Keywords
Geraniaceae; Pelargonium graveolens; essential oil; antimicrobial activity; anti-inflammatory activity; atopic dermatitis
Atopic dermatitis (AD) is a chronic inflammatory skin condition characterized by pruritus and skin barrier dysfunction. This study aims to evaluate the therapeutic potential of Pelargonium graveolens (Geraniaceae) in managing AD symptoms through its essential oil.
The chemical composition of Pelargonium graveolens flower essential oil (PFEO) was analyzed using gas chromatography-mass spectrometry (GC-MS). Its antimicrobial, antioxidant, and anti-inflammatory properties were assessed, along with the inhibitory effects of PFEO on key enzymes involved in skin repair: tyrosinase, elastase, and collagenase. An in vivo evaluation of a gel formulation containing PFEO was also conducted to assess its anti-inflammatory and analgesic efficacy.
GC-MS analysis identified major compounds in PFEO, including Geraniol (22.83%), beta-citronellol (19.51%), naphthalenemethanol (15.36%), and Geranyl tiglate (9.38%), with minor constituents such as linalool (3.81%) and neryl formate (1.31%). PFEO exhibited bacteriostatic activity against various bacterial and fungal strains, including Pseudomonas aeruginosa, Staphylococcus aureus, Methicillin-Resistant Staphylococcus aureus (MRSA), Bacillus anthracis, Streptococcus pyogenes, Staphylococcus epidermidis, Candida albicans, and Malassezia spp. The essential oil also demonstrated significant antioxidant properties and inhibited key enzymes linked to skin alterations in AD.
PFEO shows promising therapeutic potential for managing symptoms of atopic dermatitis due to its antimicrobial, antioxidant, and anti-inflammatory properties, as well as its analgesic effects. The findings support further exploration of PFEO as a natural alternative in the treatment of AD.
Geraniaceae; Pelargonium graveolens; essential oil; antimicrobial activity; anti-inflammatory activity; atopic dermatitis
Atopic dermatitis, commonly known as eczema, is a chronic inflammatory skin condition that significantly affects individuals’ quality of life across all age groups.1,2 Understanding the intricate relationship between compromised skin barriers, inflammation, and immune responses is crucial for the effective management and treatment of Atopic dermatitis.3
Skin serves as a dynamic organ, regulating various physiological processes and acting as a barrier against external elements.4 In Atopic dermatitis, altered skin barrier function due to genetic and environmental factors contributes to immune dysregulation and inflammation.5 Barrier defects, including filaggrin mutations and exposure to chemicals and microbial strains, disrupt skin integrity and predispose individuals to atopic dermatitis.6 External antigens trigger immune responses mediated by interleukins and immunoglobulins, exacerbating inflammation and atopic dermatitis symptoms.7 Atopic dermatitis presents with symptoms such as dry skin, inflamed lesions, itching, and pain, leading to visible discomfort and psychological distress. While itching is a primary symptom, the prevalence of pain in atopic dermatitis patients is increasingly recognized, further compromising their quality of life.8 Pain management in atopic dermatitis remains an underexplored area, with emerging evidence suggesting promising avenues for relief, including Janus kinase (JAK) inhibitors and biologics. Atopic dermatitis imposes a significant burden on healthcare resources due to its chronic nature and associated comorbidities. Sleep disturbances and psychological distress further exacerbate the disease burden, highlighting the need for comprehensive management strategies addressing both physical and psychological aspects of atopic dermatitis.9 Enhanced understanding of the pathophysiology of atopic dermatitis, coupled with tailored treatment approaches targeting skin barrier function, inflammation, itching, and pain, is essential for improving patient outcomes and quality of life. Addressing the multifaceted nature of atopic dermatitis requires collaborative efforts from healthcare professionals and researchers to develop effective management strategies and alleviate the burden of this chronic skin condition ( Figure 1).

A combination of topical anti-inflammatory and analgesic agents can be particularly effective in the management of atopic dermatitis, providing symptomatic relief of pain and itching as well as addressing the underlying inflammation. The approach also focuses on improving the structural integrity of damaged skin and inhibiting the growth of commensal microbes often associated with atopic dermatitis.
Natural products derived from medicinal plants are considered safe and effective, as they contain chemical components with therapeutic value.10 The complex molecular structures present in plant extracts allow them to interact with specific targets in cells. Despite the advancements in synthetic chemistry, combinatorial chemistry, and molecular modeling, medicinal plants remain a vital source of new drugs and drug leads.11 Plant research has gained increasing attention worldwide, resulting in a wealth of scientific evidence highlighting the vast potential of medicinal plants in various traditional systems. Studies have led to the isolation of chemical substances with therapeutic properties, some of which have been developed into modern drugs, while others have been used as starting materials for drug synthesis. Interestingly, even in modern pharmaceuticals, approximately 25% of drugs still have origins in plants.12 Pelargonium graveolens (Geraniaceae) essential oil, exhibiting diverse pharmacological benefits including antibacterial, antifungal, antioxidant, and anticancer effects.13–15 However, further research is warranted to fully explore its therapeutic potential due to limited studies on its biological activities.
