Keywords
Adenomyoepithelioma, breast tumor, case report, histological diagnosis, surgical management.
Adenomyoepithelioma is a rare benign tumor classified among myoepithelial and epithelial-myoepithelial tumors. While it can occur in various locations, its presence in the mammary gland is extremely uncommon. The diagnosis relies heavily on histological and immunohistochemical characteristics, as radiological findings are often non-specific.
Case 1: A 46-year-old woman presented with an ulcerated inflammatory mass in the left breast, evolving for one year. Clinical examination revealed multiple masses and ipsilateral lymphadenopathy. Initial biopsies suggested intraductal papilloma, but subsequent analysis confirmed adenomyoepithelioma after a macro biopsy. A mastectomy was performed, revealing a solid-cystic lesion with clear surgical margins, and the patient had a favorable postoperative course.
Case 2: A 38-year-old woman presented with an irregular mass at the junction of the upper quadrants of her right breast. Imaging revealed complex solid masses, leading to a diagnosis of adenomyoepithelioma with malignant transformation. A modified radical mastectomy was performed, followed by adjuvant radiochemotherapy. The patient tolerated the treatment well and exhibited good clinical progress.
Adenomyoepithelioma, though rare, can manifest with diverse clinical and imaging features, sometimes mimicking more common breast lesions. Accurate diagnosis often requires comprehensive histological examination. These cases underscore the importance of multidisciplinary evaluation and tailored surgical management to optimize patient outcomes.
Adenomyoepithelioma, breast tumor, case report, histological diagnosis, surgical management.
Adenomyoepithelioma is a rare benign tumor belonging to the group of myoepithelial and epithelial-myoepithelial tumors.1 While this tumor can develop in the salivary glands and skin appendages, its presence in the mammary gland is extremely rare.2 It usually affects women between the ages of 25 and 75, with exceptional cases reported in men.3 The radiological diagnosis is non-specific and primarily relies on histological and immunohistochemical characteristics.
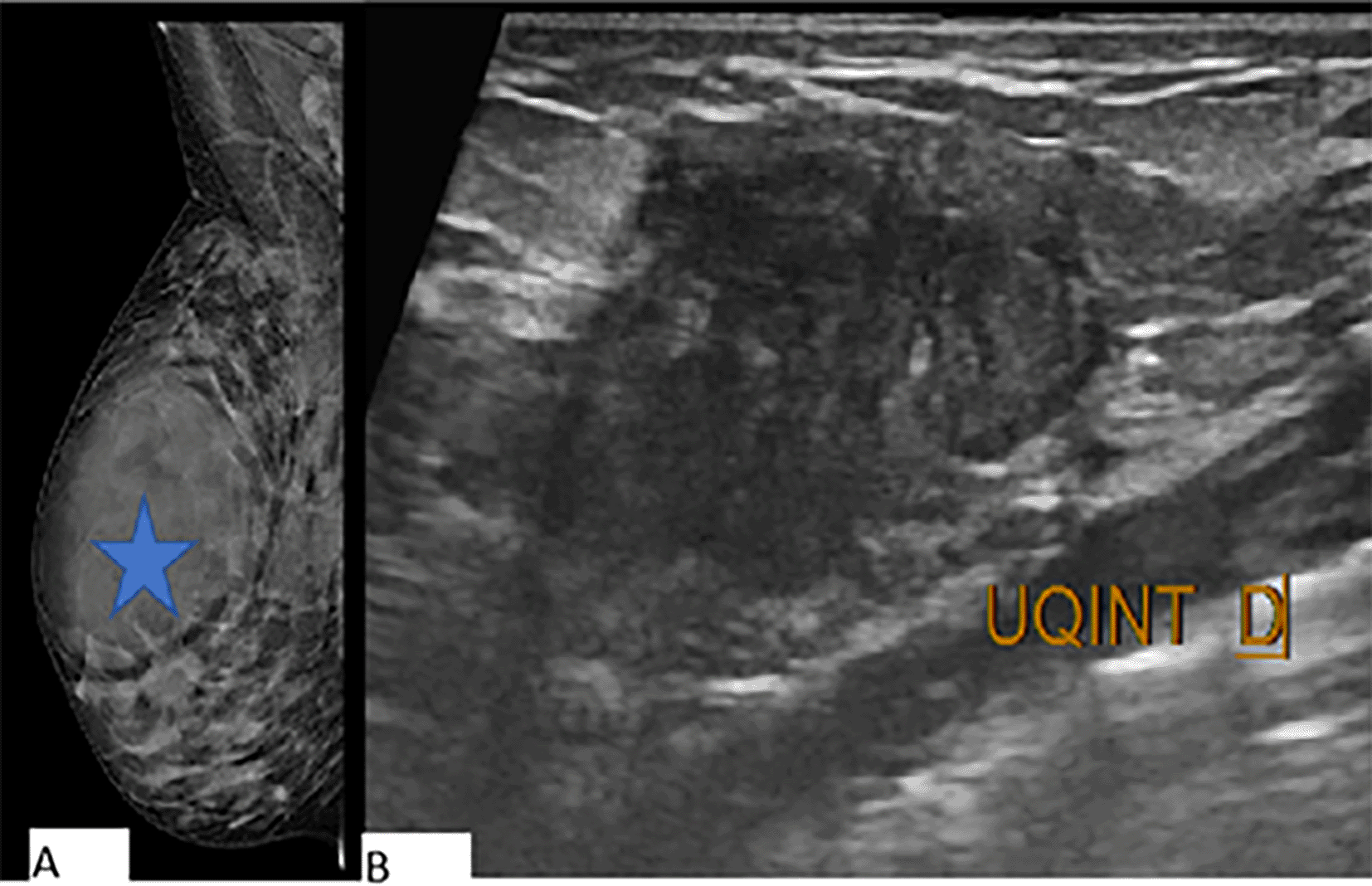
A 46-year-old woman with no significant medical history presented with an ulcerated inflammatory mass in the left breast that had been evolving for a year. Clinical examination revealed multiple masses in the left breast, involving all quadrants, along with a suspicious ipsilateral lymphadenopathy (Figure 1). Breast ultrasonography showed multiple irregular isoechoic masses with cystic components, and Doppler ultrasonography revealed increased vascularity within the tumor.
