Keywords
Hydroxamic acid, Molecular hybridization, Benzothiazole, HDACs inhibitors
Molecular hybridization in drug design is proved to be very successful approach to provide new chemical entities with potential activities and desirable physicochemical properties. Histone deacetylases (HDACs) are involved in controlling the behavior and acetylation of histone and non-histone proteins and their inhibition causes block of cell growth, differentiation, changes in gene expression, and death. Consequently, HDAC inhibitors may lead to anticytotoxic activity. An approach of synthesizing hybrid molecules of benzothiazole cross-linked with hydroxamic acid via an amino acid or aminoalkanoic acid was considered. This approach is expected to optimize the anticytotoxic activity of the proposed compounds.
Hybrid molecules of benzothiazole cross-linked with hydroxamic acid (2A-E) were synthesized and their chemical structures were confirmed by spectral analyses (FT-IR, 1H-NMR and 13C-NMR). These were subjected to molecular docking on HDAC8 (PDB ID: 1T69). Computational methods were employed using the SwissADME server to predict the ADME parameters and other physicochemical properties. The cytotoxicity evaluation was performed using MTT colorimetric assay.
Hybrids (2A-E) recorded lower ΔG (-6.322 to - 9.46) than Vorinostat (SAHA, -5.375). ΔG is an affinity scoring function (kcal/mol) and is employed to rank the candidate poses as the sum of the electrostatic and Van der Waals energies. Benzothiazole cross-linked with hydroxamic acid by a p-aminobenzoic acid (2E) has recorded the lowest docking score of -9.460 and this may refer to the possibility of high inhibitory activity. The hybrids showed no violation from Lipinski’s rule and complied with parameters with low possible passive oral absorption and no penetration into BBB. Hybrid molecules of benzothiazole recorded very interesting results, particularly, 2D and 2E which showed significant and remarkable activity.
Hybrid molecules of benzothiazole cross-linked with hydroxamic acid recorded very interesting results, particularly, compounds 2D and 2E which showed significant and remarkable activity on lung cancer cell line type A549.
Hydroxamic acid, Molecular hybridization, Benzothiazole, HDACs inhibitors
Benzothiazole (BZT) is a fused rings of benzene and thiazole and has been intensively studied, as one of the privileged pharmacophores in medicinal chemistry.1 BZT has emerged as a core structure for diversified therapeutic applications, such as, antioxidant, anticancer, antiviral anti-proliferative, anti-diabetic, analgesic, anti-malarial, anti-fungal, anti-histamine and anti-convulsant.2 BZT scaffolds showed a crucial role in the inhibition of the tumor-associated metalloenzyme carbonic anhydrase and consequently, serve as anticancer agents.3 Research has revealed that inhibition of histone deacetylases (HDACs) causes inhibition of cell growth, differentiation, changes in gene expression and death.4,5 HDACs inhibitors have relatively low toxicity toward normal cells, while, inhibiting tumor cells from proliferation and survival. The first FDA-Approved HDACs inhibitor was the hydroxamate, Vorinostat (SAHA), which is used for treating cutaneous T-cell lymphoma.6 When HDACs activity is inhibited, the balance between the conflicting activities of HATs (histone acetylates) and HDACs shifts towards the predominance of HAT activity, leading to the induction of acetylation of both histone and non-histone proteins. As a result, certain genes that influence gene expression will be influenced by the induction or silencing of transcription factor expression as well as certain proliferative and/or apoptotic genes.7 As a result, HDACIs may activate pathways that lead to apoptosis, DNA replication is hindered, the cell cycle is inhibited, proliferation is stimulated, angiogenesis is decreased and DNA fragmentation is promoted. All of which can result in the death of cancer cells.8,9 The development of hybrid multifunctional inhibitors has generated a lot of interest in the search for new medicines. Hybrid molecules have the potential to simultaneously act on two or more cancer-relevant targets, such as, metallo-proteinase, ATP binding cassette subfamily G member 2, human mitochondrial peptide deformylase, nuclear factor kappa light-chain-enhancer of activated B cells, P-glycoprotein, tubulin and vascular endothelial growth factor.10–12 Various hybrid molecules of hydroxamic acid have anti-proliferative and anticancer activities and certain hybrids possess great potency against both drug-sensitive and drug-resistant cancers.13 2-Aminobenzothiazole derivatives containing hydroxamic acid were reported to have potent histone deacetylase inhibitory and antitumor activities.14 Hydroxamic acid derivatives prepared by the reaction of 2-aminobenzo-thiazole with adipic acid have exhibited good anticancer activities against five different cell lines such MCF-7, AsPC-1, SW620, PC3 and NCI-H460.15 Due to their reduced risk of adverse drug-drug interactions than multicomponent medications, hybrid molecules can also counteract the recognized side effects associated with various hybrid elements. As a result, one of the most popular contemporary methods for preparing new anticancer drugs is hybridization.16
In view of the previous findings, a new approach of synthesizing hybrid molecules of BZT cross-linked with hydroxamic acid via an amino acid or aminoalkanoic acid, as the linkers, was considered. 2-Mercapto-benzothiazole is to be reacted with 2-bromoacetic acid to afford BZT-carboxylate. This is to be covalently linked with either of the linkers and further reaction with hydroxylamine to form new hybrid molecules through amide bond using the mixed anhydride method. This approach is expected to optimize the pharmacological activities of the proposed compounds, particularly, the antitumor activity.
