Keywords
Citronellol, inflammation, Methotrexate, nephrotoxicity, oxidative stress
Methotrexate (MTX) is a classical folic acid antagonist widely used in the treatment of malignant and non-malignant disorders. However, its clinical application is often restricted by concomitant adverse effects, including renal damage. Numerous studies have highlighted the role of oxidative stress and inflammation in mediating MTX-related nephrotoxicity. Therefore, the current study aimed to explore the possible renoprotective action of Citronellol (CT), a natural compound with prominent antioxidant and anti-inflammatory activities, against nephrotoxicity induced by MTX.
To fulfill our objective, 24 adult male Wistar rats were randomly allocated into four groups: control, MTX, 100 mg/kg CT plus MTX and 200 mg/kg CT plus MTX. At the end of the study, the experimental rats were anesthetized, blood samples were collected for biochemical assays, and the kidneys were surgically removed for biochemical and gene expression analyses, after which all rats were sacrificed by exsanguination.
Compared to the MTX-treated group, our results revealed that pre-supplementation with 100 or 200 mg/kg CT remarkably ameliorated renal damage biomarkers, including serum urea, serum creatinine, and kidney injury molecule-1 (KIM-1). In addition, pre-treatment with 100 or 200 mg/kg CT enhanced the activity of the antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase (GPx), diminished renal malondialdehyde (MDA) contents, and attenuated inflammation by suppressing renal nuclear factor kappa B (NF-κB) signaling and diminishing tumor necrosis factor-alpha (TNF-α) gene expression. Moreover, pre-treatment with 200 mg/kg CT markedly reduced interleukin-1 beta (IL-1β) gene expression.
Our findings demonstrate, for the first time, that CT can serve as a new promising agent for mitigating nephrotoxicity induced by MTX through its antioxidant and anti-inflammatory properties.
Citronellol, inflammation, Methotrexate, nephrotoxicity, oxidative stress
Methotrexate (MTX), an antagonist of folic acid, is a disease-modifying antirheumatic drug and potent chemotherapeutic agent.1,2 It is regarded as one of the most successful pharmaceuticals since it has been used in the treatment of various types of cancer, as well as autoimmune disorders, including rheumatoid arthritis, psoriasis, systemic lupus erythematosus, and refractory Crohn’s disease.1,3,4
Although MTX is known to have excellent pharmacological efficacy, some limitations have been reported regarding its therapeutic applications owing to its adverse effects that have gained considerable attention, as they are considered the main reason behind MTX treatment withdrawal, such as myelosuppression, hepatotoxicity, and renal damage.5–7
The exact etiology of MTX-induced nephrotoxicity remains unclear; however, several mechanisms have been proposed to be involved, including intratubular obstruction via crystallization of MTX and its related derivatives in the lumen of renal tubules, as well as direct tubular toxicity that has been linked to the ability of MTX to induce excessive generation of reactive oxygen species (ROS), eventually leading to various deleterious effects in the kidneys, such as lipid peroxidation, amino acid oxidation, and DNA damage.5,8,9 Moreover, excess ROS can induce activation of nuclear factor kappa B (NF-κB), a redox-sensitive transcription factor that promotes the production of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β), thereby eliciting inflammation and cell death.5,10
Given the fact that oxidative stress and inflammation play a key role in the development of MTX-induced renal injury, many researchers have suggested that agents or compounds, particularly from natural sources, that possess antioxidant and anti-inflammatory activities can have huge benefits for mitigating nephrotoxicity induced by MTX.5,11
Citronellol (CT), which has the chemical formula 3,7-dimethyl-6-octen-1-ol, is a natural monoterpene alcohol found in essential oils of diverse aromatic plants, including Cymbopogon citratus, Cymbopogon winterianus and Lippia alba.12,13 It is also present in certain types of citrus fruits, such as oranges and lemons.14
Previous in vitro and in vivo studies have documented various beneficial pharmacological effects of CT, including antioxidant, anti-inflammatory, antibacterial, antifungal, antidiabetic, and hypotensive effects.14,15 In addition, several researchers have pointed to the possible applications of CT in fields such as agriculture, food science, and medicine.14
To our knowledge, the potential protective role of CT against MTX-induced nephrotoxicity has not been reported. Hence, the current study was designed to evaluate the possible renoprotective effect of CT against nephrotoxicity in MTX-treated rats.
The current study protocol was reported in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines16 and was approved by the Research Ethics Committee of the College of Medicine, University of Baghdad (protocol number: 03-30; date of approval: October 8, 2023).
