Keywords
Dental implant, Fibroblasts, Metal Nanoparticles, Peri-implantitis, Titanium
This article is included in the Nanoscience & Nanotechnology gateway.
This article is included in the Advances in Fibroblast Research collection.
This article is included in the Nanotoxicology collection.
Despite the success of titanium (Ti) implants in dental rehabilitation, emerging evidence implicates the release of titanium oxide nanoparticles (TiO2NPs) from the implant surface as a potential contributor to the initiation of the pathogenic process leading to peri-implantitis and ultimately failure of the implants. However, a comprehensive investigation to elucidate the dose-dependent effects of TiO2NPs on cytotoxicity and inflammatory responses is lacking. The present study was undertaken to evaluate the effect of different concentrations of TiO2NPs on the cytotoxicity and inflammatory cytokine expression in Human Gingival Fibroblasts (HGFs).
HGFs were isolated from gingival tissue samples obtained from periodontally and systemically healthy subjects. Ti standard solution for ICP was diluted to create concentrations of (0.001 ppm, 0.01 ppm, 0.1 ppm, 1 ppm, 10 ppm, and 100 ppm) for cell culture media containing titanium. HGFs were then cultured in these varying concentrations for specific time periods (days 1, 3, 5, and 7) to assess cell viability. A cytotoxicity assay was performed to determine the levels and expression of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) and anti-inflammatory cytokines (IL-10, IL-12, TGF-β) using qRT-PCR and ELISA techniques.
Our findings demonstrate a concentration and duration-dependent decrease in HGF viability upon exposure to titanium nanoparticles. Notably, a 50% reduction in cell viability was observed at the highest concentration (100 ppm). qRT-PCR analysis revealed a significant upregulation of pro-inflammatory cytokines, particularly IL-1β and IL-6, in HGFs exposed to titanium. Interestingly, the expression of anti-inflammatory cytokines (IL-10, IL-12, and TGF-β) remained comparable or even equivalent compared to controls across different titanium concentrations.
The study revealed a concentration and duration-dependent influence on HGF viability and cytokine profile, suggesting potential cytotoxicity and modulation of the inflammatory response mediated by TiO2NPs. Further research is necessary to elucidate the underlying mechanisms and their implications for dental implant biocompatibility.
Dental implant, Fibroblasts, Metal Nanoparticles, Peri-implantitis, Titanium
Dental implants have become a cornerstone of modern dentistry, offering a reliable and long-term solution for tooth replacement. However, concerns regarding the failure rates of even osseointegrated dental implants continue to be a focus of research.1
The peri-implant tissue encompasses a well-defined zone encircling the implant body, typically extending approximately 1 millimeter in width.2,3 This critical interface, known as the peri-implant space, facilitates crucial interactions between the implant and the surrounding soft tissue (peri-implant mucosa) and hard tissue (alveolar bone).4,5 However, a delicate balance exists within this space. Various local and systemic factors, including bacterial biofilms, implant biomaterial and surface characteristics, and prosthetic factors, can all disrupt the implant-tissue interface by inducing an inflammatory reaction. This disruption leads to peri-implantitis, a well-established complication associated with dental implants and a leading cause of bone loss and implant failure.6–8
Titanium (Ti) is the most widely used material for dental implants due to its excellent mechanical properties and bio-inertness.9 However, recent advancements in understanding the biological and mechanical complexities of implant failure have shed light on a detrimental link between titanium release and the development of peri-implantitis. This association includes potential contributions from hypersensitivity reactions to implant materials, tribocorrosion processes at the implant-tissue interface, and the release of nanoparticles, due to fretting (type of residual stress generated by tangential micromotion at areas of contact with normal tension) and/or micromovements during mastication as well as due to triggering of surface oxidation caused by acidic environments triggered by biofilm and/or biofilm induced inflammatory process7,10–13
The presence of titanium particles and their degradation products has been increasingly documented within the peri-implant environment and surrounding tissues of patients with dental implants. These particles, identified in bone, peri-implant soft tissues, draining lymph nodes, and even lungs and spleen, are widely attributed to originate from the implant itself.14–16 Of particular concern are the unique physicochemical properties of titanium nanoparticles (Ti NPs), especially their small size and ability to interact with biological systems. These characteristics raise the possibility of Ti NPs breaching the blood-brain barrier (BBB) and potentially accessing the central nervous system (CNS).17,18
While titanium dioxide nanoparticles (TiO2 NPs) have been implicated in various adverse health outcomes, the primary focus of research has been on pulmonary toxicity.19 Emerging evidence suggests TiO2 NPs also exert significant immunotoxicity, manifesting as lymphocyte over-proliferation, macrophage infiltration, and elevated pro-inflammatory cytokines like IL-1β, IL-6, and TNF-α.20 Mechanistically, pro-inflammatory responses, reactive oxygen species (ROS) generation, and dysregulated autophagy are postulated to contribute to TiO2 NP-induced immunotoxicity.20 However, the precise mechanisms underlying these effects remain elusive.19
The periodontium, a connective tissue organ with an epithelial covering, plays a critical role in implant success.21 Fibroblasts, the predominant cell type in the periodontium, influence the health of both the implant and surrounding tissues.21 Studies suggest a link between titanium particles and peri-implantitis, an inflammatory condition around dental implants.22 While titanium particles have been found in both inflamed and non-inflamed tissues,17,23 their precise role in inflammation remains unclear. in vitro studies have demonstrated the influence of TiO2 NPs on cell viability and gene expression in various cell types.24–27
However, the concentration-dependent effects of TiO2 NPs on human gingival fibroblasts, key players in wound healing and tissue homeostasis, are yet to be explored. This research aims to address this gap by investigating the cytotoxicity and cytokine expression of human gingival fibroblasts exposed to varying concentrations of TiO2 NPs
The study was conducted with ethical approval from the institutional review board and all procedures were performed following the ethical standards of the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained and gingival tissue samples were collected from a total of three (n=3) systemically and periodontally healthy participants who reported to the Department of Periodontology. All participants were between 18 and 30 years old and presented with probing depths ≤ 3 mm, no clinical attachment loss, and less than 10% bleeding sites.
Gingival tissue explants (n=3) were collected and stored in a sterile saline solution. Following transport, tissues were rinsed extensively with phosphate-buffered saline (PBS) (Gibco-Invitrogen, Grand Island, NY, USA, Cat No. 21300-025) and sectioned into small pieces (approximately 1 × 1 mm). The tissue explants were then placed in culture dishes containing Dulbecco’s Modified Eagle Medium (DMEM) (Gibco-Invitrogen, Grand Island, NY, USA, Cat No. 11995065, 500 ml bottle) supplemented with 10% fetal bovine serum (FBS),(Gibco-Invitrogen, Grand Island, NY, USA, Cat No. 10270106), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco-Invitrogen, Grand Island, NY, USA, Cat No. 15140122). Cultures were maintained in a humidified incubator at 37°C with 5% CO2. Once adherent and confluent, gingival fibroblasts were detached using 0.25% trypsin-EDTA (Gibco-Invitrogen, Grand Island, NY, USA, Cat No. 25200072) solution and sub cultured at a 1:4 ratio in fresh medium. The culture medium was replaced every 3-4 days to maintain optimal growth conditions.
Commercially available titanium oxide nanoparticles (TiO2 NPs) with a particle size less than 100 nm (Sigma-Aldrich, St. Louis, MO, USA, Cat No. 700347, 25 gram) were used for this study. A standard solution of TiO2 NPs was prepared in the cell culture medium at a concentration of 1000 ppm (Ti concentration = 1000 ppm in 20% HCl solution). The concentration of titanium in the stock solution was verified using inductively coupled plasma optical emission spectrometry (ICP-OES). This stock solution was serially diluted to obtain working concentrations for the experiments, such as 0.001 ppm, 0.01 ppm, 0.1 ppm, 1 ppm, and 10 ppm. The pH of the culture medium was adjusted to maintain physiological conditions (~7.2) after the addition of TiO2 NPs. Based on a cytotoxicity assay, concentrations of 0.01 ppm, 0.1 ppm, and 10 ppm were selected for further investigation of their effects on inflammatory cytokine expression in gingival fibroblasts.
