assessment of the complete texts of publications that may be pertinent. Reasons for rejecting articles at both levels are recorded using a pre-established form to increase clarity and uphold a record of actions taken. Regular consensus sessions are held to resolve any differences among reviewers. Data extraction from the research included in the analysis is carried out using standardised forms by several reviewers in order to maintain uniformity. The AMSTAR method is thereafter employed to evaluate the calibre of systematic reviews, taking into account elements such as pre-established design, elimination of duplicate research selection, thorough literature search, and disclosure of conflicts of interest. Collaboration technologies such as Google Sheets, Trello, and Covidence facilitate the monitoring of progress and the provision of regular updates to team members and stakeholders. Comprehensive documentation of the screening and selection process, including the AMSTAR assessment outcomes, is kept to ensure transparency. The final report provides a detailed account of the screening process and the criteria employed. Standard Operating Procedures (SOPs) are created and adhered to at every review stage to guarantee reproducibility, with all forms, protocols, and data stored in a systematic manner. The advantages of utilising the AMSTAR tool encompass the uniform application of criteria, explicit documentation to enhance transparency, rigorous quality assessment, streamlined collaboration, and a comprehensive audit trail. These factors ultimately contribute to the production of high-quality and reproducible systematic reviews on the utilisation of machine learning techniques for early cataract detection. A Table 2 will be developed to illustrate the application of the AMSTAR tool in the selection of journal articles for a systematic review. This table will outline the assessment of each article based on the major criteria of the AMSTAR tool. The emphasis will be placed on a specific group of the AMSTAR criteria, which includes predetermined design, duplicate selection and extraction of studies, thorough search of literature, publication status (i.e., grey literature), characteristics of included studies, scientific quality of included studies, proper utilisation of meta-analytic methods, assessment of publication bias, and disclosure of conflicts of interest.
One of the objectives of this literature review was to comprehensively investigate the utilisation of artificial intelligence (AI) and machine learning (ML) technologies for the purpose of identifying cataracts. In order to accomplish this objective, an extensive examination of scholarly literature from esteemed sources such as ProQuest, IEEE, Elsevier, and PubMed was undertaken, encompassing a diverse array of scientific topics. The most recent search was conducted on July 3, 2023. The primary objective of our study was to examine the difficulties associated with the implementation of artificial intelligence (AI) in the context of cataract detection, as well as its potential influence on patient outcomes. Furthermore, we provided recommendations for further investigation in this field. The main aim of this study was to conduct a thorough analysis of the potential advantages of artificial intelligence (AI) in the diagnosis of cataracts. The data was systematically organised and presented in a structured format to enhance coherence and clarity during the evaluation phase, thereby facilitating comprehension.
In order to ascertain the dependability and calibre of nonrandomized research studies incorporated in systematic reviews and meta-analyses pertaining to the early detection or diagnosis of cataracts, we employed the AMSTAR tool along with its methodical evaluation procedure. By utilising the AMSTAR tool comments and published reviews, it is possible to ascertain potential biases and evaluate the quality of the study. The utilisation of an analytical and comparative approach greatly enhances the validity and utility of the findings obtained from our research on cataracts.
In the assessment of both established clinical and emerging AI diagnostic methodologies, a comprehensive analysis was conducted on a range of imaging modalities, including fundus imaging, slit-lamp imaging, and computed tomography (CT). Among these options, slit lamps are particularly notable for their user-friendly nature and their significant research value, as they possess the capability to capture images utilising conventional cameras. The analysis was conducted using the Murad tool, comprising of eight queries that pertain to selection, verification, causality, and reporting. According to the information provided in Table 3, a rating of five or above was deemed suitable for the evaluation. The aim of the study was to assess and present quantitative data regarding potential strategies for addressing the difficulties associated with the detection of ocular diseases, particularly cataracts. The investigation conducted by researchers involved the examination of different approaches for the identification of cataracts. This was achieved through the utilisation of a classification framework that incorporated two distinct sets of images: one comprising images with cataracts and the other consisting of images without cataracts. The primary objective was to evaluate the precision of cataract detection.