2-Mercaptobenzothiazole, p-aminobenzoic acid, 3-aminopropionic acid, 6-aminohexanoic acid were purchased from Hyperchem/China. Ethyl chloroformate (ECF, CAS ID: 541-41-3), Hydroxylamine. HCl (CAS ID: 5470-11-1), Vorinostat (suberoylanilide hydroxamic acid, SAHA, CAS ID: 149647-78-9), L-Alanine (CAS ID: 56-41-7), L-Cysteine (CAS ID: 52-90-4), aminopropionic acid (CAS ID: 107-95-9), aminohexanoic acid (CAS ID: 60-32-2) and p-amino- benzoic acid (CAS ID: 150-13-0) were purchased from sigma/Aldrich (Germany). For cell culture studies all the investigated compounds were dissolved in dimethyl sulfoxide (DMSO, 10 ml stocks) and were stored in a deep freezer at -20 °C. Experimentally, stock solutions were diluted in cell culture medium. The hybrid molecules were synthesized as described in details in the following section.
Preparation of the investigated hybrid molecules
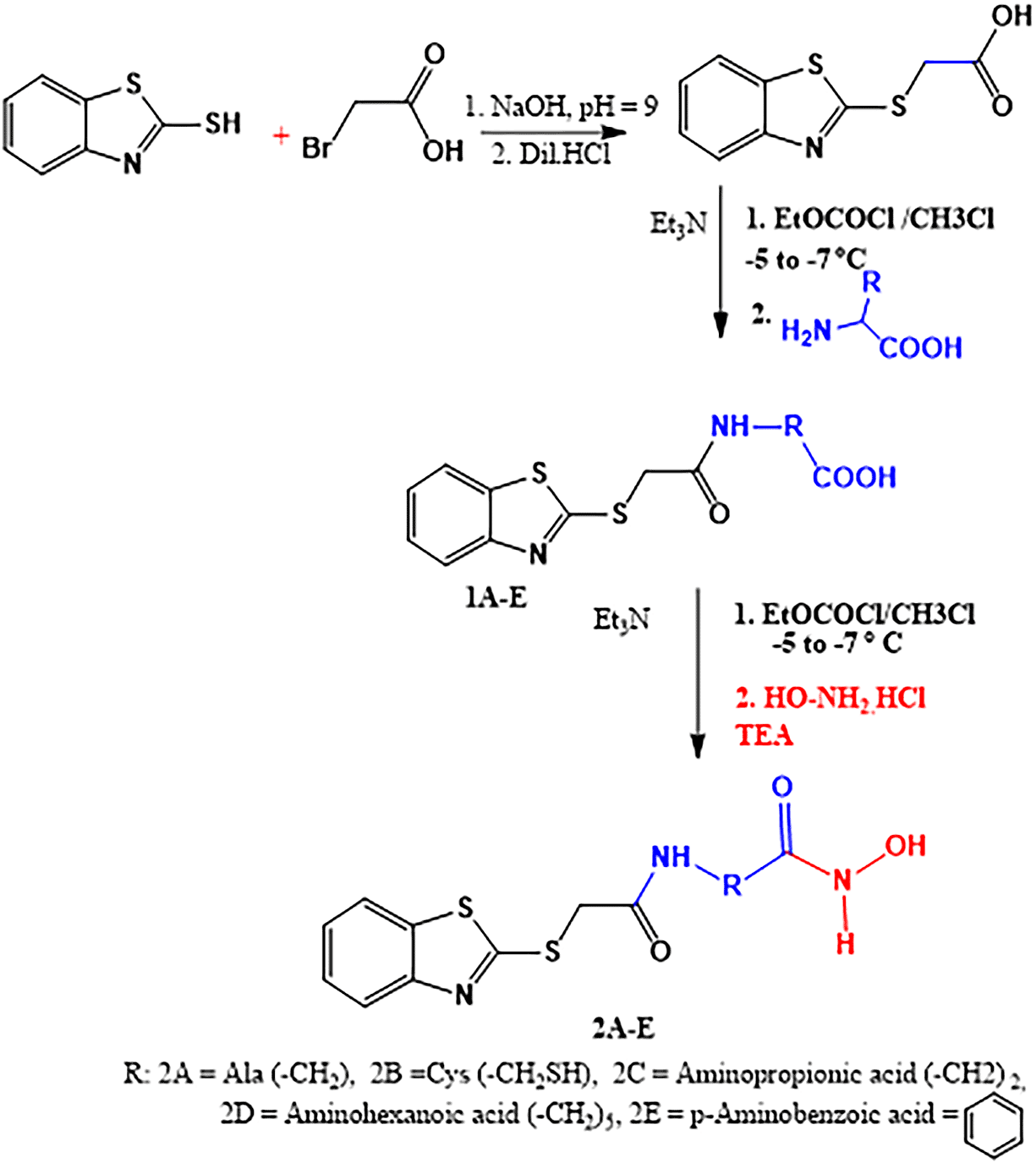
Hybrid molecules of benzothiazole cross-linked with hydroxamic acid through certain linkers, such as, L-alanine, L-cysteine, 3-aminopropionic acid, 6-aminohexanoic acid or p-aminobenzoic acid were proposed. These hybrid molecules were synthesized starting from 2-mercaptobenzothiazole, which is S-alkylated with bromoacetic acid leading to the formation of benzothiazole containing a side chain with a sulfide and a carboxyl group, as called the intermediate (2-(benzo- [d]thiazol-2-yl-thio) acetic acid). The chemical structures of the new hybrids (Scheme 1) and their SMILES (Simplified Molecular-Input Line-Entry Systems) notations were constructed using Chemdraw ultra 10.0. Vorinostat (SAHA) was used as the reference ligand.
Molecular docking
Molecular docking was performed using the Maestro software (Schrödinger, version 2023-1) and the ΔG (kcal/mol) values, as the docking scores function representing the energy required for binding to receptor, were calculated. The chemical structure of HDAC8 type 1T69 was retrieved from protein data bank, PDB, website (https://www.rcsb.org) and imported to Maestro software