Keywords
Spinal canal stenosis, laminectomy, foraminotomy, nerve root compression, postoperative pain
While a laminectomy is often mandated for spinal canal stenosis, foraminal stenosis may account for pain and functional limitations instead of central canal issues. Targeted treatment directed at this specific region may enhance patient outcomes.
Retrospective observational study of a total of 30 patients with previous decompression laminectomies for lumbar spinal canal stenosis were operated upon for foraminotomies from November 2012 to November 2018. The surgeries were clinically done, and EMG-confirmed nerve root compressions were treated with exact decompression techniques according to the anatomy and pathology in each case. Additionally, a systematic review followed PRISMA guidelines was conducted, utilizing Scopus and PubMed databases to identify studies on reoperation rates after decompression with or without fusion in lumbar spinal stenosis.
The results were fearsome post-operatively, trying significant improvement in pain, functional status, and walking distance. Pain reduction was achieved in 80% of patients by an increase in the Carnovsky rating scale with evidence of improved pain reduction and walking endurance; this points to minimal resulting complications of one complete case of foot drop and three infective cases managed conservatively. It was shown in the systematic review that fusion surgeries lowered the rates of reoperations at the index level but increased those for adjacent segment reoperations, while decompression alone had higher index-level reoperation rates due to recurrent stenosis.
Most of the emphasis on foraminal stenosis, rather than traditional laminectomies, significantly reduces postoperative pain and improves functional outcomes. This strategy brings a promising alternative to the management of recurrent symptoms in patients with lumbar spinal canal stenosis.
Spinal canal stenosis, laminectomy, foraminotomy, nerve root compression, postoperative pain
The anatomy of the lumbar spinal canal scientifically bases this notion. Relative to the volume of the spinal canal, the bundle of nerve roots is far more significant than the difference; thus, the increased mobility in CSF. However, pain, which is a common complaint with decompression laminectomies, primarily arises due to pinching at the exit of the nerve root through the intervertebral foramen and not entirely due to the too-narrow spinal canal. By dealing with foraminal stenosis alone in cases of reoperation, we intend to decompress the nerve roots and alleviate associated pain without any further impairment of the structural integrity of the spinal canal. This approach is bolstered by a rapidly accruing body of evidence that suggests, in at least some cases, targeted decompression at this level can result in enormous improvements in patient outcomes concerning reduced pain and improved functional capacity.1
The main purpose of this study is to perform an outcome evaluation of the decompression laminectomies in the treatment of foraminal stenosis in the presence of symptoms of the lumbar spinal canal stenosis, focusing particularly on whether targeted decompression effectively improves postoperative pain, functional recovery, and reoperation rate.
The current retrospective observational study has enrolled 30 patients who had previously undergone decompression laminectomies for the treatment of lumbar spinal canal stenosis and undergone surgical reoperation between November 2012 and November 2018, pegged on an eligibility criterion of those with persistent symptoms of nerve root compression despite seven months of conservative management or with recurrent symptoms within 1 to 4 years following their initial surgery.
The setting of this study was Baghdad Neuroscience Hospital, with clinical diagnosis and EMG confirmation of nerve root compression. This study followed the STROBE guidelines for observational studies.2
No statistical analysis either inferential or otherwise was carried out in this study. Actually, only descriptive statistics on the patient outcomes that included pain score, functional status, and walking distance were used in the study. This is because of the retrospective nature of this study with relatively limited sample size.
Each patient was subjected to detailed preoperative evaluation by clinical examination and EMG studies to establish the diagnosis of nerve root compressions. Imaging studies are used to locate the exact site of foraminal stenosis and preoperative planning. Details of the patient’s history, along with surgical outcomes of previous operations, were taken for planning the intervention accordingly.
Few biases were expected since diagnosing and performing with uniform techniques for all patients were considered in the prospective study. Reoperations were carried out by the same surgical team that had undertaken the initial procedures. A study size of 30 patients was adequate, considering prevalence and the volume cases handled by the institution. Data collection was from consistent medical records of the patients, EMG results, and follow-up for all groups.