A biopsy was performed twice, revealing a tumor focus with histological and immunohistochemical features consistent with intraductal papilloma. Given the clinical, radiological, and pathological discordance, the decision was made to repeat the macro biopsy. The subsequent pathological examination revealed adenomyoepithelioma.
After discussion by the multidisciplinary team and with the patient’s consent, a mastectomy was performed. Macroscopic analysis of the specimen revealed an 8 cm solid-cystic whitish lesion, along with multiple intraductal papilloma’s ranging from 0.5 to 0.7 cm. Microscopic analysis showed tumor proliferation composed of epithelial and myoepithelial cells surrounding variably sized ducts, without signs of atypia (Figure 2). The surgical margins were clear in both the superior and inferior resection areas. The patient’s postoperative course was favorable.
A 38-year-old woman, with no significant medical history, presented with an irregularly contoured mass found at the junction of the upper quadrants of her right breast. A detailed examination revealed a notable retroareolar mass in the upper quadrant of the right breast. Ultrasonography showed multiple solid hypoechoic irregular masses with angular margins, indicating complexity and potential malignancy. Mammography revealed an oval, dense, non-calcified mass with microlobulated margins (Figure 3). To further assess the nature of the masses, a breast MRI was performed. This imaging showed a significant 6 cm irregular mass with intermediate heterogeneous T2 signal. The dynamic contrast enhancement revealed heterogeneous enhancement patterns (Figure 4). A biopsy of the mass was performed, and histological examination revealed a tumor proliferation characterized by ducts of varying sizes and shapes. These ducts were interspersed with stroma that displayed a mix of dense cellularity and fibrous tissue. This histological appearance was consistent with an adenomyoepithelioma, with clear evidence of malignant transformation of the epithelial component into invasive carcinoma. Given the diagnosis and the patient’s clinical presentation, the decision was made to proceed with a modified radical mastectomy (Patey procedure). Following surgery, the patient underwent adjuvant radiochemotherapy to address any residual disease and minimize the risk of recurrence. The treatment course was well-tolerated, and the patient exhibited good clinical evolution post-therapy.
A: Mammography shows an oval, dense, non-calcified mass (arrow) with microlobulated margins.
B: Ultrasonography reveals multiple solid, hypoechoic masses with irregular, angular margins.