| Study | Dataset used | Selection domain | Comparability domain | Results/exposure domain | Score & methodology quality |
|---|---|---|---|---|---|
| Classification of cataract fundus images based on deep learning Dong et al.29 | 785I find us images from dept ophthalmology | 3-stars | 2-stars | 3-stars | Good - 8 |
| Cataract Detection using Digital Image Processing Jind al et al.88 | National Eye Institute, National Institutes Of Health hospital is the source of the data. | 4-stars | 3-stars | 3-stars | Good - 7 |
| Eye disease Detection using machine learning Ramanathan et al.89 | Does not specify the source of dataset | 1-stars | 2-stars | 2-stars | Fair - 6 |
| Automatic Detection of Eye Cataract Using Deep Convolution Neural Networks (DCNNs) Hossain et al.90 | Rand only collected from Several eye hospitals across Bangladesh | 2-stars | 1-stars | 0-stars | Poor - 4 |
This review presents a comprehensive examination of the elements taken into account when acquiring data. A total of one thousand fundus images were acquired at the Joint Shantou International Eye Centre (JSIEC) in Shantou City, Guangdong Province, China. These images were collected from open-source database (i.e., kaggle.com), which can be further explored in the Underlying data section.91 These images were part of a larger collection of 209,494 fundus images that were used for training, validation, and testing. The data source is provided, as well as a link to the Kaggle repository. In addition, we were able to utilise the Ocular Disease Intelligent Recognition (ODIR) dataset, which is a well-organized collection of ophthalmic data. This dataset consists of information from 5,000 patients, including their age, colour fundus photos of both their left and right eyes, as well as the diagnostic keywords provided by doctors. The dataset was obtained from the Kaggle repository. The fundus images in the collection were categorised into 7 distinct classes, each representing a different fundus disease or condition, including Normal (N), Diabetes (D), Glaucoma (G), Cataract (C), Age-related Macular Degeneration (A), Hypertension (H), Pathological Myopia (M), and Other diseases/abnormalities (O). Every image is categorised into a certain class according to its distinctive features. Assumptions and simplifications: We make the assumption that the dataset consisting of 5000 fundus images accurately represents a larger collection and encompasses the complete spectrum of fundus diseases and disorders often found in the general population. The dataset’s available in Kaggle repository. In addition, we also acquired dataset made up of 1000 fundus images, we made the assumption that fundus images were accurately categorised into 39 disease categories by experts in the area. The dataset presupposes the accuracy of the labels supplied to each image. Data validation is crucial for guaranteeing the integrity and dependability of the datasets. We suggest conducting a thorough validation and quality evaluation of fundus images, along with classifying them. Possible methods include expert evaluation, testing for inter-rater reliability, and comparing the dataset to established gold-standard diagnosis. Data augmentation techniques were employed to enhance the unpredictability and variety of the dataset. The objective of this strategy is to enhance the suitability and resilience of deep-learning models trained on this dataset. This section also offers accurate descriptions and comprehensive explanations of the 39 categories of fundus diseases and disorders present in the dataset. We converted the multi-class dataset into two class dataset normal image and cataract infected images, by removing other class that are infected by other ocular diseases. This will enhance understanding and facilitate the accurate application and interpretation of the findings. When gathering and using healthcare records, such as fundus images, it is essential to take ethical considerations into account. Nevertheless, the dataset in question is accessible to the public (e.g. kaggle repository). Preserving patient confidentiality necessitates adhering to suitable ethical guidelines, acquiring informed consent, and de-identifying personal identifying data. Researchers can efficiently utilise fundus imaging datasets and achieve trustworthy results from their analysis by carefully following these recommendations and thoroughly examining the assumptions and oversimplifications.
The results synthesis involved a thorough classification and examination of journal articles collected from various sources, aiming to identify significant patterns and topics relevant to the research objectives. The articles were organised based on core principles such as the methods for diagnosing cataracts, the performance criteria for algorithms, and the characteristics of the datasets.
A data charting methodology was employed to methodically condense the information obtained from the articles. We employed tables or charts to accurately display the primary discoveries of each study article, encompassing specifics such as the precision rates attained by various machine learning algorithms, the sizes of samples utilised for training and testing, and noteworthy constraints or obstacles encountered during the research endeavour.
Subsequently, the data-charting findings underwent thorough examination to reveal the recurring themes, patterns, and trends observed in multiple investigations. The primary objective was to get a comprehensive comprehension of the efficacy and constraints of AI and/or ML in cataract diagnosis.
During the synthesis procedure, meticulous attention was given to guarantee the accuracy and dependability of the findings. The inconsistencies or contradictions were carefully assessed, and attempts were made to resolve conflicts or offer clarification.
An all-encompassing methodology was employed to extract articles and evaluate their quality. The AMSTAR technique was employed to assess the methodological rigour of the systematic reviews, encompassing factors such as the comprehensiveness of the literature search, criteria for selecting studies, methodologies for extracting data, and the validity of the included studies. The study incorporated the following information: names of the authors, year of publication, place of origin, research methodology, sample size, severity of cataracts, diagnostic methodologies employed, and challenges encountered in obtaining the publications. The methodological quality of the selection, comparability, and results areas were carefully evaluated, leading to an enhancement in the overall quality of the journal selection process.
A methodical and rigorous approach was employed to handle and consolidate the journal articles, enabling the systematic examination of publications from diverse sources. This comprehensive methodology enables a thorough analysis of the existing information, leading to the discovery of significant insights and recommendations for future research and practice.
It is crucial to identify the sources of evidence in academic research. Through an extensive search conducted across various databases, we successfully identified 400 pertinent studies. After eliminating redundant data and thoroughly examining titles and abstracts, we narrowed down our selection to 130 studies that met our inclusion criteria. We have presented a flowchart (e.g. Figure 2) that outlines the criteria utilised to determine the suitability of the published journals for inclusion in our research. This was implemented to ensure transparency in the selection process. This flowchart offers a concise summary of the fundamental characteristics of the highlighted studies. Furthermore, for the goal of providing a point of reference, we have included a compilation of publications that were excluded from the comprehensive analysis of full-text documents. This compilation can be found in the Extended Data section.
Moreover, after thoroughly examining the works, we would like to propose suggestions for additional research in this field. Prior to proceeding, it would be advantageous to examine the effectiveness of several machine-learning algorithms in identifying cataracts and assess their performance in comparison to current diagnostic methods. Moreover, doing research on the effects of integrating AI and ML technologies on patient outcomes and healthcare efficiency would yield valuable insights. Moreover, conducting extensive clinical trials to authenticate the findings of smaller studies and assess the applicability of AI-based cataract diagnosis would be advantageous. It is important to thoroughly examine the ethical concerns related to the use of AI in cataract diagnosis, including issues of patient privacy and data security. These study directions would contribute to the progress of the field.