program. They were processed and their energy were minimized by the protein preparation workflow module. Through this process, unnecessary metals (except Zn+2 ions), ligands (except SAHA, which is the reference ligand), enzyme chains (except chain A), and the water molecules were deleted. The Grid was generated by the Receptor Grid Generator module, during which the binding site was defined by SAHA. The lowest docking scores, as ΔG (kcal/mol), for the investigated compounds and their images, which showed the interaction of each compound with Zn2+ ions and the amino acid residues in the binding site. Docking scores and visualization images of ligand-receptor complexes were shown on Table 1 and Figure 1. These hybrids were treated as successful candidates that have the highest binding affinities based on the lowest docking scores (∆G, kcal/mole).
General methods
Melting points were determined (uncorrected) using electrical melting point apparatus, Electro-thermal 9300, USA. The infrared spectra were recorded in KBr disc by FT-IR spectrophotometer (Shimadzu) 1H-NMR and 13C-NMR spectra were recorded using NMR Bruker 500 MHz-Avance III spectrometer and the chemical shifts were recorded in parts per million (ppm). Tetramethylsilane was used as the reference.
Chemical synthesis of the intermediate (2-(benzo [d]thiazol-2-yl-thio) acetic acid)
This compound is synthesized through S-alkylation reaction of 2-mercaptobenzothiazole with α-bromoacetic acid, and as follows;
To a stirred aqueous solution of α-bromoacetic acid (10 mmol, 1.389 g) in sodium hydroxide solution (10%, 10 mL), 2-mercaptobenzothiazole (10 mmol, 1.6725 g) in an aqueous solution of sodium hydroxide (10%, 10 mL) was added drop wise with continuous stirring. The mixture was refluxed for 3hrs and was then cooled and acidified with diluted hydrochloric acid to obtain a precipitate, which was filtered, washed excessively with distilled water and crystallized from ethanol to afford a yield of 90%, as white powder, m.p. 152-153 °C, as depicted on Figure 1.