The cytotoxicity of TiO2 NPs towards HGFs was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (HiMedia, Mumbai, India Cat No. RM1131) Briefly, HGFs were seeded in 96-well plates and allowed to adhere overnight. Cells were then exposed to varying concentrations of TiO2 NPs (e.g., 0.001 ppm, 0.01 ppm, 0.1 ppm, 1 ppm, and 10 ppm) for 1, 3, 5, and 7 days. Following incubation with MTT, the formazan crystals formed within viable cells were solubilized, and the absorbance was measured at a wavelength of 570 nm using a microplate reader (Thermo Scientific Multiskan™ FC Microplate Reader). The quantity and quality of the isolated RNA were assessed using a spectrophotometer (e.g., Nanodrop ND-1000) by measuring the absorbance at 260 nm and 280 nm. Subsequently, cDNA synthesis was performed using a reverse transcription kit (e.g., RNA PCR Core Kit) according to the manufacturer’s instructions. Briefly, PrimeScript RTase was used to convert RNA to cDNA at 42°C.
The synthesized cDNA was employed as a template for qRT-PCR to quantify the mRNA expression levels of target genes encoding interleukin (IL)-1β, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, IL-6, IL-4, transforming growth factor (TGF)-β, and IL-10. Specific primers for these target genes were designed using appropriate software to ensure optimal amplification efficiency and specificity. The primer sequences are presented in the Extended data.41
The qRT-PCR reactions were performed using a commercially available SYBR Green master mix kit (e.g., SYBR Green FastMix Kit) following the manufacturer’s protocol. A StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA) was used for thermal cycling and fluorescence detection. During qRT-PCR, SYBR Green dye fluoresces when bound to double-stranded DNA. As the PCR reaction progresses and amplifies the target DNA, the increasing amount of double-stranded DNA leads to a proportional increase in fluorescence signal. The StepOnePlus instrument monitors this fluorescence in real-time, allowing for the quantification of target gene expression throughout the PCR process.
To minimize non-specific amplification during reaction setup or pre-cycling steps, Takara Ex Taq HS polymerase (Takara Bio Inc., Kusatsu, Japan) was included in the reaction mixture. This hot-start polymerase variant remains inactive at room temperature, preventing the formation of primer-dimers or mispriming events until the reaction reaches the optimal activation temperature.
Following qRT-PCR, the amplified products exposed to various concentraions of TiO2 NPs, were loaded on a 1.5% agarose gel stained with 0.5 μg/mL ethidium bromide in Tris-acetate-EDTA (TAE) buffer (HiMedia, Mumbai, India, Cat No. ML016). Electrophoresis was performed until the loading dye migrated approximately two-thirds of the gel length. The gel was visualized using a UV documentation system (Thermo Fisher Scientific) to verify the presence and size of PCR products for the target genes (IL-1β, IFN-γ, TNF-α, IL-6, IL-4, TGF-β, and IL-10).
The levels of secreted cytokines (IL-1β, TNF-α, IL-6, IL-4, TGF-β, and IL-10) were quantified in the culture supernatants of HGFs following exposure to TiO2 NPs for 24 and 72 hours using a commercially available ELISA kit (Thermo Fisher Scientific). The experiment was performed in triplicate for each sample. The absorbance of the samples and standards was measured at 450 nm using a Spectrophotometer.
Data are presented as mean ± standard deviation (SD) from at least three independent experiments. Statistical analysis was performed using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). One-way or two-way analysis of variance (ANOVA) was used to assess differences between groups, followed by Tukey’s post-hoc test for multiple comparisons. A p-value less than 0.05 was considered statistically significant.
Human gingival fibroblasts (HGFs) were used for evaluating the cytotoxicity and the expression of selected cytokines upon the exposure of different concentrations of titanium NPs (TiO2) at different durations. The representative image of HGFs showing the fibroblastic morphology is presented in Figure 1 (Extended data).
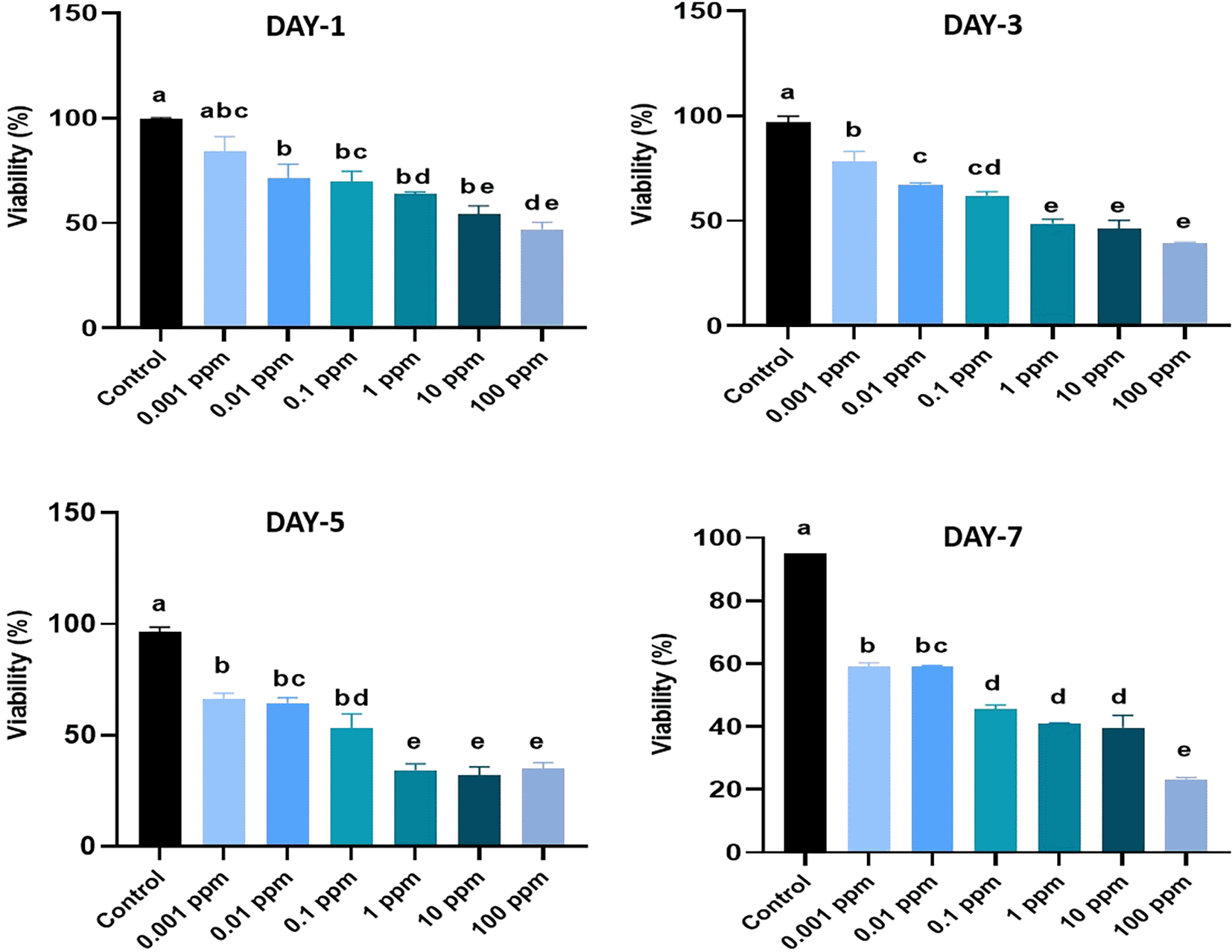
The values are presented as percentage mean ± standard deviation (SD). MTT assay was performed on day 1, 3, 5 and 7 of culture. The assay was conducted in triplicates. Different superscripts (a, b, c, d, and e) indicate a statistically significant difference at P<0.05. HGFs cultured in basal media were considered as a control.
The viability of the HGF cells were assessed as follows, On day 1, the viability of HGFs exposed to TiO2 at concentrations ranged from 0.001 ppm to 100 ppm varied from 49.5% to 78.9%. Except at 0.01 ppm, all other concentrations of TiO2-exposed HGFs showed significant (p<0.05) differences, with reduced viability compared to the control. It was also noted that 50% of the cells showed cytotoxic effects of TiO2 at higher concentrations of exposure, such as 10 ppm and 100 ppm of TiO2. However, no significant (p>0.05) differences in the viability values were observed in HGFs treated with TiO2 with concentrations ranging from 0.01 ppm to 1 ppm (Figure 1).
On day 3, the viability values of HGFs treated with TiO2 with 0.001 ppm to 100 ppm varied from 39.8% to 75.4%. A higher cytotoxicity effect of TiO2 with more than 50% of cell death was observed at concentrations of 1, 10 and 100 ppm.
On day 5, the exposure of HGFs to TiO2 at concentrations 1, 10 and 100 ppm resulted in the viability of 32.13%, 29.71% and 32.98% respectively. The highest viability of 66.1% and 68.2% were recorded in HGFs exposed to 0.01 ppm and 0.001 ppm of TiO2, respectively. However, the values were significantly lower when compared to the control (p<0.05).
