Keywords
Sorafenib, Lenvatinib, Precision medicine, Hepatocellular Carcinoma
Cancer remains a public concern and leading cause of death worldwide. Hepatocellular carcinoma, the most common form of liver cancer, is the third leading cause of cancer-related deaths. The number of cases is expected to double by 2040, according to GLOBOCAN 2020. Patients are usually diagnosed with advanced-stage HCC, which limits the number of effective treatment options. Oral-targeted therapies involving sorafenib and lenvatinib remain the first-line treatment option, which has recently been replaced by immunotherapy. Due to economic issues and ease of administration, the vast majority prefer oral systemic therapy. Sorafenib and lenvatinib, which belongs to tyrosine kinase inhibitors have few limitations of having large inter-individual variability in absorption, genetic polymorphisms, poor patient adherence to oral regimen limiting its long-term therapy. Therapeutic Drug Monitoring can overcome the limitations of TKIs and help individualise the dosage regimen with minimal adverse events. Therefore, this simple and rapid method using latest equipment enabled with ultra-high speed, high-sensitivity analysis (LCMS-8045) is expected to bring a change in clinical practice to improve the efficacy and safety of medication for optimal patient benefits.
Sorafenib, Lenvatinib, Precision medicine, Hepatocellular Carcinoma
Globally, cancer is a significant public health issue and a leading cause of death.1 Global Cancer Observatory (GLOBOCAN) estimates for 2020 indicate that 19.3 million cancer cases have occurred globally. India ranked third after China and the United States. According to GLOBOCAN’s prediction, the number of cancer cases in India is expected to rise to 2.09 million by 2040, representing a 57.5% increase from 2020.2 Hepatocellular carcinoma (HCC) is the most frequently occurring tumor among all primary liver cancers, accounting for 75–85% of cases. Patients with HCC are typically treated at an advanced stage, when a limited number of effective treatment options are available. Patients with advanced stages of HCC may benefit from systemic therapy.3–5 Despite improvements in early stage prognosis, the survival rate of patients with advanced illness remains poor.
Sorafenib and lenvatinib, tyrosine kinase inhibitors (TKIs), have been the first-line treatments for advanced HCC throughout the last decade, improving overall survival and quality of life, and have recently been replaced by immunotherapy.6,7 Sorafenib, a multiple-target tyrosine kinase inhibitor (TKI), inhibits angiogenesis by targeting proteins such as PDGFR-β, VEGFR-2, and c-KIT, and proliferation by targeting Raf-1, B-Raf, and kinase activity in the Ras/Raf/MEK/ERK signalling pathways, while extending the overall median survival of patients with advanced HCC.8 According to the US Food and Drug Administration (FDA) recommendation, the approved dose of sorafenib is 400 mg (two tablets) twice daily without food. The dose may be reduced to 400 mg once daily depending on the adverse drug reactions experienced, such as hypertension, fatigue, weight loss, rash, hand-foot skin reaction, alopecia, and pruritus.9 Lenvatinib, a multi-targeted receptor kinase inhibitor, inhibits the activity of FGFR 1, 2, 3, and 4; PDGFR α; VEGFR 1, 2, and 3; RET; and KIT. Lenvatinib binds to receptors and inhibits the PLCγ, Ras-Raf-ERK, and PI3K-AKT pathways, arresting tumor angiogenesis and cell death.10 The median overall survival was 10.7 months in the sorafenib versus 12.8 months in lenvatinib group.11,12 Lenvatinib significantly surpassed sorafenib in terms of the ORR, PFS, and TTP. Its tolerance is adequate, and the most common adverse events (AEs) associated with therapy are hypertension, diarrhea, lack of appetite, and weight loss.13 Poor adherence to oral chemotherapy is another major factor limiting long-term therapy. Inter-individual variability in pharmacokinetics is particularly pertinent in oncology, as anticancer agents are frequently administered at doses close to the maximally tolerable intensity, and most TKIs are considered to have a narrow therapeutic index.14
Therefore, individualized dosing guided by pharmacokinetics could lead to less underdosing and reduced toxicity rates owing to overdosing.15 Therapeutic Drug Monitoring (TDM) involves individualized drug dosage by maintaining plasma or drug concentrations within a therapeutic range or window. This clinical practice enables assessment of the efficacy and safety of medications for optimal patient benefits.16 Determining sorafenib and lenvatinib plasma concentrations helps provide individualized treatment through dose adjustments to improve efficacy and avoid adverse effects. Generally, Asian patients do not tolerate the recommended dose in Western patients.17–19 According to FDA recommendations, sorafenib 400 mg (two tablets) twice daily and lenvatinib according to body weight (8 mg once daily if weight < 60 kg; 12 mg once daily if weight ≥ 12 kg), only a few patients can tolerate the total dose. Our preliminary data show that even at 25% or 50% of the recommended doses, therapeutic levels are achieved with a minimal adverse effects profile, and better patient adherence can increase quality of life.