FT-IR spectra recorded the following bands; 3110-2513 (str. vib. of OH of COOH), 3074 (str. vib. of C-H aromatic ring), 2956-2896 (str. vib. of C-H aliph. (asym and sym), 1714 (str. vib. of C=O of COOH), 1602-1581-1498 (str. vib. of C=C aromatic ring), 1315 (str. vib. of C=O of COOH).
General procedure for the synthesis of BZT-Linker (1A-E)
These compounds were prepared using the mixed anhydride method,17 as depicted in Figure 1 and as follows;
The intermediate (2-(benzo [d]thiazol-2-yl-thio) acetic acid) (10 mmol) was suspended in dry chloroform (40 ml) containing triethylamine (TEA, 10 mmol) and the mixture was cooled to (-5 to -8 °C) in an ice bath. Ethyl chloroformate (10 mmol) was added dropwise during 15 min and the mixture was stirred for further 15 min. The linker (10 mmol) was dissolved in a cold distilled water containing TEA (10 mmol) and was added immediately all at once with vigorous stirring for 1hr in an ice bath and for further 3 hrs at room temperature. A two-layered yellowish-orange solution was obtained, which was separated using a separatory funnel and the aqueous layer was removed. The chloroform layer was washed with distilled water (3 × 20 ml) and was dried with anhydrous magnesium sulfate, filtered and evaporated under reduced pressure in a rotary evaporator to obtain yellowish-orange residues. The residues were washed with diethyl ether or petroleum ether few times to afford crystalline powder representing the resultant products (1A-E). These compounds were collected as follows:
BZT-Linker-Ala (1A) {(2-(benzo [d]thiazol-2-yl-thio) acetyl) alanine (C12H12O3N2S2, 296.36 g/mole)} was synthesized starting from the intermediate (2-(benzo [d]thiazol-2-yl-thio) acetic acid) and L-Alanine (10 mmol, 1.6 g), as red crystalline powder, m.p. 165-167 °C with 79% yield. FT-IR spectra (ʋ, cm−1) recorded the following characterized bands; 3461 (str. vib. of OH-COOH), 3419 (str. vib. of NH-amide), 2954-2905 (str. vib. of C-H aliph. (asym and sym), 1712 (str. vib. of C=O of COOH), 1668 (str. vib. of C=O of amide), 1589-1573-1479 (str. vib. of C=C aromatic ring).
BZT-Linker-Cys (1B) {(2-(benzo [d]thiazol-2-yl-thio) acetyl) cysteine (C12H12O3N2S3, 328.42 g/mole)} was prepared using L-Cysteine (10 mmol, 1.2 g) and collected as yellow crystalline powder, m.p. 175-177 °C with 85% yield. FT-IR spectra recorded the following bands; 3461 (str. vib. of OH-COOH), 3288 (str. vib. of NH-amide), 3062 (str. vib. of C-H aromatic ring), 2912-2871 (str. vib. of C-H aliph. (asym and sym), 1714 (str. vib. of C=O of COOH), 1641 (str. vib. of C=O of amide), 1600-1545 (str. vib. of C=C aromatic ring).
Compound 1C {3-(2-(benzo [d]thiazol-2-yl- thio) acetamido) propionic acid (C12H12O3N2S2, 296.36) was prepared by reacting the intermediate with 3-Aminopropionic acid (10 mmol, 1.6 g) and collected as orange powder, m.p. 210-212 °C with 76% yield. FT-IR spectra recorded the following bands; 3442(str. vib. of OH-COOH), 3225 (str. vib. of NH amide), 3050 (str. vib. of CH arom), 2987-2858 (str. vib. of C-H aliph. (asym and sym), 1724 (str. vib. of C=O of COOH), 1631 (str. vib. of C=O of amide), 1600-1514 (str. vib. of C=C arom).
BZT-Linker-Aminohexanoic acid (1D) {6-(2-(benzo [d]thiazol-2-yl-thio) acetamido) hexanoic acid (C15H18O3N2S2, 338 g/mole)} was prepared starting from the intermediate and 6-Aminohexanoic acid (10 mmol, 1.31 g) and collected as orange powder, m.p. 225-227 °C with 90% yield. FT-IR spectra recorded the following bands; 3427 (str. vib. of OH-COOH), 3271(str. vib. of NH of amide), 3075 (str. vib. of C-H arom), 2983-2873 (str. vib. of C-H aliph. (asym and sym), 1737 (str. vib. of C=O of COOH),1629 (str. vib. of C=O of amid),1612-1581 (str. vib. of C=C arom).