Figure 2 illustrates the characteristics of the evidence sources utilised in the analysis. Out of the studies analysed, the majority (130) employed a cross-sectional design. Significantly, we discovered a substantial quantity of recent articles (100), primarily focusing on the utilisation of artificial intelligence (AI) techniques for the diagnosis of cataracts. In addition, 30 of these publications specifically addressed the clinical aspects of diagnosing cataracts.
We compiled a dataset consisting of 1000 ocular fundus images that exhibit a range of characteristics. This dataset is intended to assist in the assessment of cataract development. The photos were sourced from the Kaggle dataset and underwent extensive processing to ascertain the presence or absence of cataracts based on their distinct characteristics. Every image was labelled to be used as a point of reference in the future. Nevertheless, it is important to acknowledge that the visual quality of the ocular photographs was inadequate to discern the gender of the persons in question. Moreover, the study included individuals aged between 40 and 65 years.
Given these findings, we propose recommendations for future research in this domain. Exploring the potential of integrating advanced imaging methods, such as high-resolution retinal imaging, may enhance the sharpness and accuracy of ocular images for diagnosing cataracts.92–96 Improving ocular image quality and precision for cataract diagnosis is critical for proper therapy. Several technical approaches can be utilised to accomplish this:
The paragraph explores many technical techniques designed to improve the clarity and precision of ocular images for cataract diagnosis. Optical Coherence Tomography (OCT) and Scheimpflug imaging are advanced techniques that produce precise cross-sectional images of the eye, allowing for clear visualisation of cataract-affected areas.96–101 Adaptive Optics is a method used to reduce optical distortions, improving the clarity and sharpness of images.102 Contrast enhancement methods are used to amplify the differences in pixel brightness, therefore enabling better differentiation between healthy and cataractous regions.103 The application of Image Registration techniques allows for the alignment of many eye images captured at different time intervals, thereby enabling accurate monitoring of cataract progression. Deep Learning-based Image Analysis utilises machine learning algorithms to train models that can detect and segment cataracts in medical images.91,104–108 The approach described allows for the extraction of quantitative measurements pertaining to the existence and intensity of cataracts. Polarimetric imaging is a method that enables the detection of distinct features of cataractous tissue, hence enhancing the capacity to differentiate it from healthy tissue.109 The process of Multimodal Imaging Fusion includes combining data from different imaging techniques to provide a detailed representation of the eye’s anatomical features and improve diagnostic110 accuracy. Real-time image processing refers to the process of optimising algorithms to provide instant feedback as images are being acquired.111 The adoption of defined imaging methods enables the achievement of uniform and reliable results across different clinics and equipment.96,112 Moreover, conducting longitudinal studies that cover a wider range of ages has the capacity to produce substantial discoveries about the development and long-lasting impacts of cataracts.113–117 Additionally, augmenting the dataset to encompass a more diverse array of individuals from various ethnicities and geographic regions has the potential to enhance the relevance of the findings to a wider community.118–120 Finally, investigating research on the correlation between the development of cataracts and systemic illnesses such as diabetes or hypertension could yield significant information regarding potential risk factors and concurrent medical problems.121–123 Engaging in the exploration of these research avenues would contribute to the advancement of our understanding of cataracts and bolster evidence-based practises in diagnosis and treatment.124,125
The objective of this evaluation was to assess the calibre and reliability of specific sources of evidence for their inclusion in research projects or literature reviews. It is important to mention that the scoping review did not conduct a thorough assessment of each individual source.
Several studies have examined the use of machine-learning techniques for cataract diagnosis. When evaluated on a dataset of 2,073 images, Li et al.69 used a convolutional neural network (CNN) on digital slit-lamp images and attained an accuracy rate of 94.1%. With a dataset of 1,410 images, Wang et al.19 used deep learning approaches on optical coherence tomography (OCT) images and attained a precision rate of 91.84%. On a dataset of 500 images, Akram et al.111 used a convolutional neural network on slit-lamp images and achieved a precision rate of 92.5%. Guo et al.106 employed various deep learning models on slit-lamp pictures, with the ResNet50 model attaining 98.4% accuracy on a 1,000-image dataset. Kavitha et al.100 used deep learning techniques on OCT images and achieved 96.7% accuracy with a collection of 500 images. For anterior segment OCT images, Zheng et al.99 proposed utilising a mix of CNN and SVM classifiers. They obtained a 95.7% accuracy rate with a dataset of 1,000 scans. Wang et al.19 created a deep learning method for slit-lamp images, reaching 95.7% precision using a dataset of 1,000 images. Khan et al.55 created a diagnostic system combining CNN and transfer learning, attaining a precision rate of 96.08% on a 1,000-image dataset. Raza et al.72 developed a deep learning system based on transfer learning, attaining a precision rate of 98.6% on a 1,000-image dataset. With a dataset of 200 images, Zarei-Ghanavati et al.73 created an AI system to detect cataracts on slit-lamp images, achieving an accuracy score of 94.1%. With a dataset of 3,456 images, Yoo et al.74 used transfer learning techniques and attained a precision rate of 95.2%. With a dataset of 5,000 images, Liu et al.104 employed CNNs on slit-lamp images and achieved an accuracy rate of 98.9%. With a dataset of 1,000 images, Li et al.69 used a deep learning methodology on slit-lamp images and attained an accuracy rate of 94.1%. On a dataset of 3,200 images, Cheng et al.112 discovered that deep learning methods outperformed traditional machine learning techniques with a precision of 96.8%.