The procedures performed were foraminotomies, which were clinically executed and verified using EMG-nerve root compressions. Consequently, a careful and detailed surgical approach was undertaken based on the patient’s anatomical findings and clinical condition. Methods of performing surgery Here are some important recommendations for the surgical technique: 1) Anesthesia and Positioning: The patients were administered anesthesia and placed in a face-down posture on the operating table. The pressure points were adequately cushioned to prevent the development of pressure ulcers and nerve damage. 2) Incision and Exposure: A vertical cut in the center of the lower back was performed, allowing access to the lumbar spine. The outermost layer of tissue was carefully separated to reveal the bony plates of the vertebrae, being cautious not to harm the surrounding soft tissues and muscles. 3) Foraminotomy Procedure: Foraminotomy was done by decompression of the nerve roots with rongeurs and a motor drill. This primarily involved removing bone and soft tissue impinging on the nerve roots at the intervertebral foramina. Guidance and accuracy were obtained by intraoperative fluoroscopy in conducting the decompression. 4) Hemostasis and Closure: Meticulous hemostasis was done and the surgical wound was closed in layers with absorbable sutures for deep tissues and non-absorbable sutures for the skin.
Following surgery, patients were managed for pain, underwent physiotherapy sessions, and were followed up regularly. Patients were observed for any signs of complications, and their recovery process was recorded. Follow-up was planned for 1-, 3-, 6-, and 12-months post-surgery and periodically after that, as required by the individual progressions of patients.
The outcome measures recorded in this study included objective pain scores, the Carnovsky rating scale for functional status, and walking distance before the onset of pain.
The systematic review follows Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1)3 and involves a thorough literature search using Scopus and PubMed to identify studies on reoperation rates after decompression with or without fusion for lumbar spinal stenosis. The search was conducted without restrictions on language or geography, and it utilized a combination of relevant keywords and MeSH terms, such as “lumbar spinal stenosis,” “reoperation,” “decompression,” and “fusion.” Boolean operators were applied to refine the search, and filters limited the results to studies on human subjects.
Inclusion criteria consisted of studies with adult participants with a diagnosis of lumbar spinal stenosis, subjected to any decompression surgery with or without fusion, where reoperation rates or related outcomes had been reported. More specifically, it included retrospective and prospective cohort studies and RCTs. Exclusions included studies unrelated to lumbar stenosis, and those with no specific data on reoperation rates, case reports, editorials.
The selection process began with an initial number of studies, followed by duplicate removal. Two independent reviewers screened the titles and abstracts for relevance, resolving disagreements through discussion or by consulting a third reviewer. Full texts of potentially relevant studies were evaluated, and five studies were ultimately selected for inclusion.
Data extraction from included studies regarding study design, patient demographics, surgical interventions, reoperation rates, complications, and clinical outcomes was independently performed by two reviewers on a predeveloped standardized form. The results were synthesized narratively, with key data presented tabularly for comparison.
The quality of the included cohort studies was evaluated using the ROBINS-I (Risk of Bias In Non-randomized Studies of Interventions) tool.4 Each study was reviewed and categorized into one of the following levels: low risk, moderate risk, serious risk, or critical risk of bias for each domain. Studies with low or moderate risk across most domains were considered to have acceptable quality, while those with serious or critical risk of bias were flagged for potential concerns. No studies were excluded solely based on ROBINS-I assessment, but the level of bias was considered when interpreting the overall findings (Table 1).5–9
| Study | Bias due to confounding | Bias in selection of participants | Bias in classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall Bias |
|---|---|---|---|---|---|---|---|---|
| Ahn et al. (2019)5 | Moderate Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Moderate Risk | Moderate Risk |
| Deyo et al. (2011)6 | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Javalkar et al. (2011)7 | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Joelson et al. (2021)8 | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Kim et al. (2020)9 | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
A qualitative synthesis was conducted to compare reoperation and complication rates and other clinical outcomes across the studies.
Using the objective pain score scale, 80% of patients showed significant improvement in pain post-surgery from 10° as presumed to be preoperatively to 6°. Only one case had a complicated foot drop following our intervention; it was a partial one preoperatively but, unfortunately, became a complete one postoperatively. Only 3 cases of postoperative infection were reported, but all were treated conservatively without reopening them. The sensory radiculopathy showed improvement in only 30% of cases; other cases required treatment with medical neuroleptic drugs. There were also improvements in the Carnovsky rating scale and the distance walked before the onset of pain (Tables 2-4).