BZT-Linker-p-Aminobenzoic acid (1E) {4-(2-(benzo [d]thiazol-2-yl-thio) acetamido) benzoic acid (C16H12O3N2S2, 344.4 g/mole)} was prepared from the intermediate and p-Amino-benzoic acid (10 mmol, 1.37g) and collected as purple powder, m.p. 180-182 °C with 70% yield. FT-IR spectra recorded the following bands; 3430 (str. vib. of OH-COOH), 3330 (str. vib. of NH of amide), 3026 (str. vib. of C-H arom), 2981-2937 (str. vib. of C-H arom), 1703 (str. vib. of C=O of COOH), 1641(str. vib. of C=O of amide), 1598-1558-1479 (str. vib. of C=C arom).
General procedure for preparing the hybrid molecules (2A-E)
The target hybrid molecules were prepared starting with compounds 1A-E and reacted with hydroxylamine to afford compounds 2A-E, as depicted in scheme 1. This reaction was performed using the mixed anhydride method (17), as previously described. Compounds 1A-E (10 mmol) in dry chloroform containing TEA (10 mmol) was reacted with ECF (10 mmol) and was then reacted with hydroxylamine. HCl (10 mmol) in an aqueous solution (10 ml) containing TEA (10 mmol).
BZT-Linker-Ala Hydroxamate (2A) {2-(2-(benzo- [d]thiazol-2-yl-thio) acetamido)-N-Hydroxy-propanamide (C12H13O3N3S2, 311.37 g/mole) was synthesized using compound 1A (10 mmol, 2.964 g) and was collected as yellow powder, m.p. 253-255 °C with 80% yield. FT-IR spectra recorded the following bands; 3452 (OH str. vib. of oxime), 3257 (NH str. vib. of oxime), 3200 (NH str. vib. of amide), 3058 (str. vib. of C-H arom), 2983-2852 (str. vib. of C-H aliph, asym and sym), 1699 (str. vib. of C=O of oxime),1616 (str. vib. of C=O of amide), 1610-1569 (str. vib. of C=C arom). 1H-NMR spectra recorded the followings signals (ppm); 1.28 (doublet, 3H, CH3), 4.25 (quartet,1H, CH), 4.92 (singlet, 2H, CH2), 7.40-8.35 (multiplate, 4H, represent phenyl), 8.85 (singlet 1H, NH of amide group), 9.20 (singlet 1H, NH of oxime group), 10.96 (singlet, 1H, OH group). 13C-NMR spectra recorded the followings signals; 13.67 (CH3), 34.59 (CH), 61.50 (CH2), 111.91-125.83 (arom. CH), 152.58 (C=N of benzothiazole), 164.54 (C=O of amide), 168.08 (C=O of oxime).
BZT-Linker-Cys Hydroxamate (2B) {2-(2-(benzo [d]thiazol-2-yl-thio) acetamido)-N-hydroxy-3-mercapto propanamide (C12H13O3N3S3, 343.43 g/mole) was synthesized starting from compound 1B (10 mmol, 3.284 g) and was collected as paige-colored powder, m.p. 235-237 °C with 77% yield. FT-IR spectra recorded the following characteristic bands; 3431 (str. vib. of OH-oxime), 3290 (NH str. vib. of oxime), 3114 (str. vib. of NH-amide), 3078 (str. vib. of C-H aromatic ring), 2964-2894 (str. vib. of C-H aliph. (asym and sym), 1699 (str. vib. of C=O of oxime), 1639 (str. vib. of C=O of amide), 1596-1554-1496 (str. vib. of C=C arom.). 1H-NMR spectra recorded the following signals; 3.48 (singlet, 1H, SH), 4.50 (triplet, 1H, CH), 4.13 (doublet, 2H, CH2), 4.88 (singlet, 2H, CH2), 7.37-8.10 (multiplate, 4H, phenyl), 8.27 (singlet, 1H, NH of amide group), 8.82 (singlet, 1H, NH of oxime group), 10.81 (singlet, 1H, OH group).
13C-NMR spectra recorded the following signals; 39.66 (CH2-SH), 40.78 (CH), 41.23 (CH2), 124,86-135.25 (CH arom), 153.32 (C=N of benzothiazole), 158.61 (C=O of amide), 167.27 (C=O of oxime).