These studies collectively highlight the potential of machine learning and deep learning techniques in cataract diagnosis, demonstrating promising accuracy rates and performance across various imaging modalities.
In order to make additional progress in the field, we propose carrying out comparison studies that directly evaluate several machine-learning algorithms to determine the best efficient method for diagnosing cataracts. In addition, broadening the dataset to encompass a wider range of individuals in terms of age, ethnicity, and geographical distribution will improve the ability of detection algorithms to be applied to a larger population. Moreover, it is important to examine the practical application and medical viability of AI-driven cataract detection, taking into account aspects such as seamless integration into existing processes, ease of usage, and cost-efficiency. Examining the ethical implications of using machine learning algorithms in therapeutic contexts, such as safeguarding patient privacy and ensuring data security, is extremely important. These research directions will enhance the ongoing progress and practical application of AI for cataract diagnosis.
When evaluating the work of different researchers or authors in the journal papers included in this review, it is important to recognise certain limitations. The aforementioned research are subject to the following constraints:
Limited Sample Size: Several studies, such as Li et al.69 with 8,287 images, Akram et al.111 with 500 images, and Zarei-Ghanavati et al.73 with 200 slit-lamp images, utilised limited datasets. The limited sample size hinders the capacity to apply the data to a broader population and capture the entire spectrum of patients with cataracts. Furthermore, there is a restricted range of datasets utilised in various investigations. For example, certain studies have exclusively concentrated on particular imaging techniques, like as OCT imaging or anterior segment OCT imaging. It is important to note that these limited datasets may not accurately capture the range of differences and intricacies seen in actual patients with cataracts. Lack of External Validation: Certain studies exclusively relied on the same dataset for both training and testing, without incorporating external datasets to validate the model.51 The absence of external validation can result in an inflated evaluation of the model’s performance and limit the practicality of the findings. Inadequate Comparative Analysis: While Cheng et al.112 conducted a comparison between deep learning and traditional machine learning algorithms, many publications did not evaluate their proposed approaches against established procedures or alternative algorithms. This limitation hampers the ability to assess the superiority or additional value of the suggested models.126 Moreover, these research predominantly concentrated on evaluating algorithm performance metrics, such as accuracy, precision, and sensitivity, using certain image datasets, without furnishing details on real-world clinical validation or the influence on patient outcomes. The selection of studies for this compilation may have been influenced by publication bias, which could have limited the coverage of the complete body of literature on the application of AI and ML techniques for cataract diagnosis.127,128 The potential bias in this study may have been influenced by the exclusion of research that had contradictory or unfavourable findings. When interpreting the findings of specific studies, it is essential to take these limitations into account. Additional research is necessary to further investigate these factors, which will improve our comprehension and the actual implementation of AI and/or ML in cataract diagnosis.
To overcome these constraints and make future progress in the field, we suggest carrying out research using extensive and varied datasets that include a wider spectrum of patients with cataracts. In addition, researchers should prioritise the significance of external validation in order to evaluate the applicability and reliability of the suggested models. Conducting comparative assessments of established methods and alternative algorithms would enhance our understanding of the performance and value of the proposed alternatives. Furthermore, it is imperative for future research to integrate practical clinical verification and examine the influence of artificial intelligence (AI) and/or machine learning (ML) on patient results. Efforts should be directed towards overcoming publication bias by including a wide range of studies, irrespective of their outcomes, in order to present a more equitable perspective of the field. By implementing these suggestions, we can enhance the understanding and practical application of artificial intelligence (AI) and machine learning (ML) in the diagnosis of cataracts.
This study offers a succinct overview of the information pertaining to the detection and evaluation of cataracts. Cataracts impact multiple ocular tissues, such as the cornea, iris, pupil, lens, and retina. Recent research has emphasised the influence of cataracts on the lens, resulting in alterations in visual acuity and the requirement for modifications in eyewear.42,45,129,130 Eye specialists utilise corneal topography to identify irregularities on the surface of the cornea, which may suggest the presence of cataracts.131 Additional possible diagnostic indicators for cataracts encompass alterations in pupil size, sensitivity to light, perception of colours, and opacity of the lens in the iris.32,132–135 Cataract formation frequently leads to visual symptoms, including diminished contrast sensitivity and heightened susceptibility to glare. These symptoms are affected by the size and reactivity of the pupil. Systematic pupillometry has demonstrated potential in monitoring the advancement of cataracts and selecting the most favourable timing for therapies.133,135,136 Ophthalmologists have a vital role in precisely identifying cataracts by doing a comprehensive examination of the lens. Methods such as Scheimpflug and anterior segment optical coherence tomography (OCT) provide for accurate imaging of the lens, making it easier to identify lens opacities.61,97 Optical Coherence Tomography (OCT) is a robust imaging technique commonly used in medical and scientific environments. Nevertheless, it is important for researchers to acknowledge the limits associated with its application in their research. These limitations include