Achieving maximum therapeutic efficacy with minimal iatrogenic toxicity is the primary objective of pharmacological treatment of patients with cancer. To achieve this goal, the dosage regimen should be adjusted for each patient. In regular clinical practice, although some individuals respond well to the standardized drug dosage regimen, others may experience lower efficacy and adverse effects.20 The association between dosage regimens and changes in drug and/or metabolite concentrations throughout the body over time has been studied using pharmacokinetics.21 Dose adaptation based on therapeutic drug concentration, that is, therapeutic drug monitoring, can improve the treatment quality. Although TDM is routinely used for antibiotics and immunosuppressants, its role in the treatment of cancer is underutilized.16 The criteria that need to be considered as candidates for TDM are tyrosine kinase inhibitors, which include large inter-individual PK variability, variable absorption rates, genetic variations in metabolizing enzymes, and interactions among co-medications.22–24 TKIs are mostly oral drugs that block the binding site of adenosine triphosphate (ATP) of tyrosine kinase receptors in malignant cells, thus preventing autophosphorylation of tyrosine residues and activating proteins involved in angiogenesis and tumor proliferation signalling pathways.25 TKIs are usually administered at fixed doses26 and orally. Steady-state methods provide a wide range of plasma concentrations with an inter-individual variability in trough concentration (Ctrough) of up to23-fold. Sorafenib, an oral multi-kinase inhibitor with a recommended dosage of 400 mg twice daily, is a highly plasma-bound drug (99.5%). The liver metabolizes sorafenib via CYP3A4 enzyme and glucuronidation. High-fat meals reduce the bioavailability of sorafenib; therefore, they are either administered one hour before food or two hours after food intake. Close monitoring of patients receiving sorafenib therapy is required to determine toxicities.8,27,28 According to previous studies and our pilot study, sorafenib concentrations ranged from 3.6 to 7 μg/mL. Improved overall survival was seen in patients with maximum sorafenib concentration Cmax ≥4.78 μg/mL.29–31 The multi-targeted inhibitor lenvatinib was approved at 12 mg/day for patients with a body weight ≥ 60 kg and 8 mg/day for patients with a body weight < 60 kg. The drug is rapidly absorbed orally within 1-4 hours of administration (Tmax). The enzymes CYP3A4 metabolize lenvatinib and are excreted in feces. Ninety-six to ninety-eight percent of the drug proteins are protein-bound. The incidence of gastrointestinal and skin toxicities is closely related to the concentration of lenvatinib.32,33 A study by Noda et al.34 demonstrated that lenvatinib’s target trough concentration in the treatment of HCC may range from 36.8 to 71.4 ng/mL in order to control disease and minimize grade 3 toxicity. Although the relationship between sorafenib and lenvatinib exposure and toxicity has been reported, data from the Indian population are lacking.
Here, we describe a sensitive, rapid, and simple LC-MS/MS method for the analysis of sorafenib and lenvatinib in human plasma using simple mobile phases (water and acetonitrile) with 0.1% formic acid, and extraction of analytes by simple protein precipitation with acetonitrile. The developed method reports a precise LC-MS/MS approach for the determination of sorafenib, sorafenib-n-oxide, and lenvatinib in hepatocellular carcinoma patients.
To elucidate the association between plasma sorafenib and lenvatinib concentrations and their clinical efficacy in patients with HCC at a tertiary care hospital.
1. To develop and validate a method for the quantification of plasma sorafenib, sorafenib N-oxide, and lenvatinib concentrations.
2. To study the relationship between trough plasma concentrations of sorafenib and lenvatinib and their clinical responses.
3. To study the relationship between trough plasma concentrations of sorafenib, sorafenib N-oxide, and lenvatinib and adverse effects.
4. To determine the efficacy parameters and correlation of trough concentrations of sorafenib and lenvatinib with treatment efficacy, patients will be analyzed for tumor response using the mRECIST criteria: (a) complete response; (b) partial response; (c) stable disease; (d) progressive disease; (e) objective response rate; and (f) disease control rate (DCR).
5. To determine the overall survival (OS) and progression-free survival (PFS) of patients treated with sorafenib and lenvatinib, the Child-Pugh class A and B groups (severity of disease) were analyzed.