| Subjective pain score | Postoperative patients’ number |
|---|---|
| 3-4 | 9(23.7 %) |
| 6-5 | 19(50%) |
| 8-7 | 7(18.3%) |
| 10-9 | 3(8%) |
| Carnovsky rating scale | Preoperative score | Postoperative score |
|---|---|---|
| 4 | 19(50%) | 1(2.6%) |
| 5 | 17(44.8%) | 1(2.6%) |
| 6 | 1(2.6%) | 1(2.6%) |
| 7 | 1(2.6%) | 7(18.4%) |
| 8 | 0 | 9(23.7%) |
| 9 | 0 | 12(31.7%) |
| 10 | 0 | 7(18.4%) |
| Time (minutes) before the onset of pain | Preoperative | postoperative |
|---|---|---|
| 0-15 | 14(36.8%) | 2(5.2%) |
| 15-30 | 20(52.6%) | 4(10.5%) |
| 30-45 | 4(10.6%) | 3(8%) |
| 45-60 | 0 | 18(47.3%) |
| >60 | 0 | 11(29%) |
Subjective pain score
The patient’s subjective pain scores improve significantly postoperatively. Assuming that each patient had a presurgical pain score of 10, in this series, 23.7% of patients described their pain score as 3-4, which was a significant reduction in their pain. 50% of the patients described their pain as moderate, with scores ranging from 5-6. A smaller group, 18.3%, experienced a pain score ranging from 7-8, and a further 8% reported pain scores between 9-10, indicating very little pain relief for these few patients. In general, most patients expressed that they had considerable pain relief after surgery.
Carnovsky rating scale
The Carnovsky rating scale of the functional status dramatically improved after surgery. Fifty percent of patients were at a score of 4, and 44.8% were at a score of 5 before the operation. After the follow-up period, these percentages dropped significantly to 2.6% for both scores. On the other hand, the number of patients scoring relatively higher in the scale increased: 18.4% scored 7, 23.7% scored 8, 31.7% scored 9, and 18.4% scored 10. This shift shows an improvement in the functional status of patients following the new approach in management.
Walking distance before the onset of pain
The walking distance before the onset of pain also exhibited considerable improvement postoperatively. Whereas 36.8% of patients could walk only up to 15 minutes and 52.6% from 15-30 minutes without pain before surgery, these numbers decreased in the post-surgery phase to 5.2% and 10.5%, respectively. More encouragingly, unlike in the preoperative phase, 47.3% of the patients were able to walk for 45-60 minutes, with 29% able to walk for over an hour without pain, which pointed out that these patients significantly improved their mobility and endurance.
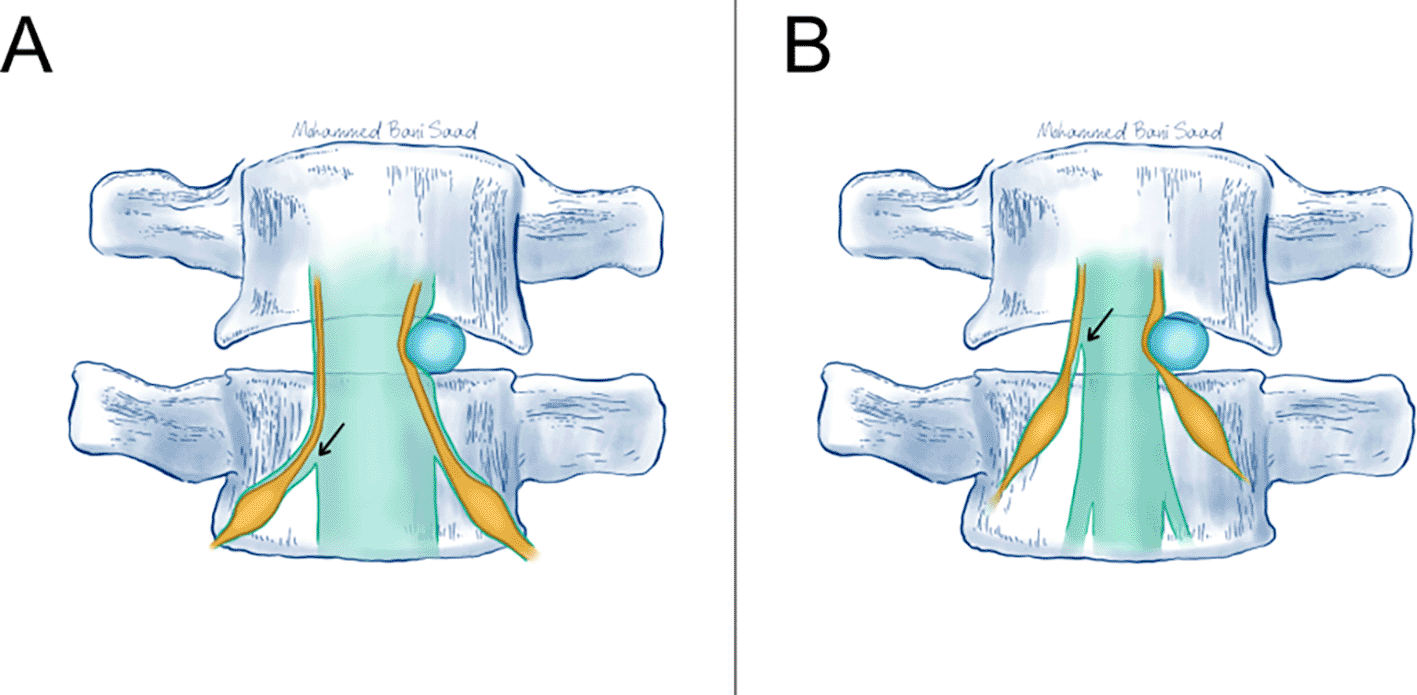
Findings from the study conclude that the new management strategy in place of conventional decompression laminectomies, foraminotomies, is proven to help patients with lumbar spinal canal stenosis significantly improve pain relief, functional status, and mobility. Figures 2 and 3 demonstrate the size of the thecal at the lumbar level and the impact of any compressive masses on the related anatomical structures. The results obtained here present a promising alternative treatment to the unsuccessful decompression surgery of the primary treatment. This may further be held out to prove foraminotomies as effective in reducing pain and improving functional outcomes in previous laminectomy patients who showed recurrent symptoms. Such targeted relief could afford lasting alleviation to patients with spinal canal stenosis, enhancing quality of life since it brings in significant improvement in terms of reduction of pain scores and functional assessments.