A total of 24 adult male Wistar rats weighing 200–280 g were included in the present study. These rats were procured from and kept in the animal house of the Iraqi Center for Cancer Research and Medical Genetics under standard conditions of relative humidity, atmospheric temperature, and light-dark transitions (12-hour light/12-hour dark). The experimental rats were supplied with commercial pellets and tap water ad libitum, throughout the study. Moreover, they were acclimatized for a period of one week before the start of the experiment.
All efforts were made to ensure the welfare of the experimental rats and minimize any pain, distress, or suffering. Such efforts included appropriate ventilation of the animal house, regular cleaning of the animal cages, replacing wood husk every other day, careful handling of all animals throughout the entire experiment, and using effective anesthetic doses of ketamine and lidocaine hydrochloride to alleviate any pain.
According to the study protocol, the experimental rats were randomly allocated into four groups, consisting of six rats each, and treated as follows:
Group I (control): rats received corn oil via oral gavage for 14 consecutive days.
Group II (MTX): rats received corn oil via oral gavage for 14 consecutive days and a single intraperitoneal (IP) injection of 20 mg/kg MTX (Seton Pharmaceuticals, UK) on the 11th day of the experiment.
Group III (100 mg/kg CT + MTX): rats received 100 mg/kg CT (Bidepharm, China) dissolved in corn oil via oral gavage for 14 consecutive days and a single IP injection of 20 mg/kg MTX on the 11th day of the experiment.
Group IV (200 mg/kg CT + MTX): rats received 200 mg/kg CT dissolved in corn oil via oral gavage for 14 consecutive days and a single IP injection of 20 mg/kg MTX on the 11th day of the experiment.
After 24 hours from the final administration, the rats were anesthetized with 150 mg/kg ketamine (Bremer Pharma GmbH, Germany) and 10 mg/kg lidocaine hydrochloride (GF Bonn GmbH, Germany). Subsequently, blood samples were collected from the inferior vena cava of each rat through an abdominal incision, and the kidneys were surgically removed for analysis, after which all rats were sacrificed by exsanguination.
Immediately following excision, each kidney was rinsed with pre-chilled phosphate-buffered saline (Sigma, USA) and blotted on filter paper to remove any residual blood. Thereafter, the renal capsule was peeled off from each kidney and tissue samples were collected for biochemical assays and gene expression analysis.
For serum isolation, each blood sample was placed in a gel and clot activator tube and left to clot for approximately 30 min at room temperature. Then, each tube was centrifuged at 3500 rpm for 15 min, after which the serum (the supernatant layer) was transferred into a pre-cooled microcentrifuge tube and stored at −20 °C until used for the biochemical assays of serum urea and creatinine levels.
For biochemical assays, renal tissue samples were homogenized in an extraction medium consisting of tissue protein extraction reagent (Cat. No. 78510, Thermo Scientific, USA) and protease inhibitor cocktail (Cat. No. 78439, Thermo Scientific, USA). First, an adequate working solution for all samples was prepared by mixing the extraction reagent with the protease inhibitor cocktail according to the volumes recommended by the manufacturers. Next, renal tissue samples were weighed, after which each sample was placed in a microcentrifuge tube, treated with the appropriate volume of the prepared working solution, homogenized with the aid of a tissue grinding pestle, and centrifuged at 10,000 rpm for 5 min, where the obtained supernatant layer from each sample was collected in a pre-cooled microcentrifuge tube and stored at −20 °C for later use.
For gene expression analysis, samples from the kidneys were placed in microcentrifuge tubes containing 1 mL of TRIzol (Cat. No. 15596026, Thermo Scientific, USA). Then, each sample was homogenized using a tissue grinding pestle and incubated for 5 min to permit protein and nucleic acid dissociation. Next, 0.2 mL of chloroform was added to each tube, after which all tubes were incubated for 2–3 min and centrifuged at 12,000 rpm for 10 min to facilitate proper separation of the formed mixture into a lower phenol-chloroform organic phase, an interphase, and an upper aqueous phase containing total RNA.
After separation, the aqueous phase from each tube was carefully transferred into a new microcentrifuge tube. Then, 0.5 mL of isopropanol was added to each tube, and subsequently, all tubes were incubated for 10 min and centrifuged at 12,000 rpm for 10 min to allow total RNA to precipitate in the form of white gel-like pellets at the bottom of the tubes.