On day 7, the viability of HGFs treated with TiO2 at concentrations ranging from 0.1 ppm to 100 ppm resulted in higher cytotoxicity with values varying from 46.47% to 22.84%. Further, the highest viability values of 59.92% at 0.001 ppm and 59.01% at 0.01 ppm were observed in HGFs when compared to the control (92.2%).
The proliferation rates of HGFs were analysed in terms of absorbance values measured at different time points following their exposure to TiO2 at varying concentrations. A higher absorbance value of 0.4347 was recorded in HGFs treated with 0.001 ppm of TiO2 and lowest absorbance value of 0.3225 was recorded in HGFs exposed to 100 ppm of TiO2. On day 3, the highest absorbance value of 0.4203 was recorded in HGFs treated with 0.001 ppm of TiO2 and the lowest absorbance value of 0.3044 was recorded in HGFs exposed to 100 ppm of TiO2. On day 5 the highest absorbance value of 0.3877 was recorded in HGFs treated with 0.001 ppm and the lowest absorbance value of 0.2655 was recorded in HGFs exposed to 10 ppm of TiO2.
On day 7, the highest absorbance value of 0.3654 was recorded in HGFs treated with 0.001 ppm and the lowest absorbance value of 0.2456 was recorded in HGFs exposed to 100 ppm of TiO2 (Figure 2).
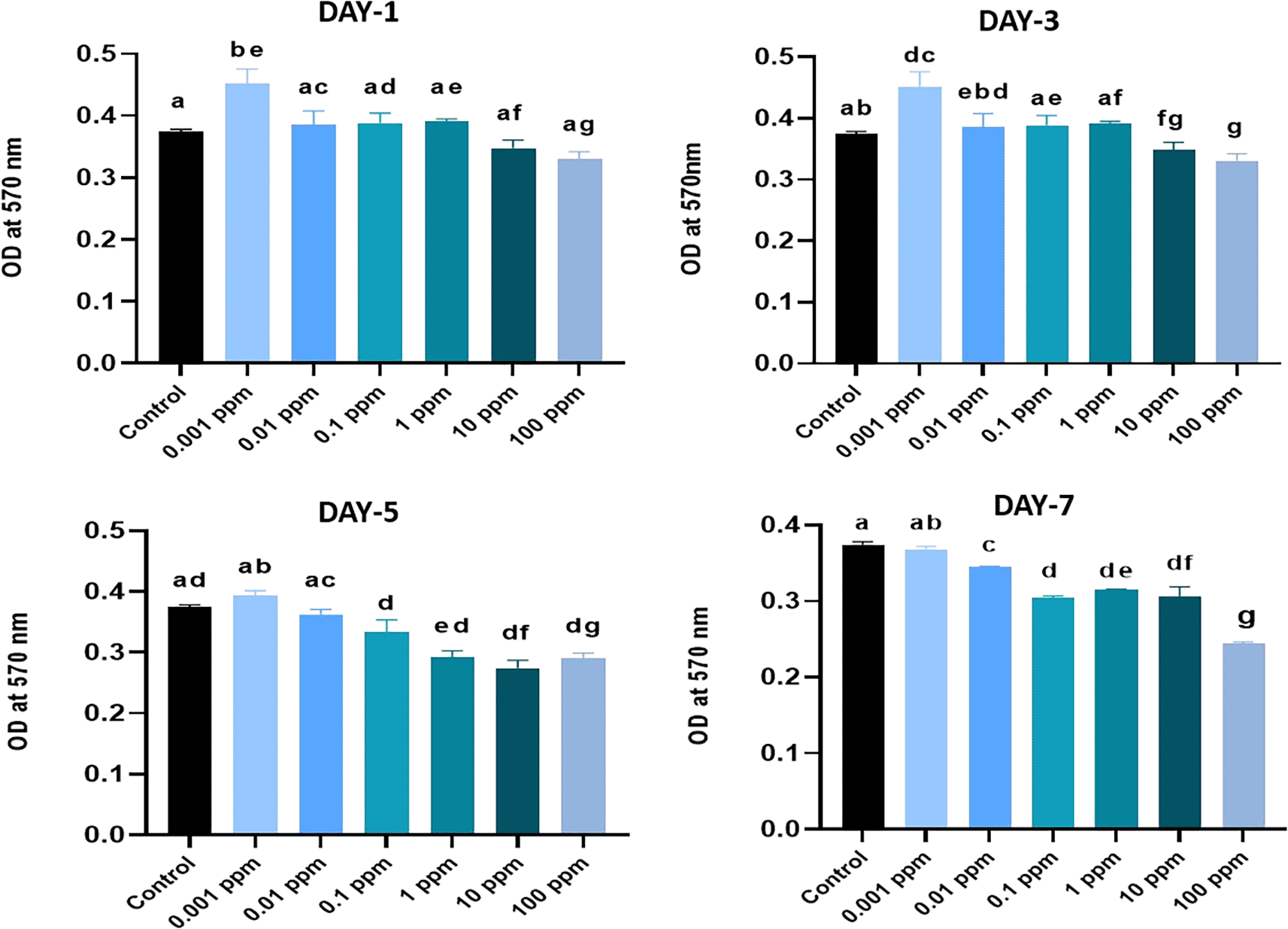
MTT assay was performed on day 1, 3, 5 and 7 of culture, and the optical density (OD) values are presented. The assay was conducted in triplicates. Different superscripts (a, b, c, d, e, and g) represent a statistically significant difference with P<0.05. HGFs cultured in basal media was considered as a control.
Expression of inflammatory cytokines by qRT-PCR
The effect of TiO2 on the mRNA expressions of selected proinflammatory and anti-inflammatory markers in HGFs exposed to selected concentrations, such as 0.01 ppm, 0.1 ppm, and 10 ppm was analyzed at 24 hrs and 72 hrs by qRT-PCR. The mRNA expressions of target cytokines were normalized to 18s rRNA which was used as a reference or housekeeping gene.
Expression of IL-1 beta analyzed at 24 hrs of exposure revealed that the expression of IL-1 beta was significantly (P<0.05) higher at 0.01 ppm. However, at 72 hrs, the expression levels were not significant (p>0.05) in TiO2-treated HGFs in all tested concentrations in comparison to control (Figure 3). Expression of TNF-alpha analyzed at 24 hrs was significantly (P<0.05) higher in control cells than in TiO2-exposed HGFs. However, at 72 hrs, the levels of TNF-alpha were not significant (p>0.05) in TiO2-treated HGFs in comparison to control (Figure 4). Expression of IL-6 analyzed at 24 hrs and 72 hrs following the exposure of HGFs to 0.01 ppm, 0.1 ppm, and 10 ppm of TiO2. The results indicated that the expression of IL-6 at both 24 hrs and 72 hrs of exposure was not statistically significant (p>0.05). However, the expression levels of IL-6 were higher in TiO2-treated HGFs than in control (Figure 5).