BZT-Linker-Aminopropionic acid (2C) {3-(2-(benzo [d]thiazol-2-yl-thio) acetamido)-N-hydroxypropanamide (C12H13O3N3S2, 311.37 g/mole)} was prepared starting from compound 1C (10 mmol, 2.964 g) and was collected as dark brown powder, m.p. 219-221 °C with 88% yield. FT-IR spectra recorded the following bands; 3315 (OH str. vib. of oxime), 3188 (NH str. vib. of oxime), 3112 (NH str. vib. of amide), 3053 (str. vib. of C-H arom.), 2981-2837 (str. vib. of C-H aliph. (asym and sym), 1704 (str. vib. of C=O of oxime), 1679 (str. vib. of C=O of amide), 1600-1531-1500 (str. vib. of C=C arom.). 1H-NMR spectra recorded the following signals; 2.16 (triplet, 2H, CH2-C=O), 3.58 (triplet, 2H, CH2-N), 4.32(singlet, 2H, CH2-S), 7.32-8.20 (multiplate, 4H, phenyl, 8.90 (singlet, 1H, NH of amide), 8.94 (singlet, 1H, NH of oxime), 9.86 (singlet, 1H, OH).
13C-NMR spectra recorded the following signals; 19.05 (CH2-C=O), 38.30 (CH2-N), 56.53 (CH2-S), 119.31-129.34 (CH arom), 152.97 (C=N of benzothiazole), 166.37 (C=O of amide), 166.59 (C=O of oxime).
BZT-Linker-Aminohexanoic acid (2D) {6-(2-(benzo [d]thiazol-2-yl-thio) acetamido)-N-hydroxyhexanamide (C15H19O3N3S2, 353.45 g/mol) was synthesized from compound 1D (10 mmol, 3.38 g) and was collected as chocolate-color powder, m.p. 269-271 °C with 85% yield. FT-IR spectra recorded the following bands; 34446 (str. vib. of OH-oxime), 3359 (str. vib. of NH-oxime), 3184 (NH str. vib. of amide), 3060 (str. vib. of C-H arom.), 2985-2918 (str. vib. of C-H aliph. (asym and sym), 1704 (str. vib. of C=O of oxime), 1649 (str. vib. of C=O of amide), 1599-1583(str. vib. of C=C arom.). 1H-NMR spectra recorded the following signals; 1.25-2.11(Multiplate, 6H, 3(CH2), 4.14 (triplet, 2H, CH2-C=O), 4.19 (triplet, 2H, CH2-N), 4.87 (singlet, 2H, CH2-S),7.35-8.05 (multiplate, 4H, phenyl), 8.32(singlet, 1H, NH of amide), 8.86 (singlet, 1H, NH of oxime), 10.20 (singlet, 1H, OH). 13C-NMR spectra recorded the followings; 13.78 (CH2), 18.25 (CH2), 20.89 (CH2), 24.60 (CH2 C=O), 35.86 (CH2-N), 55.86 (CH2-S), 111.86-134.90 (CH arom), 151.03 (C=N of benzothiazole), 158.84 (C=O of amide), 162.32 (C=O of oxime).
BZT-Linker-p-Aminobenzoic acid Hydroxamate (2E) {4-(2-(benzo [d]thiazol-2-yl-thio) acetamido)-N-Hydroxy benzamide (C16H13O3N3S2, 359.42 g/mole) was prepared starting from compound 1E (10 mmol, 3.44 g) and was collected as white powder, m.p. 280-282 °C with 90% yield. FT-IR spectra recorded the following bands; 3444 (OH str. vib. of oxime), 3404 (NH str. vib. of oxime), 3295 (NH str. vib. of oxime), 3022 (str. vib. of C-H arom), 2972-2925 (str. vib. of C-H aliph. (asym and sym), 1685 (str. vib. of C=O of oxime), 1625 (str. vib. of C=O of amide), 1597-1546 (str. vib. of C=C arom). 1H-NMR spectra recorded the following signals; 4.12 (singlet, 2H, CH2-S), 7.28-7.30 (doublet, 2H, CH far C=O), 7.31-7.82 (multiplate, 4H, CH of benzothiazole),7.91-7.94 (doublet, 2H, CH near C=O), 8.35 (singlet, 1H, NH of amide group), 9.35 (singlet, 1H, NH of oxime), 10.78 (singlet, 1H, OH). 13C-NMR spectra recorded the following signals; 41.12 (CH2-S), 116.28-137.12 (CH arom), 153.61 (C=N of benzothiazole), 158.62 (C=O of amide), 167.22 (C=O of oxime).
Cell culture
Detailed information as well as culture and MTT seeding conditions for used cell lines are provided. All cells were grown under standard cell culture conditions and regularly checked for mycoplasma contamination. The medium was supplemented with 10% fetal bovine calf serum.