Next, the supernatant layers were discarded from all tubes and the formed pellets were resuspended by adding 0.5 mL of 70% ethanol into each tube. Then, all tubes were vortexed briefly and centrifuged at 10,000 rpm for 5 min, after which the supernatant layers were discarded from all tubes and the RNA pellets were left to air-dry for 10 min at room temperature. Later on, RNA pellets were solubilized by adding 50 μL nuclease-free water into each tube, and lastly, all tubes were incubated in a heat block set at 55 °C for 10 min and stored at −20 °C for later use.
Serum urea and creatinine concentrations were measured colorimetrically using colorimetric assay kits (Linear Chemicals, Spain). In addition, the levels of kidney injury molecule-1 (KIM-1), NF-κB, and oxidative stress biomarkers, including superoxide dismutase (SOD), glutathione peroxidase (GPx), and malondialdehyde (MDA), were quantified in kidney tissue homogenates using enzyme-linked immunosorbent assay (ELISA) kits (Cloud-Clone Corp., USA). All assays were performed in accordance with the manufacturer’s instructions.
The relative mRNA expression levels of renal TNF-α and IL-1β were assessed by performing two-step reverse transcription-quantitative polymerase chain reaction (RT-qPCR) according to the manufacturer’s instructions. Briefly, for each renal tissue sample, the extracted RNA was reverse-transcribed into cDNA using GoScript™ Reverse Transcription System (Promega, USA), after which the synthesized cDNA was quantified using QuantiFluor® dsDNA System (Promega, USA). Next, for each gene expression analysis, a qPCR mix was assembled by combining the cDNA template with GoTaq® qPCR Master Mix (Promega, USA) as well as the gene forward and reverse primers (Macrogen, South Korea), provided that beta-actin (β-actin) was used as a housekeeping gene to normalize mRNA expression levels of the target genes. Subsequently, quantitative analysis was performed using a magnetic induction cycler (MIC) for qPCR (Bio Molecular Systems, Australia). Moreover, the cycling conditions included initial denaturation at 95 °C/5 min, followed by 40 cycles of denaturation at 95 °C/20 s, annealing at 55 °C/20 s, 65 °C/20 s, and 60 °C/20 s for TNF-α, IL-1β, and β-actin primers, respectively, and subsequent extension at 72 °C/20 s. The cDNA amplification plots were assessed using micPCR software (version 2.10.0), and the thresholds were automatically determined to obtain the cycle threshold (Ct) values. Finally, the 2−ΔΔCt method was used to analyze the relative mRNA expression data. The primer sequences used in the present study are listed in Table 1.