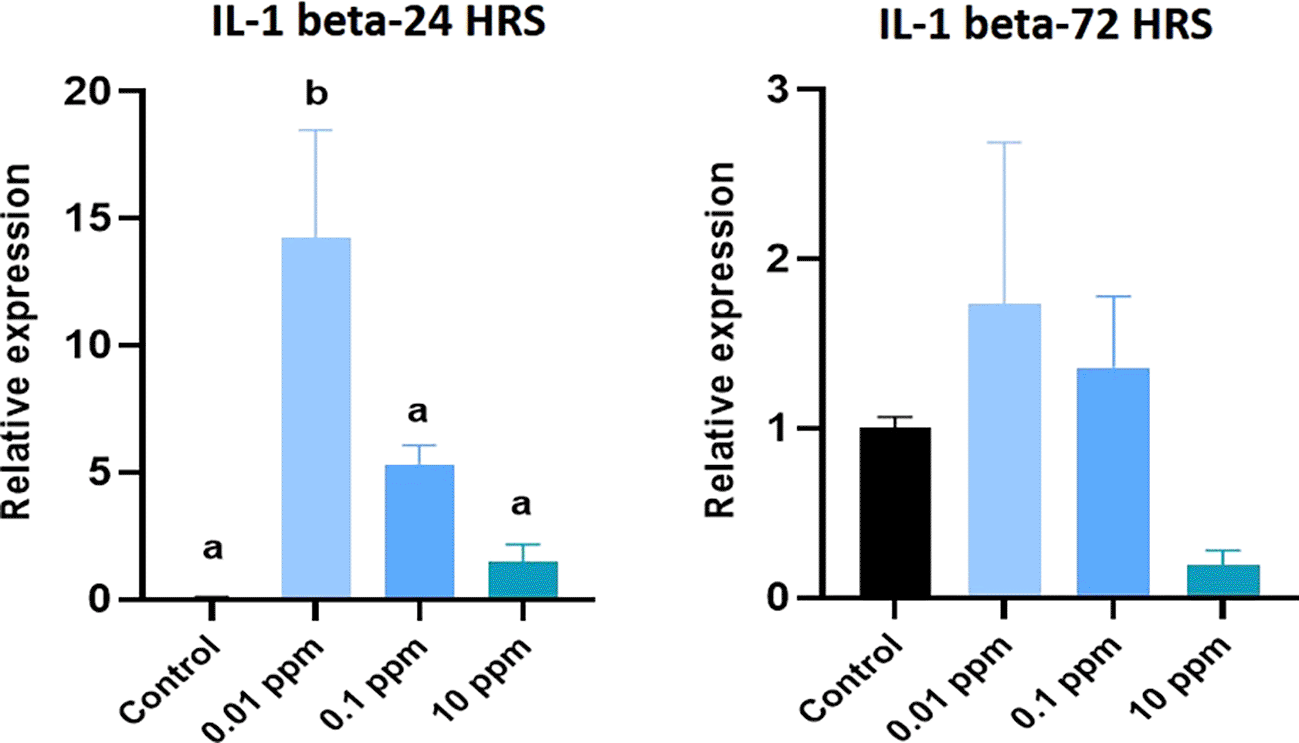
HGFs were stimulated with or without 0.01 ppm, 0.1 ppm and 10 ppm of TiO2. Gene levels of IL-1 beta at 24 hrs and 72 hrs were analyzed. The relative expression levels were normalized to the reference gene, 18s rRNA. The values are expressed as mean ± standard deviation (SD). Superscripts a and b indicate statistically significant differences at P<0.05.
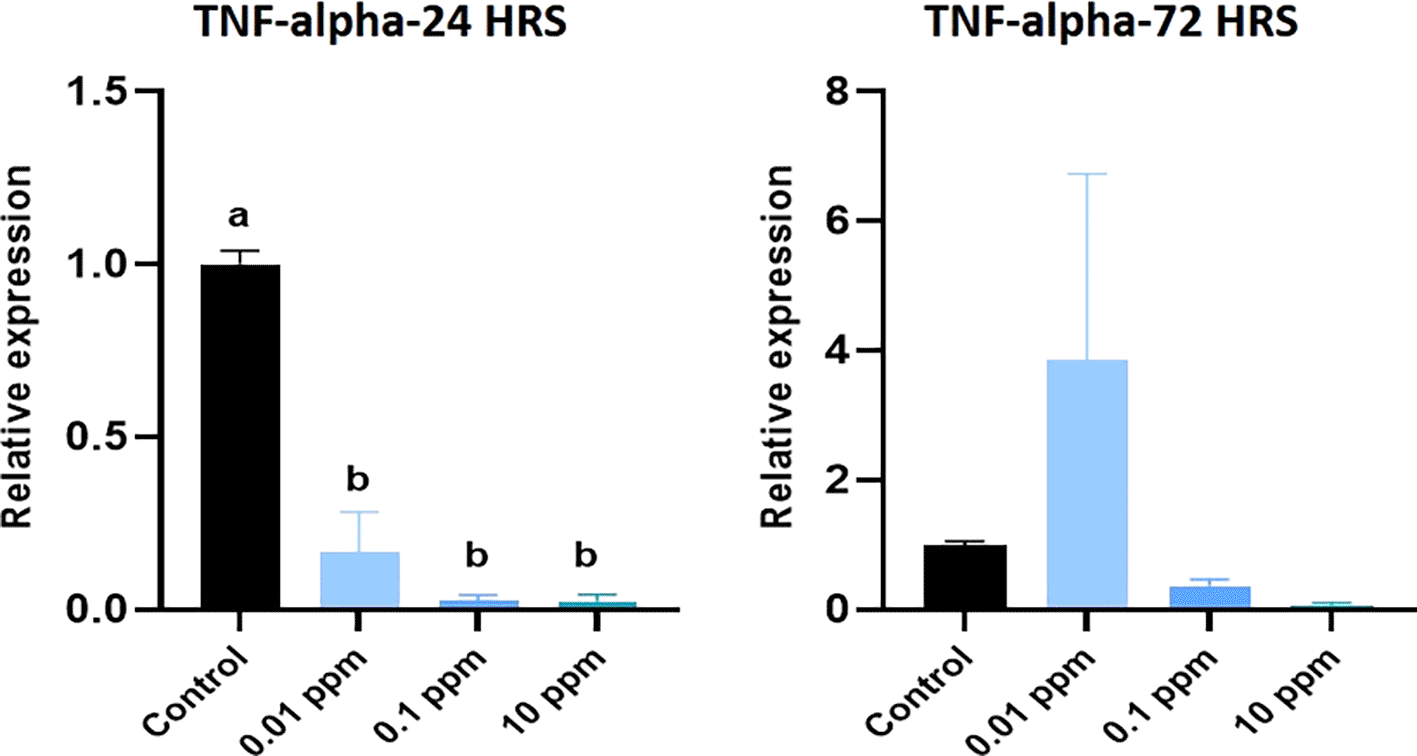
HGFs were stimulated with or without 0.01 ppm, 0.1 ppm, and 10 ppm of TiO2. Gene levels of TNF-alpha at 24 hrs and 72 hrs were analyzed. The relative expression levels were normalized to the reference gene, 18s rRNA. The values are expressed as mean ± standard deviation (SD). Superscripts a and b indicate statistically significant differences at P<0.05.
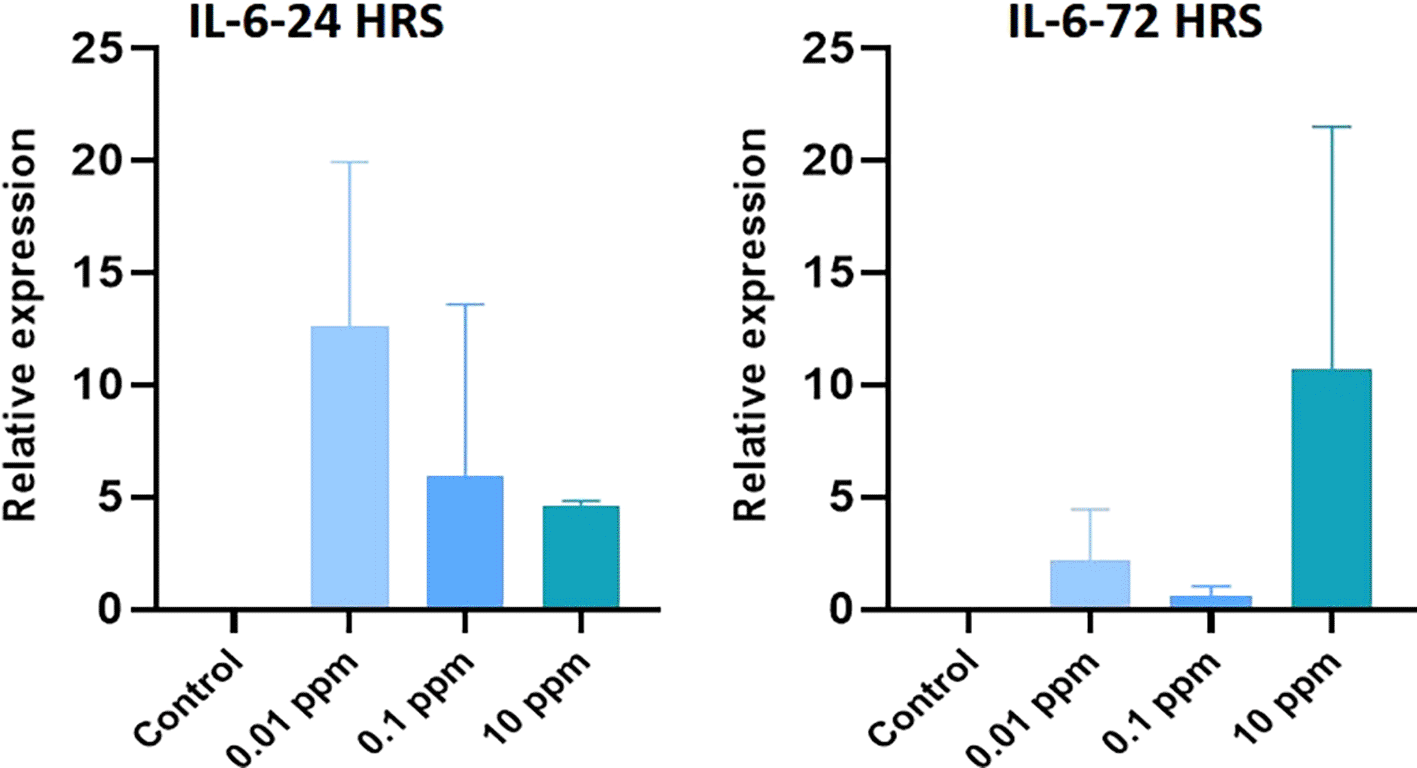
HGFs were stimulated with or without 0.01 ppm, 0.1 ppm and 10 ppm of TiO2. Gene levels of IL-6 at 24 hrs and 72 hrs were analyzed. The relative expression levels were normalized to the reference gene, 18s rRNA. The values are expressed as mean ± standard deviation (SD).