For three years, this prospective, interventional study was conducted in the Department of Medical Oncology, Amrita Institute of Medical Sciences, Kochi. The study protocol was approved by the Ethics Committee of Amrita School of Medicine (ECASM-AIMS-2021-293) on 15-06-2021 and was conducted as per principles of the Declaration of Helsinki and guidelines of the Indian Council of Medical Research.
The study population includes patients visiting the Medical Oncology Department of AIMS and were diagnosed with unresectable hepatocellular carcinoma treated with sorafenib and lenvatinib.
To be eligible for inclusion in this study, participants must meet the following criteria: (a) an adult patient diagnosed with unresectable hepatocellular carcinoma; (b) Patients with Child-Pugh scores of A and B; (c) Patients with Barcelona Clinic Liver Cancer (BCLC B) and (BCLC C); (d) patients who had a good performance status (ECOG-PS: 0-2); and (e) patients or their caregivers willing to provide informed signed consent.
The exclusion criteria were as follows: (a) incomplete patient data profile, (b) neurological or psychiatric conditions, and (c) decompensated liver cirrhosis.
Demographic, initial presenting symptoms, laboratory, staging, and radiological details will be collected using the Amrita Hospital Information System (AHIS) [Amrita Hospital, Kochi]. The adverse events profilewill be monitored during each visit through verbal communication. The sorafenib and lenvatinib drug concentrations will be determined using LC-MS/MS apparatus (Shimadzu LCMS-8045, Japan) of Sophisticated Analytical Instrument Facility (Mahatma Gandhi University, Kottayam).
Procedure
The following steps will be-
(a) Stock solution preparation
• A weighed quantity (2.5 mg) of sorafenib/sorafenib-N-oxide/lenvatinib API [Clearsynth Lab Ltd, Chennai] will be dissolved in a suitable solvent (10 mL of 0.1% formic acid in water and acetonitrile) [Nice Chemicals, Kochi, Kerala] to obtain a stock concentration of 250 ppb. The stock solution will be then sonicated, filtered, and different concentrations 10,20,40,80 and 100 ppb will be made.29,34,35
(b) Sample preparation for validation
• Frozen samples will be thawed at room temperature.
• The sample will be prepared by spiking different concentrations of standard drug in 100μL of healthy subject blood, followed by separation of plasma.
• The most common extraction method for protein precipitation using acetonitrile was chosen over methanol because it showed better chromatographic separation. 100 μL of acetonitrile were added to 100 μL of the plasma sample. The resulting mixture was vortexed for 5 min and centrifuged for 5 minutes at 3,000 rpm at room temperature.
• The supernatant was reconstituted in 100 μL of mobile phase containing 0.1% formic acid in water and methanol and transferred to autosampler vials. Vials were placed into an autosampler (maintained at 48°C) and 10 μL of each vial will be injected.
(c) Method validation.
• Healthy blood samples containing different concentrations of the standard drug will be injected into the equipment. A linear regression graph was used to plot the peak area versus the concentration. The slope, intercept, and correlation coefficient will be calculated using the standard curve.
(d) Chromatographic and mass spectrometer conditions
• To achieve the desired chromatographic separation, peak shape, and signal intensity, method optimization will be carried out using an LC System [Shimadzu LC-MS 8045, Japan] with a Shimadzu HILIC column (150 × 2.1 mm; 3 μm) for sorafenib and a Shimadzu C8 (2 μM) column for lenvatinib with a gradient of water and acetonitrile containing 0.1% formic acid at a flow rate of 0.4 mL/min with a total run time of 5 min. The HILIC column is used for the extraction of polar compounds, such as sorafenib. The column has several advantages such as faster and efficient analysis, low-viscosity eluent usage, high cost, and high dependence on acetonitrile solvent.
• An AB SCIEX 4000 Q TRAP mass spectrometer [Spectralab Scientific Inc, US] meeting the legal requirements for medical devices will be used for detection equipped with pneumatically assisted electrospray ionization (ESI) operating in the positive mode. A multiple reaction monitoring (MRM) with m/z 465→252 (sorafenib); 480.9→286.05 (sorafenib-N-oxide) and 427→370 (lenvatinib).
• The MS/MS conditions were as follows: interface voltage: 4 kV; source temperature, 300°C; and oven temperature, 40°C. Analytical results will be recorded and processed using Analyst Software 1.6.3 MD [Sciex; Spectralab Scientific Inc, US].