Cell viability assays
Cells were plated (depending on the cell model 2–7x 103 cells/well) in 96-well plates and allowed to recover for 24 h, 48 h and 72 h. Subsequently, the indicated drugs or their combinations were added. After 72 h exposure, the proportion of viable cells was determined by MTT assay.18 Cytotoxicity was expressed as IC50 (μg/ml) values calculated from full dose-response curves using Graph Pad Prism 8 software. The new hybrid molecules (2A-E) were tested for their anticancer activities using a colorimetric assay on the A549 human lung cancer cell.18 MTT stock solution was added and the mixture was stored to generate purple formazan crystals for 2-4 h at 37°C. MTT solution was correctly disposed, formazan crystal was mixed in DMSO (100 μl) containing SDS (10%) and acetic acid (1%). To completely dissolve the formazan crystals, the plates were agitated on the top of a shaker for 10 min. The procedure was done in 96-well flat plates at different concentrations (500-0.97 μM) of the investigated compounds and the IC50 values were calculated after treating the cells with the compounds for 24, 48 and 72 hrs. Cell culture medium was carefully removed after being treated with the hybrids. The optical density was obtained at 570 nm and a Safire 2 Microplate reader were used to calculate the concentrations. The experiments were carried out in triplicate independently. The results are presented on Table 4. The dose-response curves were done by Prism Pad 9 using nonlinear regression analysis for the synthesized molecules using A549 cells are shown on Figure 3.
All the investigated compounds (2A-E) have shown very interesting results of interaction pattern with the amino acids of HDAC8 (PDB ID: 1T69). These compounds have recorded high binding affinities, as indicated from the low docking scores, expressed as ΔG (Kcal/mol) than SAHA (Table 1). Moreover, compound 2E (BZT linked to hydroxamic acid by p-aminobenzoic acid, recorded the lowest docking score -9.460). This may indicate that this compound has the highest HDAC8 inhibitory activity. The interaction of compound 2E with the target HDAC8 type 1T69 was shown on Figure 2, and revealed the amino acids that were involved in this interaction. This compound recorded the best binding score that has been obtained in comparison with other compounds.
The SwissADME server was employed for the prediction of the physicochemical and ADME properties of the investigated compounds in comparison with SAHA. The pharmacokinetic parameters of these target compounds have shown variation in properties according to chemical structures, as illustrated on Tables 2 and 3. The predicted results have indicated that there was no violation of Lipinski’s rule19 and all the investigated compounds complied with all parameters. However, the results have predicted low passive oral absorption and no penetration into the blood brain barrier (BBB), as shown on Table 3. This may be due to the violation of other rules such as Veber, Egan, and Muegge. All target compounds have violated these rules because their total polar surface area (TPSA) is >131.6 for Egan, and >140 for Veber. Additionally, compound 2B has violated Muegge rule because it has a TPSA of >150 (Table 2).
The predicted low oral bioavailability of the target compounds may suggest that the potent compounds could be used through other route of administration. Compounds 2B and 2D may be considered as P-gp substrates. However, compounds 2A, 2C and 2D may exhibit a lower incidence of cellular resistance. SAHA did not show a predictive inhibitory activity to any of the CYP enzymes used in this study. While, compounds 2B, 2D and 2E have shown possible predictive inhibitory activities (Table 3). It is worth mentioning that the cap group and the linker of the presently investigated compounds are considered more versatile due to the presence of more hydrogen bond acceptors and donors. Besides, the optimal length, which is favorable for the interaction with the target enzyme, as indicated by the well-confirmed structure-activity relationships of HDACs inhibitors.20,21
The interaction of the investigated compounds and SAHA with HDAC8 type 1T69 were comparable to a large extent showing that these target compounds bound to the target enzyme on approximately the same amino acids (Table 1). This may indicate that these compounds may have certain inhibitory activity resembling that of SAHA. However, compound 2E has recorded a remarkable result of binding affinity (docking score) of –9.460, and hence, is expected to have a much higher activity than SAHA. Moreover, this hybrid molecule has interacted to the target enzyme in a much similar way in comparison with SAHA (Figure 2). SAHA has interacted on Asp101, His142, Tyr306, Zinc378 of the target enzyme. While, compound 2E has interacted with His142, Tyr306, Zinc 378 with a very high docking score than SAHA (Table 1).