Expression of IL-10 analyzed at 24 hrs was significantly (P<0.05) higher in HGFs cultured with TiO2. Especially, TiO2 at 0.01 ppm resulted in higher expression of IL-10, and higher levels of 1 ppm and 10 ppm did not show any significant (P>0.05) difference in expression levels with control. At 72 hrs, a similar expression pattern of IL-10 was observed (Figure 6). Expression of TGF- beta analyzed at 24 hrs was not significant (p>0.05) in TiO2 treated HGFs when compared with control. However, at 72 hrs, the expression of TGF- beta was significantly (P<0.05) higher in controls with 0.01 ppm of TiO2 than in 0.1 ppm and 10 ppm (Figure 7). Expression of IL-12 analyzed at 24 hrs did not show any significant difference among TiO2 treated HGFs and Controls. However, at 72 hrs, the expression of IL-12 was significantly (P<0.05) higher in HGFs exposed to TiO2 than in control cells (Figure 8).
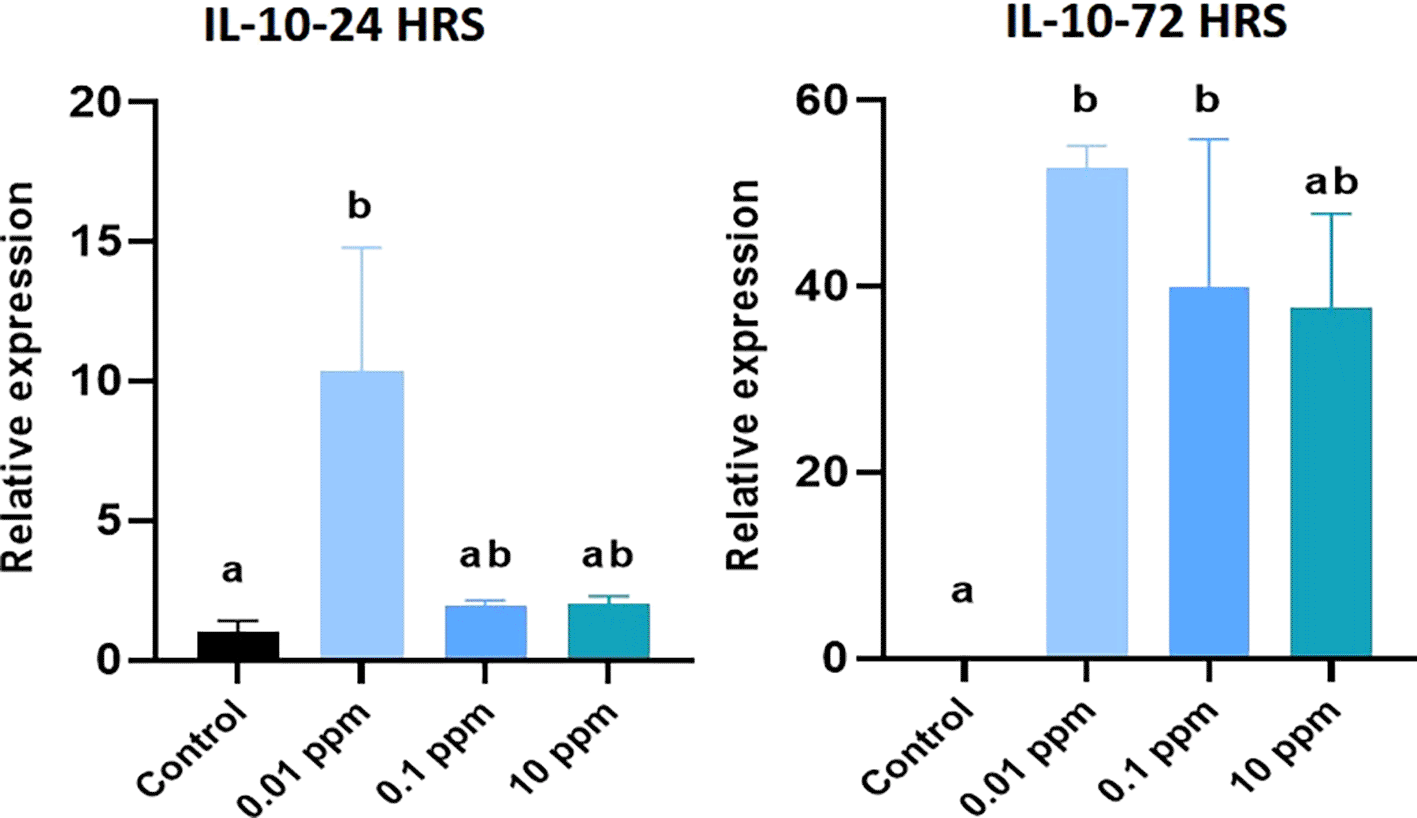
HGFs were stimulated with or without 0.01 ppm, 0.1 ppm and 10 ppm of TiO2. Gene levels of IL-10 beta at 24 hrs and 72 hrs were analyzed. The relative expression levels were normalized to the reference gene, 18s rRNA. The values are expressed as mean ± standard deviation (SD). Superscripts a and b indicate statistically significant differences at P<0.05.
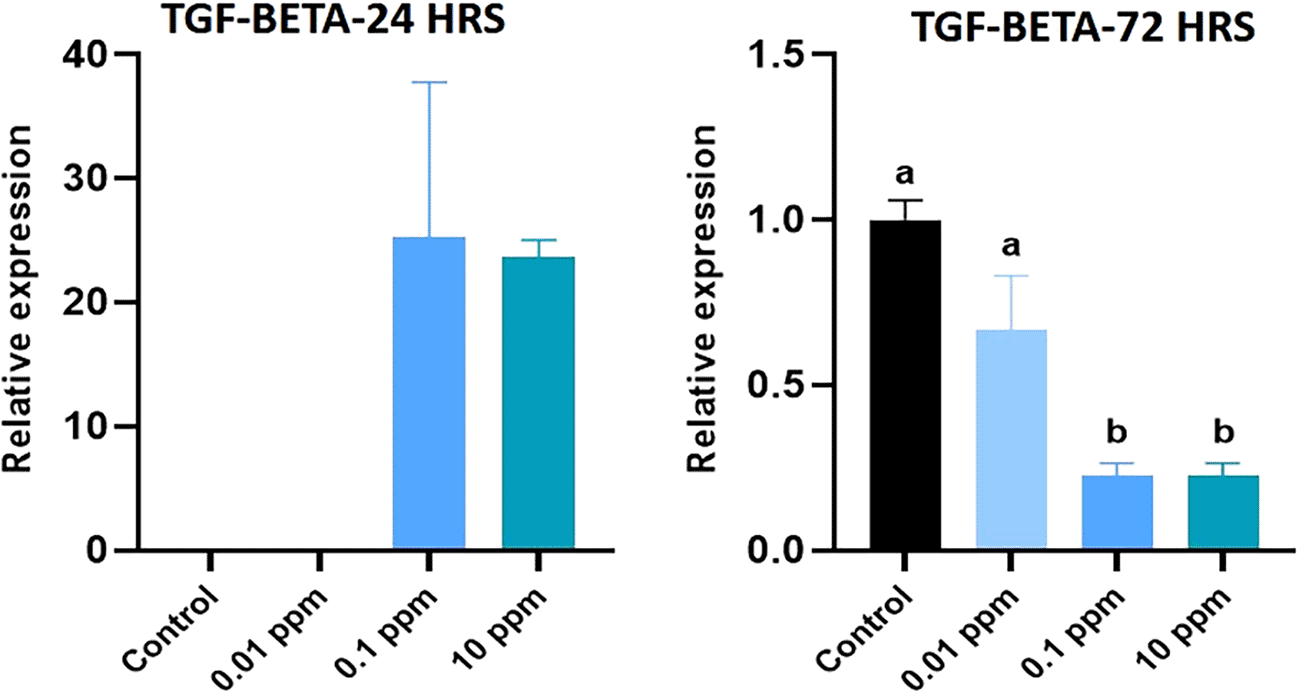
HGFs were stimulated with or without 0.01, 0.1 and 10 ppm of TiO2. Gene levels of TGF-beta at 24 hrs and 72 hrs were analyzed. The relative expression levels were normalized to the reference gene, 18s rRNA. The values are expressed as mean ± standard deviation (SD). Superscripts a and b indicate statistically significant differences at P<0.05.