(e) Blood sample analysis
• Blood samples will be collected into EDTA test tubes by venipuncture from HCC patients satisfying the inclusion criteria during their 1st hospital visit, 7 days after drug administration, and at different time intervals (especially when an ADR is experienced).
• The blood will be centrifuged at 3,000 rpm for 5 minutes to separate plasma.
• From this aliquot, 10 μL of plasma will be taken mixed with 10 μL acetonitrile to precipitate plasma proteins.
• The resulting mixture was vortexed for 5 min and centrifuged for 5 minutes at 3,000 rpm at room temperature.
• The clear supernatant (20 μL) from each patient sample was transferred to an autosampler tube and reconstituted with 80 μL of mobile phase of 0.1% formic acid in water, and methanol will be injected into the column for analysis.
Clinical study protocol
During the initial visit to the Department of Medical Oncology, the investigator informs the objectives and expected benefits of the study. The volunteers are given the opportunity to decide regarding the participation in the study. Once included in the study, the blood samples are collected from the patient once the steady state concentration is achieved (2 weeks/1month). The adverse event profile, laboratory parameters, and radiological findings will be monitored during the visit. The blood sample will be analyzed and according to the reported plasma drug concentration, dose adjustments (dose increment/reduction) will be recommended.
The design of the study is illustrated in Figure 1.
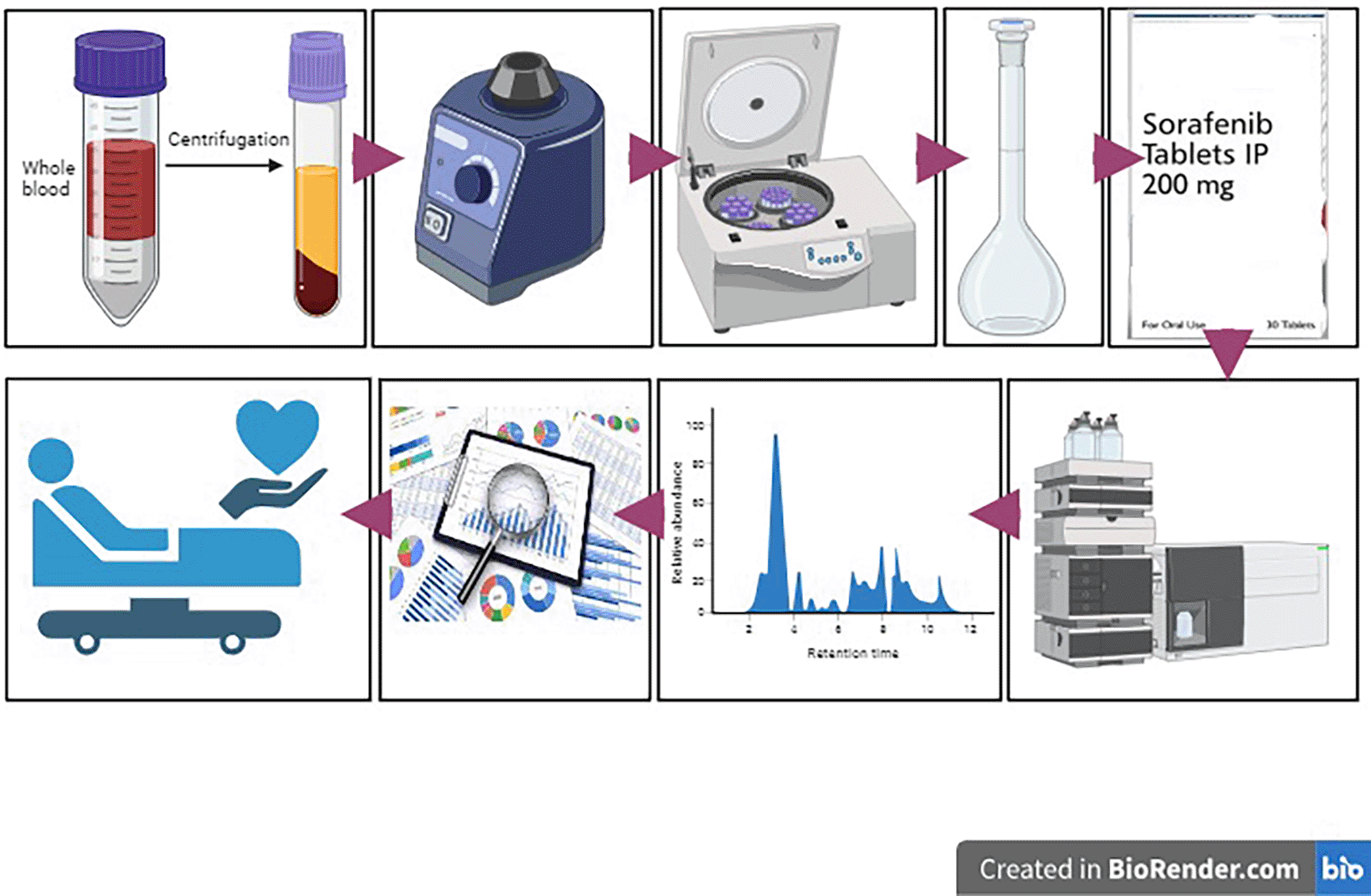
Sample size
Based on the mean and SD, the steady-state concentration of sorafenib at dose 400 mg/d (6.1±3.9 mg/L) and at dose level 200 mg/d (4.5±4.5 mg/L) in patients with HCC who were continuously administered sorafenib orally was observed in an earlier publication,36 with 20% allowable error and 95% confidence, the mean sample size comes to 39 for the dose 400 mg/d and 96 for the dose 200 mg/d, respectively. The minimum sample size was 96.