GraphPad Prism software (version 9.1.5) was used to perform the statistical analysis. The numeric data obtained from the current study were expressed as the mean ± standard error of the mean (SEM) for each group and analyzed using one-way analysis of variance (one-way ANOVA) to evaluate the statistical significance of the results, followed by Tukey’s post-hoc analysis for multiple comparisons. P values of less than 0.05 were considered to be statistically significant.
As presented in Table 2 and Figure 1, the means of serum urea and creatinine levels were significantly increased in MTX-treated rats compared to those of the control group. Similarly, MTX administration significantly elevated the mean of KIM-1 levels in the kidneys of our experimental rats. However, pre-treatment with either 100 or 200 mg/kg CT significantly ameliorated all the above-mentioned biomarkers, compared to the group affected by MTX.17
| Group | Urea (mg/dL) | Creatinine (mg/dL) | KIM-1 (pg/mL) |
|---|---|---|---|
| Control | 34.94 ± 1.61 | 1.34 ± 0.17 | 467.41 ± 53.55 |
| MTX | 49.45 ± 1.41a**** | 2.42 ± 0.13a** | 1891.51 ± 101.31a**** |
| 100 mg/kg CT + MTX | 39.22 ± 2.62b** | 1.60 ± 0.25b* | 777.70 ± 112.08b**** |
| 200 mg/kg CT + MTX | 36.16 ± 0.79b*** | 1.31 ± 0.14b** | 440.64 ± 62.90b**** |
As shown in Table 3 and Figure 2, the kidneys of MTX-treated rats showed a significant decline in the means of SOD and GPx levels, together with a significant elevation in the mean of MDA contents when compared to those of the control group. Meanwhile, rats that received either 100 or 200 mg/kg CT prior to MTX administration showed a significant increase in the means of renal SOD and GPx levels, along with a significant decline in the mean of renal MDA contents when compared to those of the MTX group.17
| Group | SOD (ng/mL) | GPx (ng/mL) | MDA (ng/mL) |
|---|---|---|---|
| Control | 7.36 ± 0.08 | 84.10 ± 1.98 | 3.22 ± 0.27 |
| MTX | 1.83 ± 0.12a**** | 36.88 ± 3.34a**** | 16.07 ± 1.54a**** |
| 100 mg/kg CT + MTX | 4.06 ± 0.50a****b*** | 53.42 ± 6.76a***b* | 8.13 ± 1.62a*b*** |
| 200 mg/kg CT + MTX | 3.94 ± 0.27a****b*** | 70.72 ± 1.38b****c* | 6.72 ± 0.25b**** |
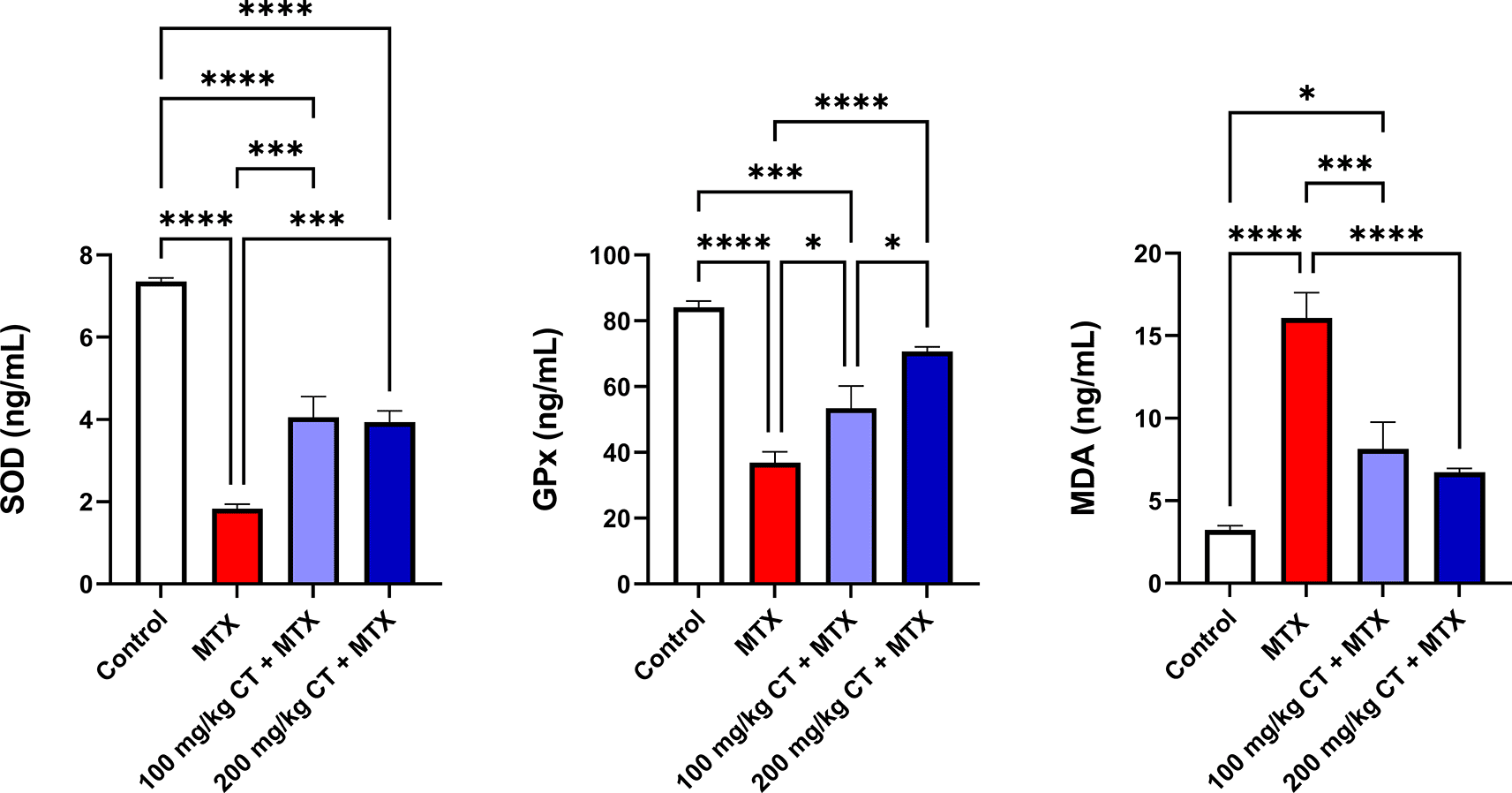
Data are presented as the mean ± standard error of the mean (SEM), n = 6. *P < 0.05; ***P < 0.001; ****P < 0.0001. CT, citronellol; GPx, glutathione peroxidase; MDA, malondialdehyde; MTX, methotrexate; SOD, superoxide dismutase.