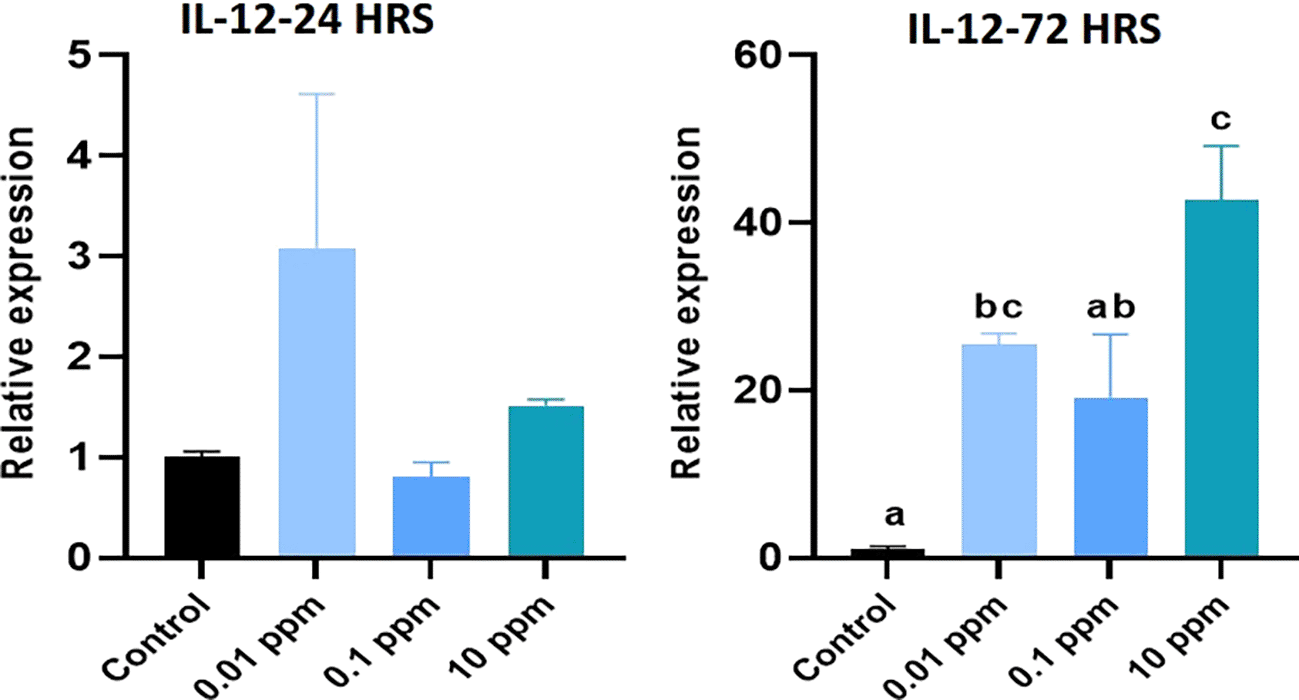
HGFs were stimulated with or without 0.01 ppm, 0.1 ppm and 10 ppm of TiO2. Gene levels of IL-12 at 24 hrs and 72 hrs were analyzed. The relative expression levels were normalized to the reference gene, 18s rRNA. The values are expressed as mean ± standard deviation (SD). Superscripts a and b indicate statistically significant differences at P<0.05.
The levels of IL-1 beta analyzed at 24 hrs and 72 hrs was not significant (P>0.05) in TiO2 treated HGFs in comparison to control. The level of TNF- alpha analyzed at 24 hrs and 72 hrs was not significant (P>0.05) in TiO2 treated HGFs in comparison to control cells. The level of IL-6 analyzed at 24 hrs significantly (P<0.05) higher expression at 10 ppm of TiO2. At 72 hrs, the level of IL-6 was not statistically significant (P>0.05) in TiO2 treated HGFs at 0.01 ppm and 0.1 ppm in comparison to control cells (Figure 9).
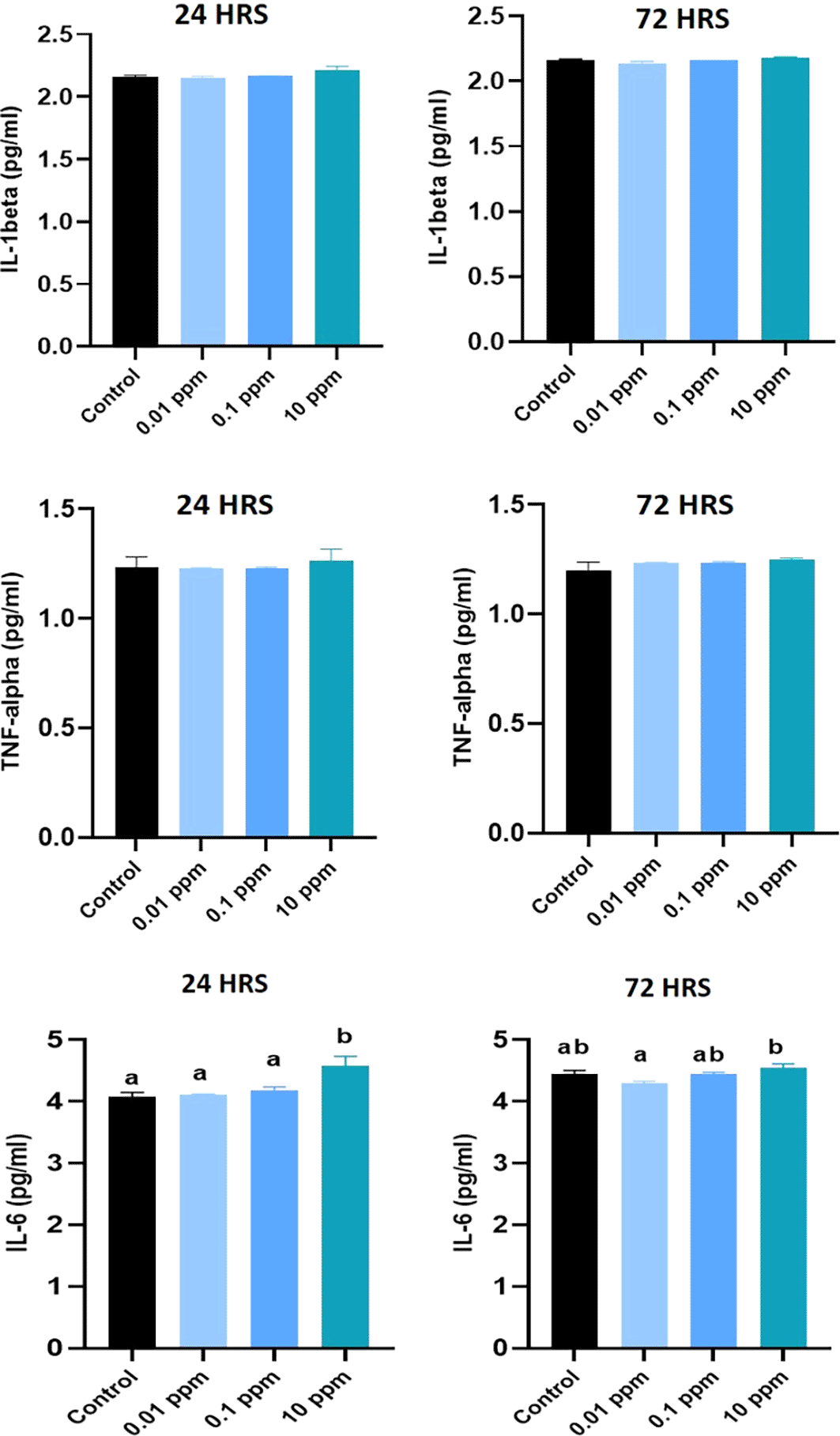
Effect of TiO2 nanoparticles on the cytokine expression of pro-inflammatory cytokines (IL-1 β, TNF- α, and IL-6 respectively), in human gingival fibroblasts (HGFs) exposed to different concentrations and durations. HGFs were stimulated with or without 0.01, 0.1 and 10 ppm of TiO2. Cytokine levels of IL-1 β, TNF- α, and IL-6 at 24 hrs and 72 hrs were analyzed by ELISA. The values are presented as pg/ml, and expressed as mean ± standard deviation (SD). Different superscripts (a, b) indicate a statistically significant difference at P<0.05.