It has a potential space to accommodate any pressure by mass lesion, almost 75% of its diameter before compression on the nerve roots occur. Prepared by Dr. Mohammed A. Bani Saad.
This review synthesized data from five key studies examining reoperation rates following spinal decompression with or without fusion in patients with lumbar spinal stenosis. The results demonstrate varying outcomes in terms of reoperation rates, complications, and patient-reported outcomes, depending on the type of surgical intervention and patient characteristics (Table 5).5–9
| Article | Study type | Timeframe of study | Sample size | Country | Age (Mean ± SD) | Gender distribution (number & percentage) | Condition treated | Main symptoms | Surgical intervention | Outcome measures | Complications | Follow-up duration | Key Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahn et al. 20195 | Retrospective Cohort | January 2006 - December 2016 | 536 | Korea | 67 ± 9.5 | Men: 278 (51.9%), Women: 258 (48.1%) | Lumbar spinal stenosis at L4-L5, L5-S1 with or without degenerative spondylolisthesis. | Radiculopathy, back pain, leg pain | Decompression and Fusion | Oswestry Disability Index (ODI), VAS | 4.2% reoperation rate, pseudarthrosis | 5 years | Fusion associated with lower reoperation rates at the index level. |
| Deyo et al. 20116 | Prospective Cohort | March 1997 - May 2007 | 825 | United States | 69 ± 8.7 | Men: 402 (48.7%), Women: 423 (51.3%) | Lumbar spinal stenosis (L3-L4, L4-L5, L5-S1) with or without spondylolisthesis. | Neurogenic claudication, leg pain | Decompression and/or Fusion | Pain scores, ODI, SF-36 | 5.5% reoperation rate, implant complications | 8 years | Reoperation more frequent in patients with fusion. |
| Javalkar et al. 20117 | Retrospective Study | April 2000 - October 2010 | 690 | United States | 66 ± 10.2 | Men: 360 (52.2%), Women: 330 (47.8%) | Lumbar spinal stenosis primarily at L4-L5, involving adjacent segments in some cases. | Radicular pain, neurogenic claudication | Decompression, Fusion in some cases | Pain scores, ODI | 5.8% reoperation rate, adjacent segment disease | 6 years | Decompression alone associated with higher adjacent segment disease. |
| Joelson et al. 20218 | National Register Study | January 2007 - December 2017 | 6,532 | Sweden | 65 ± 9.1 | Men: 2,795 (42.8%), Women: 3,737 (57.2%) | L4-L5 spinal stenosis with or without degenerative spondylolisthesis. | Back pain, leg pain | Decompression with/without Fusion | Oswestry Disability Index (ODI), EQ 5D | 3-6% reoperation rates, implant failure | 10 years | Fusion increases reoperation rates at adjacent segments. |
| Kim et al. 20209 | Retrospective Cohort | May 2001 - March 2015 | 987 | Korea | 65.8 ± 11.7 | Men: 415 (42.1%), Women: 572 (57.9%) | Degenerative lumbar spinal stenosis (primarily L4-L5, L5-S1), some cases with multilevel involvement. | Back pain, leg pain | Decompression, Fusion | Oswestry Disability Index (ODI), VAS | 4.86% reoperation rate, proximal junctional kyphosis | 3-5 years | Fusion associated with better outcomes for back pain but higher complication rates. |
Reoperation rates