The data displayed in Table 4 and Figure 3 revealed a significant increase in the mean of renal NF-κB levels in MTX-treated rats compared to that of the control group. Moreover, a significant elevation in the means of TNF-α and IL-1β relative mRNA expression levels was observed in the kidneys of rats challenged with MTX compared to those of the control group. Interestingly, administration of either 100 or 200 mg/kg CT prior to MTX injection resulted in a significant reduction in the means of renal NF-κB levels and TNF-α relative mRNA expression levels compared to those of the MTX group. However, the impact of CT on the mean of IL-1β relative mRNA expression levels was observed to be statistically significant only at a dose of 200 mg/kg when compared to that of the MTX group.17
| Group | NF-κB (ng/mL) | TNF-α relative mRNA expression | IL-1β relative mRNA expression |
|---|---|---|---|
| Control | 5.20 ± 0.32 | 1.00 ± 0.39 | 1.00 ± 0.33 |
| MTX | 28.31 ± 1.07a**** | 22.81 ± 6.67a** | 5.20 ± 1.09a** |
| 100 mg/kg CT + MTX | 5.65 ± 0.29 b**** | 7.61 ± 2.44b* | 4.42 ± 0.79a* |
| 200 mg/kg CT + MTX | 4.29 ± 0.28 b**** | 6.54 ± 2.03b* | 1.90 ± 0.73b* |
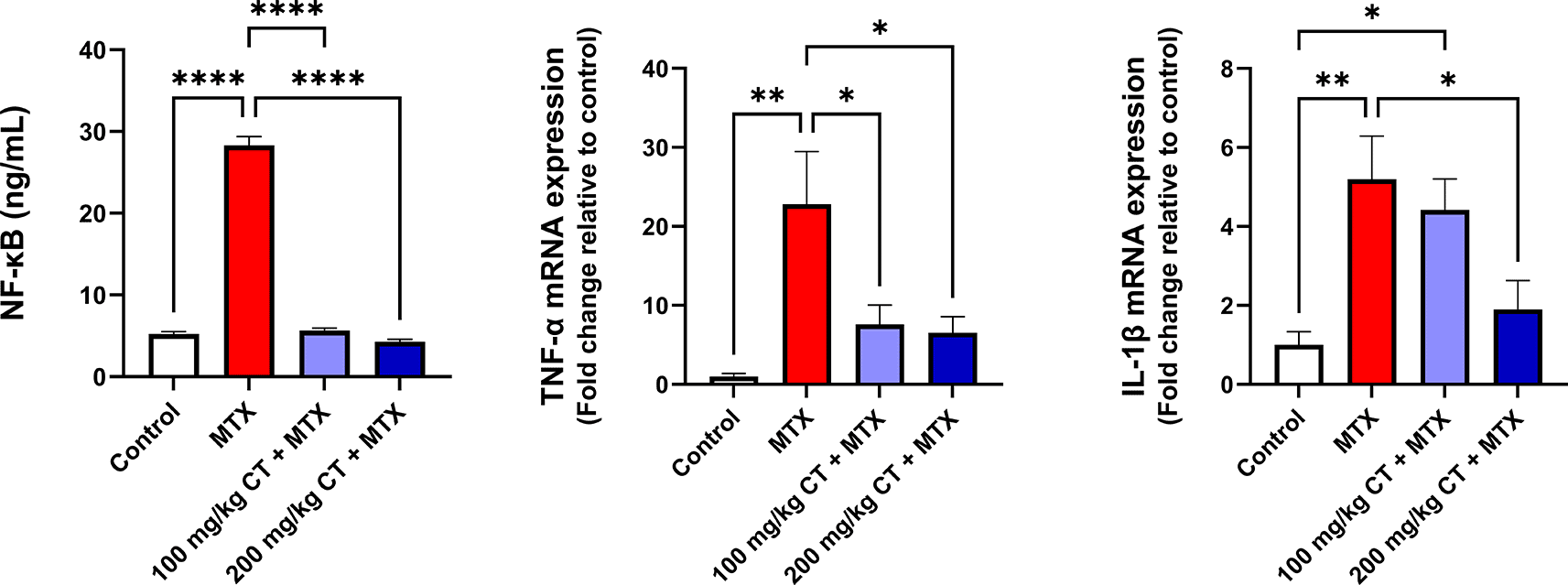
Data are presented as the mean ± standard error of the mean (SEM), n = 6. *P < 0.05; **P < 0.01; ****P < 0.0001. CT, citronellol; IL-1β, interleukin-1 beta; MTX, methotrexate; NF- κB, nuclear factor kappa B; TNF-α, tumor necrosis factor-alpha.