The level of IL-10 analyzed at 24 hrs revealed that the expression of IL-10 at 24 hrs and 72 hrs in all tested concentrations was not statistically significant (P>0.05) in TiO2 treated HGFs in comparison to control cells. The level of TGF-beta analyzed at 24 hrs showed statistically significant difference (P<0.05) among TiO2 treated HGFs in comparison to control cells. However, at 72 hrs, no statistically significant (P>0.05) differences were found between the control cells and TiO2 treated HGFs (Figure 10).
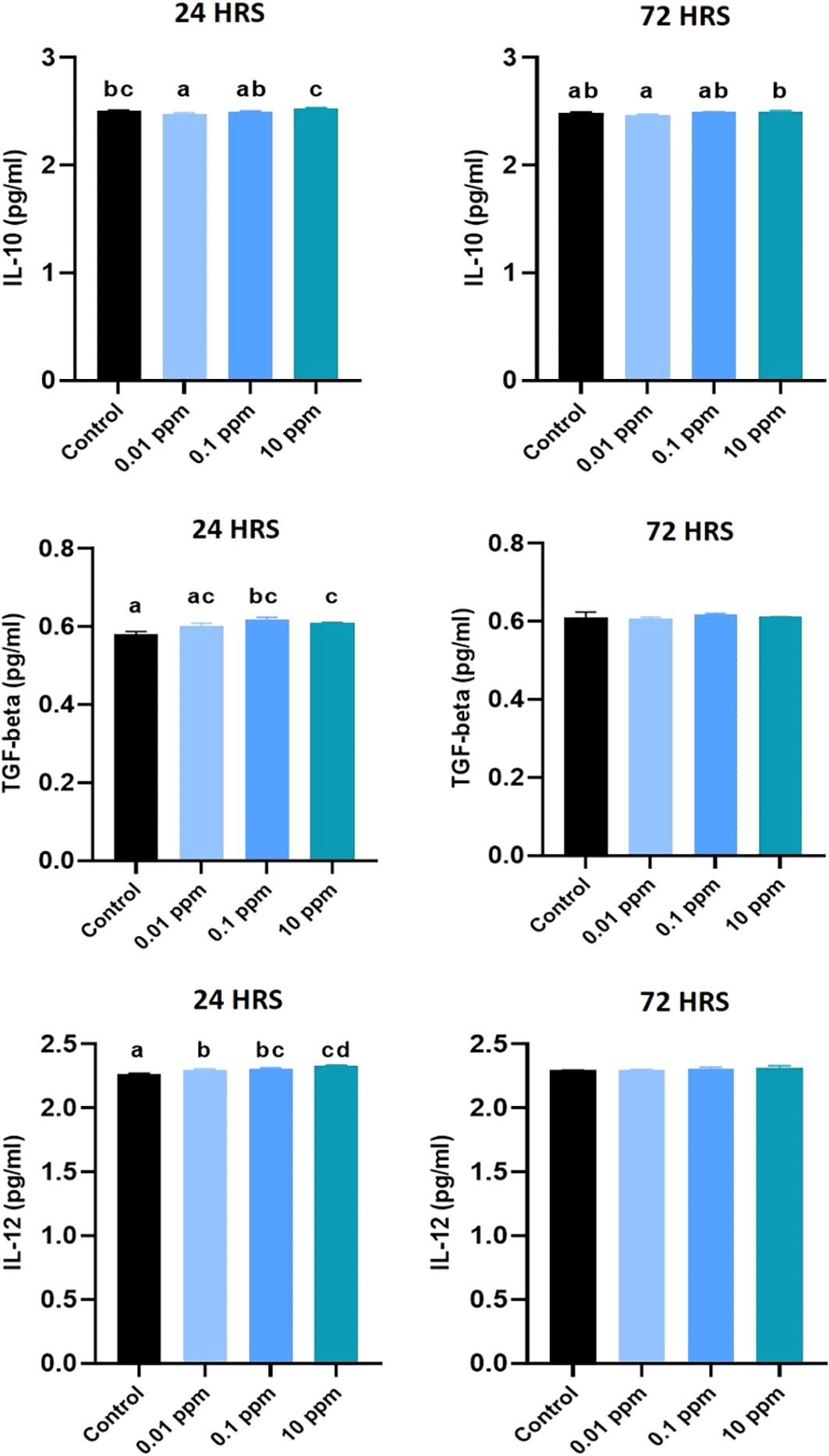
HGFs were stimulated with or without 0.01, 0.1, and 10 ppm of TiO2. Cytokine levels of IL-12 at 24 hours and 72 hours were analyzed by ELISA. The values are presented as pg/ml and expressed as mean ± standard deviation (SD). Different superscripts (a, b, c) indicate a statistically significant difference at P<0.05.
The level of IL-12 analyzed at 24 hrs was statistically significant (P<0.05) in TiO2 treated HGFs in comparison to control cells. However, at 72 hrs, no statistically significant (P>0.05) differences in IL-12 levels were found between the control and TiO2 treated HGFs (Figure 10).
Implants are widely employed as the restorative modality for replacing missing natural teeth. In the early 1970s, implant therapy was primarily considered as the treatment option for completely edentulous patients. However, in recent years, patients with partial edentulism have also become candidates for dental implants. There are various types of implants with several newer biomaterials used as zirconia, roxolid, surface-modified titanium, etc.
Alloy-incorporated titanium mainly Ti-6Al-4V is considered as the most commonly used metal for the manufacture of dental implants because of the superior biocompatibility and physical properties such as low elastic modulus.26,27 Titanium implants in bone have been shown to continually emit Ti particles typically starting from a few hours to several months after the implant placement, that could exert a negative influence to the surrounding tissues.28 In general, Ti particles are insoluble in nature therefore their elimination from the body is challenging and they typically get scattered and accumulate in the hard and soft tissue surrounding the implant. Elevated concentrations of Ti particles become excessive, oral intraepithelial homeostasis is lost resulting in a exacerbate inflammation of the adjacent tissues, and causes dynamic imbalance between osteoblasts and osteoclasts. In addition, the Ti particles that gets released from the implant surface are not only confined to the tissue surrounding the implant, but they also travel with the circulation and accumulate gradually in the peripheral organs, generating systemic and allergic reactions.28 TiO2 NPs may diffuse into the peri-implant area triggering an inflammatory response and resorption of the alveolar bone, which mediates the development of peri-implantitis.29
Peri-implantitis, a significant complication affecting up to 20% of patients and 30% of implants,30 is characterized by inflammation in the tissues surrounding dental implants. The World Workshop on Periodontology defines healthy peri-implant tissues as those exhibiting no bleeding on probing and minimal bone loss.31 In contrast, peri-implant mucositis presents with bleeding on probing but minimal bone loss. Peri-implantitis, however, is a more severe condition characterized by bleeding or suppuration upon probing, increased probing depth, and significant bone loss.31
HGFs and periodontal cells serve as representatives of periodontium and therefore are widely used for in vitro research in periodontology.32 HGFs are considered as the major cellular components responsible for the repair of damages tissue and for creating a seal around the implants from the oral microflora.33 In the present study, TiO2 NPs particles of varied concentrations were used to study their reaction of human gingival fibroblasts for various durations. Evaluation of the cytotoxic effects, cytokine and gene expression was carried out in the present study. In the present study, TiO2 NPs particles of varied concentrations were used to study their interaction with human gingival fibroblasts for various duration, to elucidate the concentration-dependent cytotoxicity and modulation of the inflammatory response elicited by TiO2 NPs in human gingival fibroblasts (HGFs).