constraints on depth, suboptimal performance in highly scattering tissues, limited lateral resolution, and a confined field of view. Artefacts have the capacity to modify OCT images, which can make it challenging to interpret them.40,137,138 Additionally, OCT may exhibit inadequate contrast in some tissues and could be costly and challenging to get in certain research settings.101 Despite the aforementioned constraints, OCT proves valuable in numerous scientific domains, notably ophthalmology, dermatology, and cardiology. Researchers can overcome these constraints by employing sound experimental design, employing effective image processing techniques, and validating their findings through the use of alternate imaging techniques. Gaining a comprehensive understanding of the advantages and drawbacks of optical coherence tomography (OCT) is crucial for making well-informed assessments in research endeavours. Cataract symptoms frequently manifest in the retina, namely in the macula. Ophthalmic examination and imaging techniques aid in the detection of alterations in lens transparency, lens disc opacity, and concomitant retinal diseases. Cataracts have a substantial impact on visual function, namely on visual acuity, contrast sensitivity, and the health of the retina.136 Visual field and contrast sensitivity are useful for detecting cataract-related problems. Healthcare personnel must possess a comprehensive understanding of different ocular components and their role in the development of cataracts. This information is essential for promptly identifying the condition, offering appropriate treatment choices, and educating patients.139 An in-depth analysis of ocular tissues, including the cornea, iris, pupil, lens, and retina, is crucial for the early identification of cataracts. This examination also offers vital information about the development, advancement, and management of cataracts.63,93,94,133
Nevertheless, there are specific constraints that need to be acknowledged when conducting diagnostic research on cataracts. Selection bias arises when datasets fail to sufficiently encompass the full patient population, resulting in the over- or underrepresentation of particular groups.98 The lack of consistency in the diagnostic criteria, measurement methodology, and outcome assessments makes it difficult to compare the data.98,136 The dataset restrictions diminish the statistical power and can lead to imprecise or inconclusive findings. More extensive datasets with increased statistical power are necessary to evaluate the effectiveness of the diagnostic measures.113,114,140,141 The dataset restrictions diminish the statistical power and can lead to imprecise or inconclusive findings. More extensive datasets with increased statistical power are necessary to evaluate the effectiveness of the diagnostic measures.84,142–144 Furthermore, the research findings cannot be extrapolated to different demographic groups due to the limited diversity of the photos utilised for cataract diagnosis. This include concerns related to resolution, age, race, and other medical conditions.132,136 It is essential to verify the accuracy of the diagnostic algorithms by utilising varied datasets or real-world clinical environments.49,127 These limitations undermine the reliability, usefulness, and generalizability of research on cataract diagnosis. Overcoming these constraints is vital for improving cataract diagnosis. It is essential to acknowledge and overcome these obstacles when proposing alternative techniques. To address potential selection bias, researchers should ensure that sample datasets represent a larger study population and avoid the disproportionate inclusion of specific subgroups.145 Standardised methods and methodologies enhance the comparability of the data and the reliability of the conclusions drawn from them. Increasing the sample size through multicentre studies and collaborations enhances statistical power, reduces errors, and improves diagnostic precision.105,121
Longitudinal studies, which monitor patients over a period of time, are essential for comprehending the progression of cataracts, the effectiveness of treatments, and the lasting consequences. A diverse array of research viewpoints and external validation are needed for a thorough analysis. To mitigate publication bias, researchers and academic publications should strive to disseminate both positive and negative findings. Adopting pre-registration and transparent reporting practices can decrease publication bias and enhance the precision and efficacy of diagnostic techniques. Evaluating the effectiveness of new treatments requires making comparisons with established diagnostic approaches. Assessing the viability, cost-effectiveness, and patient outcomes is essential for establishing the efficacy of diagnostic procedures. Long-term evaluations of diagnostic procedures are necessary to comprehend their influence on visual function, effectiveness of treatment, and general quality of life. To improve the accuracy and reliability of cataract diagnosis, researchers can overcome these restrictions, resulting in better patient outcomes and developments in the profession.
For preprocessing and classifying eye images with the VGG19 model in (i.e., Figure 3), there is a methodical process that makes use of the model’s deep learning abilities. To start, images of the eyes are put into groups based on their unique physical features (cataract or non-cataract) to create a cataract classification. We suggested using the powerful VGG19 model, a Convolutional Neural Network (CNN), to do a number of tasks as part of a deep learning workflow. These tasks include finding anomalies, making heatmaps, and classifying them. The process starts with loading a image of the eye using libraries such as OpenCV or PIL. This is followed by steps to prepare the image so that the region of interest (ROI) can be found by cropping it. After that, the VGG19 model is used to extract features and find anomalies. This lets the ROI be analysed for anomalies using the recovered features. Then, using tools like Grad-CAM, a grid is made to find these strange patterns. The calculated cataract score is shown on a visual representation of the abnormalities that have been found. Image processing methods are also used to do other tests, such as measuring the pupil and lens density. Pixel intensity and other criteria are used to decide the end classification. This makes sure that the process of categorising is thorough and correct.