Nephrotoxicity induced by high-dose MTX, which has been defined as “the administration of MTX in doses exceeding 500 mg/m2”, represents a serious medical emergency, particularly among patients in hospital settings, with a high chance of progressing to irreversible renal injury.8,18 Moreover, kidney intoxication induced by MTX can retard renal elimination of the drug itself, thereby increasing the probability of developing more severe adverse events, such as myelosuppression, neurotoxicity, and liver damage.10
In agreement with previous pre-clinical studies,2,11 administration of 20 mg/kg MTX provoked renal damage in our experimental rats, which was indicated by the apparent elevation in serum urea and creatinine levels. These biomarkers have traditionally been utilized as reliable measurements in assessing renal impairment, given the fact that these metabolic wastes tend to be retained in the human body upon the development of kidney injury.8,19,20 Furthermore, MTX-related nephrotoxicity was further confirmed by the marked upregulation of KIM-1, a transmembrane protein that has been reported to be highly elevated in cases of renal injuries, particularly proximal convoluted tubular injuries.5,11 Interestingly, pre-treatment with 100 or 200 mg/kg CT remarkably attenuated the nephrotoxic potential of MTX, as evidenced by the effective reduction in serum urea, serum creatinine, and KIM-1 levels. Our results are in accordance with two previous studies that have demonstrated the ability of CT to ameliorate renal damage biomarkers in animal models of glycerol-induced rhabdomyolysis and gentamicin-induced nephrotoxicity.13,21
Another hallmark of MTX-induced nephrotoxicity is the oxidative stress phenomenon, which is described as a state of disproportion between the formation of ROS and the ability of the biological system to remove these reactive molecules.11,22,23 It is regarded as a detrimental process involved in the development and progression of a wide range of pathological conditions.23,24 Moreover, it represents one of the major mechanisms by which MTX induces damage in the kidneys.11
Previous studies have reported that MTX can promote excessive ROS formation by activating NADPH oxidase, stimulating neutrophils, inducing mitochondrial dysfunction, suppressing homocysteine remethylation, and diminishing intracellular NADPH levels.11,25 In turn, the generated ROS can cause massive destruction throughout the cell by promoting DNA damage, oxidizing amino acid side chains, forming protein-protein cross-linkages, and inactivating antioxidant enzymes.5,26,27 In addition, ROS can induce lipid peroxidation, which occurs upon their interaction with cellular membrane phospholipids.28,29 This process, in turn, can induce alterations in the cellular membrane composition, structure, and dynamics. Moreover, it can lead to the inactivation of membrane-bound enzymes and receptors.5,30
In this context, our findings revealed that MTX administration caused a significant increase in renal MDA, an extremely reactive toxic product that has been widely utilized as a biomarker of lipid peroxidation in biomedical researches.28,31,32 In addition, the levels of the antioxidant enzymes SOD and GPx were significantly decreased in the kidneys of MTX-intoxicated rats. In contrast, pre-supplementation with 100 or 200 mg/kg CT effectively reduced the ability of MTX to induce lipid peroxidation in the kidneys and boosted renal SOD and GPx levels.
Accordingly, previous in vitro and in vivo studies have reported the antioxidant potential of CT, which was attributed to the ability of the compound to scavenge free radicals by means of its reducing power.33,34 Furthermore, CT was found to enhance the gene expression of nuclear factor-erythroid 2-related factor 2 (Nrf2), a key regulator of the transcription of certain cytoprotective and antioxidant genes, including SOD, GPx, catalase (CAT), and heme oxygenase 1 (HO-1).5,9,33 Under non-stressful conditions, Nrf2 remains confined to the cytosol by binding to Kelch-like ECH-associated protein 1 (Keap1).9,35 This coupling enhances Nrf2 degradation through the action of the ubiquitin-dependent proteasomal system.5,35 However, upon activation, Nrf2 splits away from Keap1 and translocates into the nucleus, after which it binds to the antioxidant response element (ARE) and activates the gene expression of multiple antioxidant enzymes.2,35 Hence, we assume that the renoprotective effect of CT against MTX-related nephrotoxicity is mediated, at least in part, by its powerful antioxidant action.