Reoperation rates varied significantly between patients who underwent decompression alone and those who underwent decompression with fusion. Fusion generally reduced reoperation rates at the index level but increased the likelihood of reoperations at adjacent segments due to adjacent segment disease (ASD). For instance, fusion often resulted in more stability at the treated level, particularly in cases with degenerative spondylolisthesis (DS), leading to fewer reoperations at that level. However, the increased mechanical stress on adjacent spinal segments due to fusion frequently led to degeneration over time, with reoperation rates at adjacent levels being notably higher in fusion patients. One study8 reported rates of adjacent segment reoperation as high as 9.7% for fusion compared to 4.2% for decompression alone. On the other hand, higher reoperation rates at the index level were noted for decompression alone, particularly in those procedures where instability was left unidentified. Rates for reoperation after a decompression alone were recorded to range from 4.0% to 6.2%,8,9 with recurrent symptoms and incomplete decompression being the more common reasons that necessitate further surgical interventions. Without fusion, the spine may remain vulnerable to degeneration at the treated level, which often necessitated additional surgeries.3
Complications and risk factors
It was expected that the type of surgery further modulated the rate and nature of complications. Fusion patients had a higher risk for pseudarthrosis, which is characterized by the failure of bone fusion, PJK, and implant failure.9 One of the most common reasons for reoperation following fusion surgeries was PJK, or abnormal curvature that developed above a fusion; this occurred in as many as 40% of various cohorts’ reoperations.9 The use of multiple levels of the spine during a fusion greatly increased the likelihood of its complication. In fact, multilevel surgery was a significant risk factor for subsequent reoperations.9 In contrast, decompression often created a need for reoperations due to problems such as recurrent stenosis or incomplete decompression.9 Recurrent stenosis occurred up to 48.8% in one study, and further intervention was needed to relieve the symptoms.9 Further, another significant cause for additional surgeries was the instability in the patients who first underwent decompression alone.7 The need for consideration of the choice of operation according to the patient’s condition is thus pointed out, especially in those cases when spinal instability can be an issue.
Comorbidities such as diabetes and cardiovascular diseases further increased the odds for reoperations, especially in cases of fusion patients.9 Deyo et al. (2011)6 stated that an older patient with additional health conditions presents more complications. These complications, along with implant-related failures, contribute a lot to the risk of reoperation.
Clinical outcomes and patient-reported measures
Patient-reported outcomes significantly improved for both groups, but fusion tended to be somewhat better regarding pain relief and patient satisfaction. Generally speaking, the patients who underwent fusion reported more significant improvements in back pain, which may be explained by the stabilizing effect of the surgery. In the series by Kim et al. (2020),9 medially, improvement in back pain was significantly better, with a mean improvement score of 3.9 ± 1.7 compared with a mean of 2.5 ± 1.5 for decompression alone. Fusion patients also reported higher satisfaction rates: 85.5% for fusion patients vs 72.3% for decompression patients.
Despite these enhancements, fusion had a higher rate of complications, which included pseudarthrosis and failures of the instrumentations, that might diminish the patient’s satisfaction when reoperation is required.3 However, for complication-free patients, fusion offered more durable relief from mechanical back pain than decompression alone.