This section offers a comprehensive description of the dataset that was used, including how it was prepared, implemented, evaluated, and how the high-performing model compares to other models in terms of performance. This study use the Ocular Disease Intelligent Recognition (ODIR) database from Kaggle.com. The database consists of organised ophthalmic data for 5,000 patients, including demographic details like age and colour fundus images of both eyes. Medical staff enhanced each patient record by adding diagnostic keywords. Shanggong Medical Technology Co., Ltd. created a database that has an exact copy of patient information collected from various hospitals and medical locations around China. Fundus images exhibit different levels of resolution due to the use of various camera combinations. An adept ophthalmologist meticulously annotated the photographs. The eight patient categories include normal vision, diabetes, glaucoma, cataract, age-related macular degeneration, hypertension, pathological myopia, and other diseases. To enhance the identification and categorization of cataract conditions, a specific group of 2168 images was chosen for further examination. This subset exclusively includes images from two categories: Cataract (C) with 1168 images and Normal (N) with 1000 images. A total of over 2000 images underwent a curation procedure in which noisy and unclear ones were deleted. The preparatory stages involve the following steps: downsizing the photographs by 75×75 pixels to standardise their dimensions according to the model’s specifications and make computation easier. Normalisation is a procedure that transforms the pixel values of an image to a standard range, usually from 0 to 1. This might potentially enhance the accuracy of the model and provide a consistent training process. The models receive training for a period of 50 epochs. These images are meant to be used for further research purposes. The preprocessed dataset consists of little over 2,000 colour fundus images that represent demographics and either cataract or non-cataract conditions. This dataset is used for training, validation and/or testing purposes. The data was carefully structured and separated into training and testing sets. The diagnostic criteria were assigned numerical values, with 0 representing normal eyes and 1 indicating the presence of cataracts. The appropriate word for this process is label encoding. We employed a singular splitting technique to measure the classification’s performance, utilising an 80% training set and a 20% testing set. We expedited the process of model training and experimentation by leveraging Google Collab in tandem with a T4 GPU. We developed models after preprocessing the dataset, the VGG19 classification performance and predictive performance was compared with the following deep learning models ResNet50, DenseNet201, Inceptionv3, and EfficientNetB0 using Python (Google Collab) and the Keras framework. To enhance the performance of the suggested model, we employed Adam Optimizer with a batch size of 15 and a learning rate of 0.001. Evaluating the model, we consider accuracy, recall (also known as sensitivity or true positive rate), precision (the model’s ability to correctly identify positive cases), and F1 score (a metric that combines recall and precision to provide a balanced representation of the model’s performance by calculating their harmonic mean). The following provides a description of them:
In the context of equation 1,2,3 and 4, TP represents true positive, TN represents true negative, FP represents false positive, and FN represents false negative. The effectiveness of the paradigm being evaluated by calculating Accuracy, Recall, Precision and F1_score: We provide a thorough evaluation of the VGG19 model’s effectiveness in this section. We conduct a comparative analysis of our model and eight other pretrained models for the purpose of cataract detection and classification, using datasets that are exactly the same and standard size. The experimental results are compared with the latest improvements in cataract detection and classification approaches.
The aggregated empirical findings (i.e., Table 4) demonstrate that the proposed methodology attained a binary classification accuracy of 93.12% (using VGG19) within a mere 50 epochs, effectively distinguishing between normal and cataract states. Based on the situation, demonstrated bythe confusion matrix in (i.e., Figure 4), the model produced the following predictions: 5 cases were predicted as false negatives (FN). This means that all real cataract cases were correctly detected by the model, indicating that no cataract diagnoses were missed. The model incorrectly identified 10 instances as cataracts when they were actually not cataracts (False Positive - FP). Additionally, there were 15 occurrences that the model did not identify as cataracts, even if they were. 85 Predicted Cases (TP): These cases correspond to actual cataracts that were correctly identified by the programme. The 118 anticipated cases in are classified as true negatives, indicating that the algorithm correctly recognised the absence of cataracts. The results demonstrate that the model successfully decreases the frequency of missed cataract diagnoses (FN), ensuring that no authentic cataract cases are disregarded. However, labelling non-cataract cases as cataracts (FP) may be overly sensitive. To enhance the accuracy of the model in predicting cataracts, it is recommended to fine-tune it, specifically to reduce the occurrence of False Positive (FP) cases. This adjustment will lead to a model that is more precise and reliable. To assess efficiency in terms of model size, number of layers, and average execution time, all pretrained models were compared to the proposed model. The results of this comparison are displayed in (i.e., Table 4), Comparison of existing and high-performing (VGG19) models. The average execution time is 212 milliseconds, indicating its suitability for real-time or near-real-time jobs that need quick inference. The specified characteristics enhance the efficiency of the suggested model’s memory and computing resources, while also maintaining excellent performance in many tasks such as image recognition, classification, and other relevant activities. Furthermore, the VGG19 has satisfactory performance when dealing with small datasets. The result below (i.e., Figures 4 and 5) for the high performing and proposed deep learning VGG19 model, displayed metrics confusion matrix, the ROC curve, model loss and model accuracy.
In order to improve the effectiveness and precision of our model’s predictions, we might consider adding additional epochs to the training phase. This enhances the model’s ability to capture intricate patterns, hence enhancing its capabilities. While extending the training by adding more epochs can bring benefits, it is crucial to maintain a balanced and nuanced approach. Overfitting can occur when the model is trained for too many epochs, causing it to struggle in adjusting to new data. We conducted a preliminary investigation consisting of fifty epochs. Although we recognise that this may not be sufficient, our aim is to increase the number of epochs in order to achieve higher accuracy while avoiding overfitting. To evaluate our model’s performance, we analyse the Generalisation Gap, which quantifies the difference between the accuracy acquired during training and the accuracy achieved during validation. Desirable is a small difference, as it indicates the model’s effectiveness in generalising. Our model has achieved a low and sensible generalisation gap, with a validation accuracy of 0.9312 and a training accuracy of 0.9310. The VGG19 model has a generalisation gap of 0.0002, indicating that it demonstrates satisfactory generalisation when making predictions on a new dataset.