In addition to oxidative stress, mounting evidence has indicated the role of inflammation in MTX-induced kidney injury. In this regard, ROS have been reported to induce activation of NF-κB, a family of redox-sensitive transcription factor proteins that function as dimers to modulate diverse cellular processes, such as inflammation, immunity, and cell death.36–38
Under physiological conditions, NF-κB dimers remain secluded in the cytoplasm in the form of an inactive complex with a member of inhibitor proteins known as inhibitors of NF-κB (IκBs).39–41 However, in response to a stimulus, IκB molecule gets phosphorylated by IκB kinase (IKK), eventually leading to the liberation of NF-κB dimers for nuclear translocation, after which they bind to specific DNA sequences and activate the transcription of various genes, including those encoding the pro-inflammatory cytokines TNF-α and IL-1β.38,42 These cytokines, in turn, can activate macrophages and neutrophils, promote infiltration of inflammatory cells, induce upregulation of adhesion molecules, and enhance ROS formation, thereby raising havoc throughout all cells.43,44
Consistently, our results showed that administration of MTX highly intensified renal NF-κB levels and enhanced the gene expression of TNF-α and IL-1β in the kidneys of our experimental rats. Meanwhile, pre-treatment with 100 or 200 mg/kg CT remarkably diminished renal NF-κB signaling and reduced the gene expression of TNF-α. Moreover, pre-treatment with 200 mg/kg CT markedly reduced IL-1β gene expression. Accordingly, previous studies have reported the ability of CT to hamper renal NF-κB activation and revert the gene expression of TNF-α and IL-1β in animal models of glycerol-induced rhabdomyolysis and gentamicin-induced nephrotoxicity.13,21 Additionally, CT has been demonstrated to exert anti-inflammatory actions in other pre-clinical models of various pathological conditions, including rotenone-induced Parkinson’s disease, doxorubicin-cardiac toxicity, and Freund’s complete adjuvant-induced rheumatoid arthritis.14,33,45
Considering the fact that overproduction of ROS is the chief culprit behind activation of NF-κB and its downstream pro-inflammatory cytokines,11,43 it is noteworthy to presume that the anti-inflammatory activity of CT is related to its potent antioxidant potential, since it has been proven that the compound can scavenge free radicals in several in vitro experiments conducted by Jagdale et al. (2015).34 In addition, previous studies have indicated that CT can enhance Nrf2 signaling, which plays a critical role in regulating the gene expression of various antioxidants that can counteract massive ROS production11,33 Hence, we propose that activation of Nrf2 signaling plays a vital role in mediating the anti-inflammatory effect, and in turn, the renoprotective effect of CT against MTX-related nephrotoxicity via inhibition of the ROS/NF-κB signaling cascade (Figure 4).