Decompression-only surgeries also showed significant gains, particularly in alleviating the leg pain of neurogenic claudication. While the relief from back pain was generally not as robust as that seen with fusion, the decompression did involve improvement of mobility and reduction of leg pain without the additional risks associated with fusion. However, patients with untreated instability or more advanced degeneration of the spine typically required additional interventions due to the inability of decompression to prevent disease progression in those circumstances.7
With regard to reoperations, it is usually the case where ASD causes patients who underwent fusion to do reoperations at an adjacent level. Such reoperations did not usually worsen clinical outcomes. In a number of cases, even the reoperation following fusion still yielded a comparable or even better outcome in terms of pain relief and quality of life.8 This suggests that, while fusion may increase the need for further surgery at adjacent levels, the long-term benefits, particularly for back pain, remain favorable for many patients.
Timing of reoperations
The timing of reoperations also varied between the two groups. While reoperations subsequent to decompression alone generally occurred much sooner, with many patients requiring revision surgery within the first year post-operatively due to incomplete decompression or recurrent stenosis,9 reoperations after fusion tended to be somewhat later, often several years following the initial surgery, when ASD developed in a progressive way at adjacent levels.8 This is partly due to the fact that degenerative changes in adjacent segments following fusion tend to take longer, compared to the quicker recurrence of symptoms observed following decompression alone.
Potential for hybrid approaches, future research
One direction that would be very important to take in future studies is the investigation of hybrid surgical approaches that incorporate the elements of both decompression and stabilization without the rigidity of traditional fusion. Dynamic stabilization has the potential, for example, to stabilize the spine without the same magnitude of biomechanical stress on adjacent segments, with a potentially decreased risk of ASD. Minimally invasive techniques in performing fusion surgery may further reduce complications such as PJK and implant failure.9 It will permit more precise, targeted interventions that preserve more of the motion of the spine.
Future studies also need to target subgroups of patients who are most likely to benefit from each surgical variant. Factors related to age, comorbidities, and spinal anatomy can be used better in outcome prediction and intervention tailoring. For example, patients with isolated stenosis who have no evidence of instability may fare well with decompression alone, while patients with multilevel degenerative or spondylolisthesis disease may reap greater benefits from fusion. Moreover, predictive models might have a significant impact on enabling surgeons to better assess the risk of ASD and reoperation following fusion and thus enable more personalized treatment strategies.
LCS has been manifested by narrowing of the spinal canal, causing compression of the cord and nerve roots, clinically presenting with symptoms such as neurogenic claudication, pain, and sensory deficits. Radiological parameters, including but not limited to the dural sac cross-sectional area,10 morphological grading, and the sedimentation sign, have conventionally been used for quantifying the extent of stenosis. While these are important imaging modalities, they provide information on the anatomical severity of the condition, but often a limited correlation with the clinical presentation, like pain intensity and functional impairment in a patient. This therefore calls for an appropriate diagnosis that should adopt both clinical evaluations and radiologic findings for good diagnosis and treatment for LCS.10
This study investigated targeted decompression for foraminal stenosis in patients with previous laminectomies. The results showed significant improvements in pain relief, functional recovery, and walking endurance. While fusion reduced reoperation rates at the index level but increased the rates at adjacent segments, decompression alone was associated with higher reoperation rates due to recurrent stenosis. Targeted decompression, however, was found to improve patient outcomes effectively.
Recurrent symptoms following lumbar decompression surgery are not new, especially in patients with lumbar canal stenosis. Management has always been considered a challenge in spinal surgery. This study offers a very strong approach to such challenges by focusing on foraminal stenosis as the main contributor to post-laminectomy pain and neurological symptoms. Traditionally, the emphasis in reoperations has fallen more on central canal decompression. However, our results show that foraminal stenosis, when treated precisely by foraminotomy procedures, offers a better solution for symptom relief for those patients with prior laminectomies. The results of the study were significant in improving pain relief, functional capacity, and walking endurance following targeted foraminotomies. Close to 80% of patients enjoyed a reduction in their pain score while improvements in the Carnovsky rating scale underlined functional recovery attained post-surgery. This approach, as confirmed by the increased walking distances and improved pain scores, underlines the potential offered by foraminal decompression in providing long-term relief from nerve root compression without recourse to more invasive procedures like fusion.
One of the major findings in this study is the low rate of complications attributed to foraminotomies. With only one case of postoperative foot drop and three cases of infection that were conservatively managed, the procedure seems to carry fewer risks in comparison with fusion surgeries, which more often are associated with more complex complications such as pseudarthrosis and ASD.10 Second, it maintains the structural integrity of the spinal canal and heals the actual cause of nerve compression. This approach limits the mechanical stress that may further lead to deterioration, which is commonly witnessed in the use of fusion surgeries.