This study has found various constraints and difficulties linked to cataract detection methods. Although these tactics show potential, they encounter substantial barriers that impede their effective execution. In order to optimise the advantages of these methods, it is essential to acknowledge and tackle their constraints. This section delves into the primary impediments and limitations that impact the proposed strategies.
A significant barrier is the limited availability of financial and infrastructure resources, which hinders the extensive utilisation of advanced imaging technology and artificial intelligence (AI) algorithms for the identification of cataracts. The presence of inadequate resources and healthcare services in various areas leads to inequity, which in turn hampers the implementation of diagnostic technology. Proficient training and expertise among healthcare practitioners are crucial for the effective implementation of new imaging technology and artificial intelligence techniques. Nevertheless, attaining expertise in these technologies demands a significant amount of time and presents difficulties in terms of learning skills and ongoing education. To tackle these problems, a substantial investment in training and professional development is necessary. Although public awareness initiatives and educational programmes have the potential to be helpful, it is still a big barrier to get widespread patient information and engagement. Patients’ participation in and compliance with screening activities can be impeded by linguistic hurdles, cultural attitudes, and socioeconomic inequities. Conquering these challenges is essential for the achievement of these undertakings.
Achieving consistency in diagnostic criteria and methodologies across varied healthcare settings and geographic regions poses a significant challenge. Variations in the implementation and evaluation of diagnostic methods might undermine the dependability of the findings and hinder the establishment of globally recognised benchmarks. In order to close these disparities and promote the establishment of standards, it is imperative to foster cooperation among healthcare experts and stakeholders. Ensuring the acquisition and handling of delicate medical information is crucial for the use of AI technology; nonetheless, this gives rise to apprehensions over confidentiality and data security. Ensuring patient confidentiality and preventing unauthorised access and breaches are crucial factors to be taken into account. Implementing stringent data privacy and security protocols is an intricate undertaking that introduces novel obstacles. Achieving a harmonious equilibrium between the integration of technology and the protection of patient confidentiality necessitates the implementation of thorough strategies and unwavering compliance with privacy laws. Introducing new technology and collaborative care models must be in line with regulatory frameworks and legal norms. This includes gaining the required authorization, addressing liability concerns, and complying with local regulations. Complicated administrative procedures and obstacles can cause delays and higher administrative workloads, requiring concerted efforts to effectively handle these regulatory challenges.
Incorporating novel methodologies and advanced technologies into the existing healthcare system necessitates meticulous strategic planning and collaborative effort. This may involve the cooperation of professionals from different disciplines, reorganising the structure of the company, and making modifications to the processes in healthcare settings. Nevertheless, it is crucial to recognise that these suggested modifications can encounter resistance or practical obstacles, highlighting the importance of competent leadership and active involvement from the key stakeholders to ensure successful execution. Thorough scientific validation and compelling evidence are required to ascertain the effectiveness and influence of these actions. Additional research is necessary to evaluate the efficacy, cost-effectiveness, and clinical results of these innovative methods. Conducting such investigations necessitates a substantial investment of time, resources, attention, and commitment to guarantee a thorough assessment. Notwithstanding these constraints, it is imperative to provide resources and engage in research and collaboration to surmount these obstacles. Investments in infrastructure and education can accelerate the identification of early cataracts. Healthcare institutions can achieve higher rates of detection, quicker therapeutic interventions, and better visual outcomes in individuals with cataracts by acknowledging and resolving these obstacles. The advantages of these endeavours surpass their constraints, emphasising the significance of further advancements in dependable cataract detection systems.
Promising advancements are anticipated in future research endeavours focused on the diagnosis and treatment of cataracts. Various approaches are presently under examination to improve the precision, dependability, and accessibility of diagnostic instruments and therapies for cataracts. Our conversation will centre upon the following areas:
Further investigation is required to comprehend how individuals with different levels of cataract actively pursue medical help in order to understand patient engagement. Having this knowledge is essential for identifying patients who may postpone or not fully utilise cataract therapy, which can result in vision loss or blindness. An essential aspect of expanding awareness, improving diagnostic accuracy, and promoting treatment accessibility is to investigate the root causes of patient hesitancy in seeking help. By implementing specific procedures, it is possible to successfully tackle this problem and enhance the diagnosis, treatment, and result of cataract patients.
Researchers are currently studying the combination of different datasets and modalities, such as wearable devices like spectacles and contact lenses, to improve the identification and monitoring of cataracts. Telemedicine systems and data-driven cataract detection tools have the ability to screen and monitor patients in faraway areas. Additional investigation in these domains has the potential to enhance efficiency and accessibility in the diagnosis and monitoring of cataracts.
Further research is needed to comprehensively investigate the influence of lifestyle factors, including dietary patterns, levels of physical activity, smoking habits, and exposure to UV radiation, on the development and advancement of cataracts. Assessing the efficacy of cataract screening and treatment strategies, particularly in underprivileged rural and low-income regions, is of utmost importance. Moreover, the investigation of alternative methodologies, such as gene and stem cell therapies, shows potential in the domain of cataract treatment.
Recent developments in diagnostic technologies have explored various noninvasive methods, including optical coherence tomography (OCT), machine learning algorithms, tear fluid analysis, spectroscopy, Raman spectroscopy, and hyperspectral imaging, to determine their potential in diagnosing and assessing the extent of cataracts. These groundbreaking techniques have the capacity to transform the process of identifying and assessing cataracts.