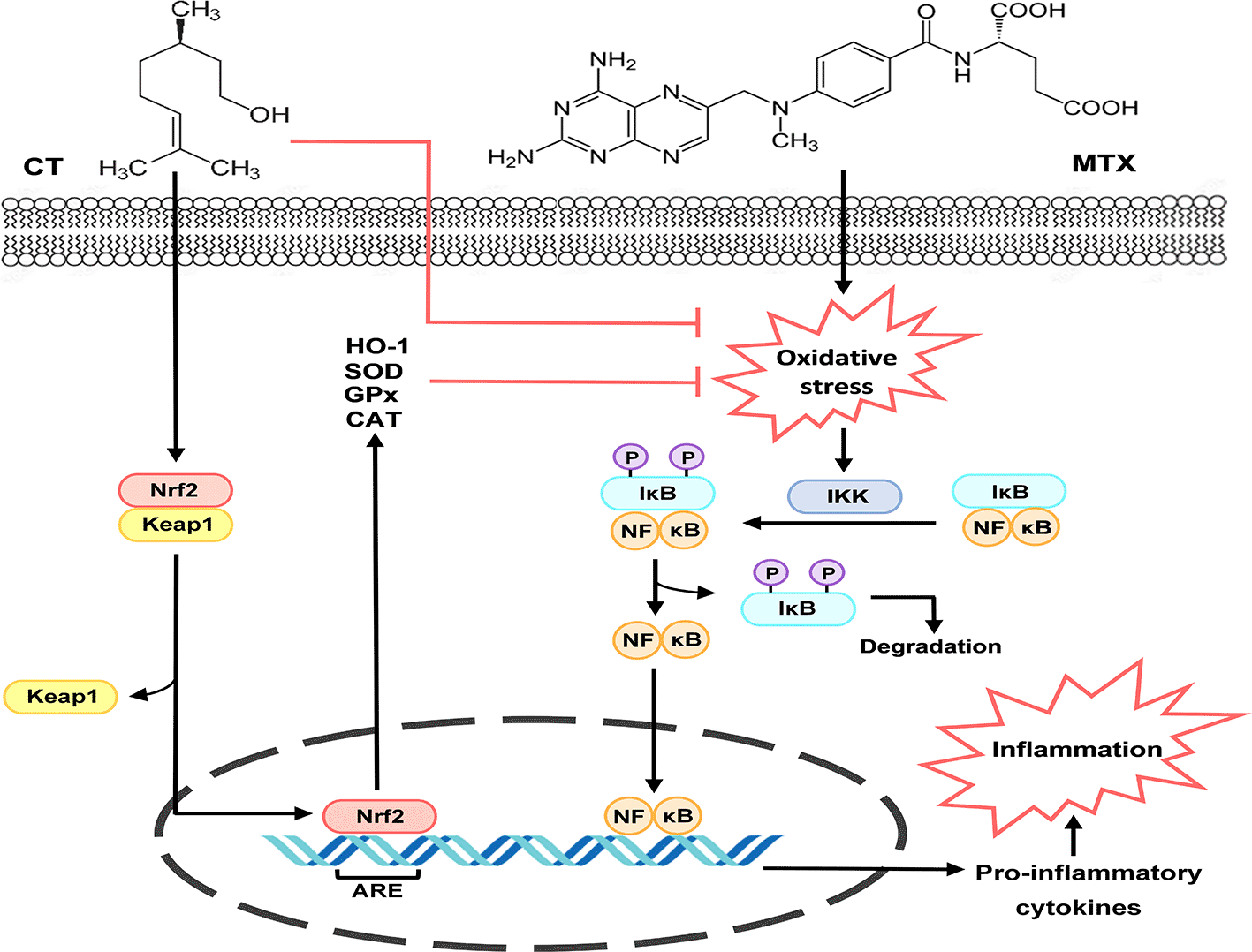
ARE, antioxidant response element; CAT, catalase; CT, citronellol; GPx, glutathione peroxidase; HO-1, heme oxygenase 1; IκB, inhibitor of NF-κB; IKK, IκB kinase; Keap1, Kelch-like ECH associated protein 1; MTX, methotrexate; NF-κB, nuclear factor kappa B; Nrf2, nuclear factor-erythroid 2-related factor 2; P, phosphate; SOD, superoxide dismutase.
The current study illustrates, for the first time, the ameliorative action of CT against renal damage induced by MTX. Our findings revealed that CT supplementation prior to MTX administration can alleviate renal damage biomarkers, enhance the endogenous enzymatic antioxidant defenses, diminish lipid peroxidation, and suppress renal NF-κB signaling with consequent inflammation-limiting effects. Therefore, we would like to emphasize that CT has high potential to serve as an adjunct modality for mitigating MTX-related nephrotoxicity. However, further investigations are warranted to highlight the comprehensive molecular mechanisms underlying the protective action of CT against nephrotoxicity provoked by MTX.
The current study protocol was reported in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines16 and was approved by the Research Ethics Committee of the College of Medicine, University of Baghdad (protocol number: 03-30; date of approval: October 8, 2023).
Zenodo: Dataset for biochemical assays and gene expression analysis. https://doi.org/10.5281/zenodo.13728740. 17
This project contains the following underlying data:
The data are available under the terms of the Creative Commons Attribution 4.0 International license (CC BY 4.0).
NCBI Protein: Tumor necrosis factor-alpha (TNF-α). Accession number NM_012675.3; http://identifiers.org/ncbiprotein:NM_012675.3 46
NCBI Protein: Interleukin-1 beta (IL-1β). Accession number NM_031512.2; http://identifiers.org/ncbiprotein:NM_031512.2 47
NCBI Protein: Beta-actin (β-actin). Accession number NM_031144.3; http://identifiers.org/ncbiprotein:NM_031144.3 48
Zenodo: ARRIVE checklist for oral pre-treatment with Citronellol ameliorates Methotrexate-induced nephrotoxicity in Wistar rats via targeting oxidative stress and inflammation. https://doi.org/10.5281/zenodo.13764641. 16
The data are available under the terms of the Creative Commons Attribution 4.0 International license (CC BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)