Results from this systematic review, which is run in tandem with our clinical study, therefore support our conclusion by indicating that while the literature evidence for fusion surgeries is effective in stabilizing the spine, it increases the risk of reoperation at adjacent levels because of ASD, and decompression alone, though associated with higher reoperation rates at the index level, provides a less invasive option with fewer long-term complications. Our findings indicated that foraminotomies may represent an effective compromise, offering the advantages of decompression without the mechanical disadvantages of fusion. This was further brought forth in work by Alimi et al. in 2015, who reviewed the management of recurrent lumbar spinal stenosis and reported good results with the minimally invasive laminectomy techniques.11 This type of study would imply the need to go forward with planning on an individual basis. This now offers the hope of a procedure that is less invasive, which can give even better benefits in managing recurrent stenosis.
Various studies reported that appropriate foraminal decompression may reduce neurological deficits and postoperative pain. Haddadi et al. (2016)12 had established that targeted decompression significantly enhances patient satisfaction and functional outcomes. Furthermore, Pietrantonio et al. (2019)13 present long-term results in terms of reduced complication rates and improvement in quality of life by using minimally invasive decompression techniques.
Comparing series, such as that by Lauryssen et al. (2012),14 have shown that facet-sparing lumbar decompression via minimally invasive techniques tends to achieve better results than does classical decompression. Ours is another finding to support those results and to further indicate that the focused foraminotomies contribute to better post-operative recovery and patient satisfaction. Srinivasan et al. (2021),15 too, have shown similar effectiveness of minimally invasive lumbar laminectomy and foraminotomy in providing significant pain relief and functional improvement.
The advantages of the foraminotomies over the laminectomy approaches are that they have shorter operative times, less intraoperative blood loss, postoperative pain, and faster recovery periods. Preserving spinal stability and avoiding extensive bone removal are essential factors in better outcomes and fewer complications.16 Such an approach to selective foraminotomies aligns closely with personalized medicine. By tailoring interventional surgery to the specific anatomical and pathological conditions of each case, surgeons can manage treatment more effectively and achieve better, individualized results. This patient-tailored approach results in less tissue damage and improved therapeutic outcomes.17
Advances in imaging and surgical technologies—like intraoperative neuromonitoring and highly resolved imaging—have made foraminotomies more precise and safer. Future research into these technological advances should be conducted to establish how they affect surgical outcomes in these patients. Extensive, multicenter studies still need to validate these values in long-term gains; techniques need refining for even better outcomes for patients.14
Despite these encouraging findings, the limitations of this study must be acknowledged. The sample size, although considered adequate for statistical significance, is relatively small and the follow-up period may not capture the long-term outcomes of this surgical approach. Further, the subjects of this study had all undergone prior laminectomies, and further research is needed to explore if such findings apply to primary surgeries for lumbar canal stenosis.
In the end, this study serves as good evidence that a targeted foraminal decompression approach offers massiveantages to patients with recurrent symptoms post-lumbar decompression over traditional reoperation strategies. Addressing the true etiology of nerve root compression, namely foraminal stenosis, allows surgeons to realize even greater pain relief and improve functional outcomes in their patients while reducing the requirement for further invasive procedures. These findings, if possible, should further be corroborated in research using larger and more diverse patient populations and also determining the long-term effectiveness of this approach in preventing further spinal degeneration.
This may be considered a unique investigation in the management of those patients in whom relaminectomies reopened for spinal canal stenosis and foraminotomies present a different approach, not considered previously. In this study, significant pain relief and functional improvement were achieved by accurately disclosing at least the site of nerve root compression, thus minimizing the requirement for further interventions and invasive treatments.
This method has been quite successful in drastically reducing rates of recurrence and offering long-term solutions for patients who have recurrent lumbar spinal stenosis, hence a favorite option for treatment. However, it is an unromantic plausible procedure that precisely addresses the root cause of the symptoms while maintaining the core structural integrity of the canal.
The Ethics Committee (University of Baghdad, College of Medicine) further stated that the current study does not require approval because it is a retrospective and has no new intervention. Moreover, verbal and written consent was obtained from all participants in this study.
The raw data is available at:
Moneer, F. (2024). Strategy for reopening of spinal stenosis cases. [Data set]. Zenodo. 10.5281/zenodo.13925403 18
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)