The determination of the half-maximal inhibitory concentrations (IC50) of the hybrid molecules has revealed that compounds 2B, 2D and 2E have showed significant and remarkable activity on lung cancer cell line type A549, potent than SAHA results, particularly after 72 hrs incubation time (Table 4).
Moreover, dose-response curves were constructed to evaluate the relationship between the percent inhibition of the cell line activity of A549 by the synthesized compounds in comparison with SAHA (Figure 3). The results revealed that there is a great similarity in the pattern of the relationship. This is an expected result due to the great similarity in chemical structures of this series of compounds and SAHA.
The highest predicted activity of compound 2E may be due to the rigidity of the aromatic ring which enforces the molecule to take a specific pose directed toward Zinc ions, forming a stronger interaction, as shown in Figure 2. Moreover, this compound may form a π- π interaction (π-π stacking) with the aromatic amino acid residues (such as L-Tyrosine, L-Phenylalanine and L-Histidine) lining the hydrophobic tunnel of the active site near the Zinc ions, as previously reported.22 The significance of the aromatic linker has been evident in developing several HDACs inhibitors, such as, tubastatin A, belinostat and quisinostat.23
The predicted results of low passive oral absorption and no penetration into the blood-brain barrier (BBB) may be due to the violation of other rules such as Veber, Egan and Muegge. All the target compounds (2A-E) have violated these rules because their total polar surface area (TPSA) is >131.6 for Egan, and >140 for Veber. Moreover, compound 2B has violated Muegge rule because of the high TPSA of >150 (Table 2). Results have indicated that SAHA did not show a predictive inhibitory activity to any of the CYP enzymes, while, compounds 2B, 2D and 2E have shown possible predictive inhibitory activities. These results may be due to the presence of versatile groups, such as, -SH of L-Cysteine in compound 2B, (-CH2 -)4 present in compound 2D and p-aminobenzoic acid present in compound 2E, which added more hydrogen bond acceptors and donors. Interestingly, the aromatic ring in the hybrid molecule 2E contributed to binding affinity to target enzyme to record better docking score and also to activity against lung cancer cell line type A549. Contribution of an aromatic moiety to binding affinity and actual in vitro antibacterial activities has also been noticed in previous findings.24,25
Besides, the long chain of (-CH2-)4 provides hydrophobicity that is favorable for perfect binding or interaction with target enzyme. A similar case was noticed with the FDA-Approved drug, Panobinostat as powerful HDAC inhibitor.26 This situation is highly indicated by the well-confirmed structure-activity relationships of HDACs inhibitors.27 It was reported that the presence of 6-aminohexanoic hydroxamate, prominently induced the differentiation of mouse neuroblastoma cells at sub-millimolar concentrations.28 A similar case was noticed with compound 2D, which include benzothiazole-linked to aminohexanoic hydroxamate and afforded significant activity on lung cancer cell line A549 (Table 4). Previously, a reported work stated that new benzothiazole analogues containing aliphatic side chain and based on SAHA exhibited potent HDAC inhibition and cytotoxicity against five cancer cell lines, namely colon cancer SW620, breast cancer MCF-7, prostate cancer PC-3, pancreatic cancer AsPC-1 and lung cancer NCI-H460.29
New hybrid molecules of Benzothiazole cross-linked with hydroxamic acid through an amino acid or aminoalkanoic acid linkers were successfully prepared and their binding affinities to HDAC8 (PDB ID: 1T69) were higher than the reference ligand, SAHA. Moreover, the aromatic linker in compound 2E has contributed to much better binding and causes the highest docking score than SAHA and the other hybrid molecules. Compounds 2A, 2C and 2D were not shown to be P-gp substrates. Therefore, this work may introduce new hybrid molecules 2B, 2D and 2E with significant anticancer activity when compared with SAHA. Moreover, compound 2E is a promising and potential successful candidate.
Zenodo: Cytotoxicity Evaluation, https://doi.org/10.5281/zenodo.11190227. 30
Zenodo: Spectral Analyses, https://doi.org/10.5281/zenodo.11190182. 31
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We gratefully acknowledge Dr. Wrood Salim Al-khfajy for performing the in vitro cytotoxicity evaluation.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Computer-Aided Drug Design, Molecular Docking, MD Simulation, Cancer Research.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Banerjee S, Baidya S, Jha T, Ghosh B, et al.: Arylcarboxamide Derivatives as Promising HDAC8 Inhibitors: An Overview in Light of Structure-activity Relationship and Binding Mode of Interaction Analysis. Medicinal Chemistry. 2024; 21. Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Drug design , synthesis, QSAR
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 11 Oct 24 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)