To summarise, addressing the challenges and restrictions in cataract diagnosis and treatment necessitates substantial academic study, public engagement, and technology advancement. Through the investigation of these study domains, healthcare professionals have the potential to enhance the precision, accessibility, and results of cataract therapy, ultimately augmenting the visual health and overall standard of living for individuals with cataracts. Persistent endeavours in this trajectory are crucial for the progression of cataract diagnosis and therapy.
To summarise, the improvement of cataract diagnosis and patient outcomes necessitates the resolution of the previously mentioned constraints and obstacles. In order to accomplish this objective, the following suggestions are put forth:
Sufficient financial resources should be allocated by governments, healthcare institutions, and stakeholders to ensure the availability of current imaging equipment, AI systems, and the essential infrastructure for cataract diagnosis. This technique effectively tackles limitations in resources and enables wider adoption of these solutions. It is imperative to create extensive training programmes that will provide healthcare practitioners with the requisite skills to proficiently utilise and comprehend modern imaging technology and AI methodologies. Continuing professional development courses are also advised to ensure that healthcare personnel stay updated on the newest breakthroughs in cataract diagnosis. Public awareness measures should be implemented through educational programmes to increase knowledge among the general population about the significance of timely detection of cataracts and frequent eye screenings. It is imperative to make concerted efforts to surmount linguistic and cultural obstacles in order to guarantee the dissemination of crucial information to marginalised people. Stakeholder Collaboration: The collaboration between researchers, physicians, industry specialists, and regulatory bodies is essential for the progress of cataract diagnosis and therapy. These collaborations have the potential to result in the creation of novel diagnostic tools, enhanced risk assessment methods, and cutting-edge imaging technology. Environmental factors, like as UV radiation and climate change, should be thoroughly examined to determine their influence on the development and advancement of cataracts. Additional investigation is required to comprehend the implications of climate change on ocular problems, specifically in regions with elevated temperatures. Randomised information collected from diverse environmental settings can offer useful insights into the extent and frequency of cataracts in different geographic regions. This, in turn, facilitates the creation of personalised approaches for the identification and treatment of cataracts. Further investigation is necessary to comprehend the correlation between immune-related disorders, such as rheumatoid arthritis, lupus, diabetes, and cataract formation. It is imperative to do research on the impact of these factors on the advancement of cataracts and the efficacy of remedial interventions. Essential to the development of efficient offline screening tools are those that are user-friendly, capable of swiftly capturing ocular pictures, and delivering diagnostic results. These gadgets would be advantageous for patients, particularly those residing in remote rural regions with restricted availability of professional healthcare services. Timely identification of cataracts can result in immediate medical treatment, enhanced results, and a decrease in the worldwide consequences of this ailment. Develop standardised diagnostic criteria and protocols: collaborative efforts are required to produce universally accepted diagnostic criteria, protocols, and guidelines for the diagnosis of cataracts. Implementing standardisation efforts will reduce inconsistencies in operating procedures and improve the integration of diagnostic methodologies. Ensuring the privacy and security of patient data: strong measures must be taken to guarantee the privacy and security of patient data. This entails implementing data privacy laws and creating robust mechanisms for the collecting, storage, and dissemination of data. Comprehensive investigation and verification are essential to assess the efficiency, cost-effectiveness, and clinical results of innovative methods for diagnosing cataracts. Accurate data should be used to guide the deployment of these therapies and to inform choices about healthcare policies.
Healthcare organisations can effectively overcome the strategic restrictions associated with cataract diagnosis by following these principles. The objective is to enhance the rate of early identification, expedite prompt treatment, and enhance visual results for persons with cataracts.
The fundus dataset used, which consists of 1000 photos of the fundus, is an open dataset.91 This dataset can be accessed using the following link: https://www.kaggle.com/datasets/linchundan/fundusimage1000.
All these 1000 fundus images which belong to 39 classes are come from the Joint Shantou International Eye Centre (JSIEC), Shantou city, Guangdong province, China.
The dataset is shared under a Database Contents License (DbCL) v1.0.
https://www.kaggle.com/datasets/akshayramakrishnan28/cataract-classification-dataset
Mendeley: The PRISMA checklist and Evaluation of searched papers of ‘An Assessment of Contemporary Methods and Data-Enabled Approaches for Early Cataract Detection’. https://doi.org/10.17632/y5hs9c24v3.1.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Partly
References
1. Esam Noori W, Albahri A: Towards Trustworthy Myopia Detection: Integration Methodology of Deep Learning Approach, XAI Visualization, and User Interface System. Applied Data Science and Analysis. 2023. 1-15 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Computer Sceince, AI, and ML
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 2 (revision) 02 Aug 24 |
|
|
Version 1 17 Aug 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
One of the most impressive aspects of this research is its meticulous analysis of data-enabled approaches. The authors have effectively demonstrated how leveraging big data, machine learning, and artificial intelligence can significantly enhance the accuracy and early detection of cataracts. The paper's exploration of these advanced technologies is both insightful and forward-thinking, illustrating the potential for these methods to transform the landscape of ophthalmology.
One of the most impressive aspects of this research is its meticulous analysis of data-enabled approaches. The authors have effectively demonstrated how leveraging big data, machine learning, and artificial intelligence can significantly enhance the accuracy and early detection of cataracts. The paper's exploration of these advanced technologies is both insightful and forward-thinking, illustrating the potential for these methods to transform the landscape of ophthalmology.