Adenomyoepithelioma is a rare tumor, accounting for 0.5% of breast tumors in peri- and postmenopausal women. The first case was reported by Hamperl in 1970.4 On imaging, adenomyoepithelioma generally appears as a lobulated mass, often well-circumscribed, hypoechoic on ultrasound, with irregular, microlobulated, or indistinct margins. Color Doppler ultrasound shows increased vascularization, mainly peripheral rather than central, or a lack of vascularization.5,6 According to a study by Zhang et al. involving three cases of benign adenomyoepithelioma, MRI demonstrated poorly defined masses with homogeneous enhancement and “wash-out” dynamic phase characteristics.7 However, distinguishing between benign and malignant forms remains difficult in imaging as well as in histopathology, particularly in micro-biopsies due to the heterogeneity of the disease.8,9
Several classifications have been proposed for mammary adenomyoepithelioma: the WHO classification in 2019, followed by Rakha et al. in 2021, who distinguished three categories—benign, atypical, and malignant adenomyoepithelioma—based on the mitotic index and cytonuclear atypia. Recently, Jeremy in 2024 proposed differentiating between carcinoma in situ and invasive and microinvasive carcinoma within the malignant forms.2,10,11
Benign adenomyoepithelioma is characterized macroscopically by a well-circumscribed tumor with a biphasic proliferation of both epithelial and myoepithelial cells. The myoepithelial component is predominant, showing no atypical features and a low mitotic index. Based on its growth pattern, the tumor can be categorized into tubular, lobular, or spindle cell variants.12 Immunohistochemistry is key to identifying the epithelial component, marked by proteins such as AE1/AE3 cytokeratin, CK7, CK8, EMA, and CEA. The myoepithelial component is identified with markers like S100, SMA, SMMHC, p63, CK5/14, and smooth muscle myosin heavy chain.13 The tumor may also exhibit positivity for estrogen and/or progesterone receptors. Electron microscopy further demonstrates myoepithelial differentiation through structures like myofibrils with dense bodies, pinocytotic vesicles, desmosomes or tight junctions, and a mosaic-patterned basal membrane.14
Eva in 2020 described two cases of benign adenomyoepithelioma. In the first case, a 63-year-old woman was diagnosed with adenomyoepithelioma after a lumpectomy for a fluctuating mass in her right breast. The second case involved a 72-year-old woman initially diagnosed with a fibroadenoma in the left breast, which turned out to be adenomyoepithelioma after surgery. In both cases, immunohistochemistry confirmed the presence of epithelial and myoepithelial cells, and surgical margins were clear.14 Chen in 2021 described a rare form of benign adenomyopithelioma in a 51-year-old woman who presented with a mass in the upper outer quadrant of her right breast resembling a benign cyst on imaging. The final diagnosis of adenomyoepithelioma was confirmed postoperatively through pathology and immunohistochemistry.15
Malignant transformation may occur in tumors larger than 1.6 cm.16 Malignant adenomyoepithelioma presents various macroscopic features. The tumor can be well-circumscribed in carcinoma in situ but poorly defined when associated with invasive carcinoma, with a larger size than benign adenofibromas. Histologically, malignant adenomyoepithelioma is characterized by more than 10 mitoses per 10 high-power fields at 40× magnification, with moderate to severe atypia. It is important to differentiate two distinct entities with different prognoses and histological criteria: malignant in situ and invasive adenomyoepithelioma.11 Adenomyoepithelioma in situ features a proliferation of epithelial or myoepithelial components (or one of them) in structures resembling ducts, without foci of carcinoma infiltrating the connective tissue. The latter defines invasive adenomyoepithelioma, which may be epithelial in the case of non-specific invasive carcinoma or myoepithelial in metaplastic or mixed carcinoma. Atypical adenomyoepitheliomas lack the criteria necessary to be classified as malignant adenomyoepitheliomas. They exhibit myoepithelial cell atypia with fewer than 10 mitoses per 10 high-power fields at 40× magnification.
From a molecular standpoint, several gene mutations have been identified that influence the tumor’s hormonal status. The HRAS p.Q61K hotspot mutation promotes myoepithelial differentiation and is associated with the absence of estrogen receptor expression. Mutations in PIK3CA or PIK3R1 can subsequently occur, promoting the acquisition of a myoepithelial phenotype. In adenomyoepitheliomas expressing the estrogen receptor, the initial genetic abnormality is, in 60% of cases, an activating mutation in PIK3CA or AKT1. More rarely, an HMGA2 fusion transcript may be observed in the absence of HRAS, PIK3CA, and AKT1 mutations.11,17
In the literature, sixty-six cases of malignant adenomyoepithelioma have been reported, with an average age of 66.2 years and a tumor size of 8 cm. Among them, nearly 40% have a mixed epithelial and myoepithelial component, 23% have epithelial tumors, and 36% have myoepithelial tumors. According to Jeremy in 2024, 50% of cases are invasive malignant adenomyoepitheliomas, 48% are carcinomas in situ, and only 2% are microinvasive.11 The prognosis depends on the benign or malignant nature of the adenomyoepithelioma, with a risk of recurrence of 10% for benign forms and 35% for malignant forms.18,19 The prognosis is worse when the myoepithelial component predominates, especially if it is of high grade. The most common metastatic sites reported in the literature are the lungs (55% of cases), brain (22%), and bone (11%).20
Given the uncertain and unpredictable potential for malignant transformation and the risk of local recurrence, conservative excision with clear margins is currently the most appropriate surgical treatment.21,22 For malignant adenomyoepithelioma, treatment involves complete resection with radiotherapy in the case of conservative surgery and lymph node dissection if the tumor is infiltrating or microinvasive. Hormone therapy may be offered for tumors expressing estrogen receptors. Chemotherapy, however, has limited efficacy.11
Adenomyoepithelioma encompasses a heterogeneous group of tumors forming a continuous spectrum that includes benign, atypical, and malignant forms. Although generally benign, it can present risks of local or distant recurrence. The morphological analysis of the resection specimen allows for a better prognosis of the tumor, which can also rely on histological and molecular criteria.
The patient signed informed consent for the publication of this case report and any associated images.
The authors are sincerely grateful to the patient for giving the permission to share this informative report.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)