In the present study, viability of the HGFs were evaluated by an MTT assay in an interval of day 1, 3, 5 and 7 with varying concentrations of TiO2 ranging from 0.001 ppm to 100 ppm. The results demonstrated that the cytotoxicity of the HGFs have been affected on exposure to TiO2 nanoparticles when compared to the control group. This detrimental impact on HGFs also depends on the different concentrations of TiO2 nanoparticles as well their exposure to different time intervals. The finding of deterministic effects of Ti on the HGF cell lines can be matched with the finding of Choi MG et al. who reported similar negative impact of TiO2 nanoparticles on various cell lines including fibroblasts and osteoblasts activity.34 He et al. also reported the toxic effects of Ti particles on the survival of PDL cells, which is similar to the present study.35
A significant decrease in the viability of HGF was noted with higher the concentration of TiO2 NPs and a longer exposure time led to a greater reduction in the cellular viability. It was also found that the proliferation of HGF at the end of 7 days, was the least in 100 ppm concentration of TiO2. This finding was in accordance with the study conducted by Jin et al. who had reported a direct proportional effect of the concentration and duration of TiO2 exposure on cytotoxicity.36 The cytotoxic effects of TiO2 NPs could be due to their ability to induce production of reactive oxides resulting in an elevated oxidative stress of the cells that could lead to damage of nucleic acids and lipids, interfering with signaling transduction pathways and modulating transcription factors (such as nuclear factor-B and activator protein-1). Subsequently, these mechanisms activate the antioxidant defense system and potentially cause cell death due to oxidative DNA damage.36
In the present study, A higher absorbance value of 0.4347 was recorded in HGFs treated with 0.001 ppm of TiO2 and the lowest absorbance value of 0.3225 was recorded in HGFs exposed to 100 ppm of TiO2, representing the proliferation of HGFs. Reduction in the proliferation capacity was noted with increase in the concentration of TiO2. These findings were supported by in vitro studies that have demonstrated that titanium particles at very minimal concentrations can stimulate fibroblast growth. Whereas higher concentrations inhibit the proliferation.37 The increased concentration TiO2 can suppress the proliferation of fibroblasts by mediating various growth factors at the cellular level, however, the mechanisms are poorly understood.
Literature evidences have reported that Ti-based particles could induce the proinflammatory response similar to an allergic reaction. Recent researchers have found that TiO2 NPs can elicit an inflammatory response, without the assistance of binding proteins to toll like receptor -4 (TLR-4), indicating the strong potential of TiO2 NPs to cause inflammation.21,38 In the present study, the expression of TNF- alpha was found to be reduced in HGFs exposed to TiO2 than the control group. But, Taira et al. had reported an increase in TNF-α on exposure to TiO2, which is in contrast to our findings.39
The expression of anti-inflammatory cytokines such as IL-10, TGF-β, IL-12 were found to be significantly higher in HGFs exposed to varied concentrations of TiO2, which demonstrates the activation of anti-inflammatory mechanism in response to the cellular changes caused due to TiO2 particles. Callejas JA et al. had reported similar high gene expression of IL-10 and TGF-β in response to Ti particles.40 IL-12 is a crucial cytokine associated with early innate immune response. It is also the primary mediator in maintaining the cell-mediated immune response, contributing to chronic inflammatory illness and studies have reported a higher expression of IL-12 genes to correlate with severe peri implantitis. Therefore, increased expression of these anti-inflammatory genes can lead to development of peri implantitis by activation of osteoclastogenesis. TNF-α is also considered as an important mediator associated with bone loss in peri-implantitis, correlating with the chronic and advanced stage of the disease. In the present study, our observation of reduced expression of TNF-α could be due to the short-term exposure to TiO2 particles (i.e only for 72 hours), rather than chronic exposure. TNF-α may also enhance osteoclast development indirectly by up-regulating the synthesis of RANKL by stromal cells and by enhancing the responsiveness of osteoclast precursors to RANKL. This may be another mechanism by which TNF-α promotes osteoclast formation.36,39
In the present study, a significant difference was noted in the expression of IL-6 upon initial exposure to TiO2 nanoparticles. The current findings are concurrent with that of Okuda et al.19 and Taira et al. in their study using DNA microarray technology reported similar overexpression of IL6 gene.39 The upregulation of expression of IL-6 could be attributed to the overexpression of heat shock protein 70 (HSP70) gene as a biologic response to TiO2 exposure due to the activity of cytotoxic mediators. It is also reported that the nanoparticles possessing magnetic properties can induce necrotic and cell death of the fibroblasts. The IL-6 gene is also upregulated by inflammation due to the release of TiO2 nanoparticles into the periodontal tissues.19 IL-1β expression is regarded as a marker representing the activation of inflammasome and cell viability. However, in this study the expression of IL-1β and IL-6 was more or less similar to that of controls by the end of 72 hours.
The results of ELISA indicated that the release of proinflammatory cytokines such as IL-1β, TNF-α, IL-6 to be unaltered in TiO2 exposed groups regardless of the concentrations on comparison with control HGFs. Also, the release of anti-inflammatory cytokines such as IL-10, TGF-β, IL-12 were almost similar in all tested concentrations of TiO2. This study reports a weaker pro and anti-inflammatory cytokine release from Human gingival fibroblast cells on exposure to TiO2. Similarly, Callejas JA et al. also reported reduced pro inflammatory cytokine release, which is relatable to our finding.40
The cytotoxic and altered gene expression of pro and anti-inflammatory mediators associated with increased concentrations of TiO2 can be related to the development of Peri-implantitis. Most studies have reported titanium particles of size 1–10 micrometer to be released from the implant surface, which are capable of diffusing into the tissues and systemic organs of the body. Recent studies have demonstrated that proinflammatory cytokines especially TNF-α, IL-1 and IL- 6 are generated during phagocytosis by local macrophages and the creation of an erosive pannus, which increases bone resorption by osteoclasts. Differentiation of macrophages into bone-resorbing osteoclast cells and subsequent bone resorption occurs extensively in the presence of TiO2 particle debris.34
From the results obtained from this study, it is evident that the cytotoxic and immunologic response associated with TiO2 is directly related to the concentration and duration of exposure. However, the present study is bound for certain inherent limitations due to the nature of the study design. Also, the present study lacks evaluation of the cytotoxic and immunologic effects based on particle size of TiO2. Further researches, comparing the effects of TiO2 released from surface modified titanium implants should be undertaken to facilitate a multi-dimensional understanding of the adverse effects of TiO2 released from dental implants
This study was conducted with ethical approval from the Institutional Ethical Committee of the AB Shetty Memorial Institute of Dental Sciences, Nitte (Deemed to be University) (Certification number: ABSM/EC/112/2021), granted on 9th January, 2021. All procedures complied with the ethical standards of the Declaration of Helsinki, 2013.
Written informed consent was obtained and gingival tissue samples were collected from a total of three (n=3) systemically and periodontally healthy participants who reported to the Department of Periodontology, following approval from the Institutional Ethics Committee (ABSM/EC/112/2021).
The data supporting the findings of this study are available in Open Science Framework with the following identifier:
OSF: Influence of titanium nanoparticles on cytotoxicity and inflammatory cytokines expression in gingival fibroblasts - An in-vitro study https://doi.org/10.17605/OSF.IO/9QJG2.
This project contains the following underlying data:
1. Data File 1: TI_V123 – Cell viability data set
2. Data File 2: TI_Ab123 – Cell proliferation
3. Data File 3: TI_PC123- PCR Data set
4. Data File 4: TI_EL123 -ELISA Data set
5. Data File 5: CRIS- CRIS Checklist for In-vitro studies
6. Data File 6: Extended Data
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Sai SR: Influence of titanium nanoparticles on cytotoxicity and inflammatory cytokines expression in gingival fibroblasts - An in vitro study [Dataset]. OSF; 2024. https://doi.org/10.17605/OSF.IO/9QJG2 41
The data supporting the findings of this study are available in Open Science Framework with the following identifier:
OSF: Influence of titanium nanoparticles on cytotoxicity and inflammatory cytokines expression in gingival fibroblasts - An in vitro study. https://doi.org/10.17605/OSF.IO/9QJG2 41
This project contains the following extended data:
1. Extended Data: Contains information on Abbreviations used, Primer sequences, cDNA synthesis, Protocol for PCR, ELISA and additional images
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Sai SR: Influence of titanium nanoparticles on cytotoxicity and inflammatory cytokines expression in gingival fibroblasts - An in vitro study [Dataset]. OSF; 2024. https://doi.org/10.17605/OSF.IO/9QJG2 41
The authors affirm that the study has been conducted and reported in accordance with the CRIS (Consolidated Reporting for In-vitro Studies) guidelines, and a detailed description of the study’s background, objectives, and significance have been provided. The manuscript includes comprehensive information on the cell lines used, culture conditions, experimental design, and methods of data analysis. We have presented our results clearly with appropriate figures and tables, and have included detailed statistical analyses. The discussion contextualizes our findings, acknowledges the study’s limitations, and proposes future research directions. All relevant references are cited, and we have disclosed any potential conflicts of interest.
Supplementary materials supporting our study are provided, and we have ensured that data availability is clearly communicated. Although our research involves only in-vitro methods, we have adhered to ethical guidelines concerning the use of cell lines.
We gratefully acknowledge the Department of Periodontology for providing the resources and facilities necessary to conduct this research.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Dental Implants, Molecular Medicine and Translational Research, Prosthodontic Dentistry, Bone Regeneration
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 03 Oct 24 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)