Based on the mean and SD, steady state concentration of lenvatinib at dose 4 mg/d (0.10031+0.10123 mg/L) and at dose level 8 mg/d (0.14361+0.1587 mg/L) in patients with HCC who were continuously administered lenvatinib orally observed in the pilot study conducted in 50 samples and with 20% allowable error and 95% confidence, the sample size comes to 98 for the dose 4 mg/d, 117 for the dose 8 mg/d respectively. Total minimum sample size comes to 117.
Statistical analysis
• The drug plasma concentration dose will be computed as mean ± SD.
• To test the statistical significant difference in the mean drug plasma concentration between 4 response of efficacy parameter, one way ANOVA followed by multiple comparison test bonferroni will be applied for skewed data or Kruskal wallis test followed bt Dunn bonferoni will be applied for skewed data.
• To test the statistical significant correlation between drug plasma concentration dose and grade of ADR, Pearson’s correlation coefficient will be applied and its statistical significance will be assessed by linear reg t test.
• To test the statistically significant difference in the median ADR grade between severity of disease of Child Pugh A class and Child Pugh B groups, Mann whitney U test will be applied.
• Overall percentage of Objective Response Rate (ORR) and Disease control rate (DCR) will be represented in number and percentage with 95%confidence Interval.
• To determine the Overall Survival (OS) and Progression free survival (PFS) of patients treated with sorafenib and lenvatinib, Kaplan Meier Analysis will be done and to compare with severity of disease in the Child Pugh A and Child Pugh B groups respectively log rank test will be applied.
Study status
The study is still in the recruitment phase. Sixty-seven patients who received sorafenib and eighty-eight patients who received lenvatinib have been recruited. In all subjects, blood samples were collected during the initial and follow-up visits.
Expected results
TDM involves combined knowledge of pharmacokinetics and pharmacodynamic activity to optimize personalized therapy. Repeated blood sampling and monitoring of adverse events are therefore necessary. This method helps to determine patient non-compliance issues and improve patient outcomes. Despite its vast applicability and impact on clinical science, TDM has not received the attention it requires. The cost involved and the tedious process of TDM limits its use in routine clinical practice. Alternate sampling techniques using dried spots that require less blood enable the care of cancer patients in rural areas. Removing the barriers to the availability, cost, and turnaround time involved in TDM will help in the wide acceptability of the method among clinicians.
Factors such as large inter-individual variability in drug absorption, genetic polymorphisms, drug interactions, and patient compliance with oral TKIs affect quality of life. Therefore, the implementation of therapeutic drug monitoring for TKIs can help reduce adverse events with maximum efficacy. The acceptance of TDM as a test in routine clinical practice can improve the survival of HCC patients with hepatocellular carcinoma. Therefore, this study has the potential to improve the clinical outcomes.
Analytical results obtained from LC-MS apparatus is recorded using using Analyst Software 1.6.3 MD [Sciex; Spectralab Scientific Inc, US] which is available at their site (https://sciex.com/support/software-support/software-downloads ) for free download.
The authors would like to thank the Sophiscated Analytical Instrument Facility, MG University, Kottayam for the LC-MS equipment and research support. We would like to thank Amrita Vishwa Vidyapeetham, Amrita Institute of Medical Sciences and Research Centre, Kochi.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for, and objectives of, the study clearly described?
Partly
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: medical oncology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 07 Nov 24 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)