This study aims to explore the pharmacological properties and dermatological applications of Pelargonium graveolens flower essential oil (PFEO) to treat skin alterations and relieve pain associated with atopic dermatitis, pigmentation problems and inflammatory conditions. The research focuses on various bioactive compounds identified by gas chromatography-mass spectrometry (GC-MS) analysis.
The blossoms of Pelargonium graveolens (Geraniaceae) were harvested in May 2023 from Sahel Boutaher, located in the Taounate Province of Northwest Morocco (34.5233° N, -4.6500° W), and identified under the vocher code RAB114770. Subsequently, these flowers underwent drying procedures under open-air conditions were meticulously shielded from light and maintained at ambient room temperature.
Hydrodistillation was conducted utilizing a Clevenger-type apparatus. A quantity of 50 g of dried Pelargonium graveolens flowers was introduced into a 2-liter flask containing 1 liter of distilled water. Following a 3-hour hydrodistillation process, the resulting PFEO was carefully collected into a flask and promptly stored in a refrigerated environment until further application.
To prepare the topical gel, 0.5% carbopol polymer was dispersed in 89% water, followed by stirring with a Laboratory dispenser (492-IV Model, ERICHSEN Gmbh, Germany) until homogeneous. The water was neutralized with triethanolamine.
Male Swiss Webster mice weighing 29.75 ± 1.70 g were selected as study subjects. The mice were housed in groups of nine in plastic cages placed in an animal house. Environmental conditions were maintained at a temperature of 25°C, with a 12-hour light/dark cycle. Mice always had access to food and water, except during experimental periods. Each animal was used only once in the study.
All animal procedures throughout the experiments were conducted in strict compliance with ethical standards and regulations to ensure the welfare of the mice. The study was considered and approved by the Institutional Ethics Committee for the Care and Use of Laboratory Animals of the Faculty of Sciences Dhar El Mehraz of the University Sidi Mohamed Ben Abdallah in Fez, Morocco (Approval NO-07/2024/LBEAS). Ethical guidelines for the handling and use of animals were established by the Institutional Ethics Committee in accordance with European Community Directive EEC/86/EEC (Union, 1986).
The analysis was conducted using a GCMS-TQ8040 NX Triple-Quadrupole GC/MS/MS system (Shimadzu Corporation®, Tokyo, Japan). Separation was achieved using a capillary column,) RTxi-5 Sil MS™ column (30-m length × 0.25-mm internal diameter × 0.25-μm film thickness) (Restek Corporation™, Bellefonte, PA, USA), with helium as the carrier gas and 1 μl volume injections. Hexane served as the dilution solvent. The ion source and interface temperatures were maintained at 200 °C and 280 °C, respectively. The experimental procedure began with a splitless injection mode, transitioning to split opening after 4 minutes, with an injection temperature of 250 °C and a pressure of 37.1 kPa. Temperature programming involved an initial hold at 50°C for 2 minutes, followed by ramps of 5°C/min to 160°C for 2 minutes and subsequently to 280°C for 2 minutes, with a total analysis duration of 50 minutes. Compound identification was facilitated by operating in full scan mode and utilizing the NIST 2019 library. All compounds were quantified from the total area of the peaks detected in each chromatogram.
The antioxidant potential of PFEO was evaluated through three distinct methodologies, namely Total Antioxidant Capacity (TAC), 2,2-Diphenyl-1-picrylhydrazyl radical scavenging (DPPH), and Ferric Reducing Antioxidant Power (FRAP), adhering closely to established protocols.16–18 Percentage inhibition (I%) was calculated using the following equation (1) and quantitative outcomes are reported as IC50 in mg/mL. All assays were conducted in triplicate, and ascorbic acid was employed as the positive control.
: Absorbance of negative control.
: Absorbance of test sample.
Bacterial strains
PFEO underwent testing against a spectrum of seven clinical bacterial strains, namely Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, Bacillus anthracis, Methicillin-Resistant Staphylococcus aureus (MRSA), and Streptococcus pyogenes, as well as two yeast strains, Candida albicans and Malassezia spp, sourced from the University Hospital of Fes.
Agar well diffusion method
The antibacterial properties of PFEO were qualitatively assessed using the agar-well diffusion method, following the protocol outlined by Ref. 19. In brief, microbial inoculum was evenly spread on Luria-Bertani (LB) agar for bacterial strains or sabouraud agar medium with chloramphenicol for fungi. A sterile well, measuring 5 mm in diameter, was created, into which 80 microliters of PFEO solution (50 μL/ml) was introduced. Incubation at 37°C for 24 hours facilitated the diffusion of the antimicrobial agent into the agar medium, consequently restraining microbial proliferation. The resultant zone of inhibition values was measured in millimeters and ampicillin and fluconazole were used as positive controls.
MIC determination
The qualitative antimicrobial evaluation of PFEO followed a protocol outlined by Ref. 19. In a 96-well plate, the antimicrobial agents (PFEO, ampicillin and fluconazole) concentrations ranging from 32 to 0.01 μL/ml were dispensed, along with bacterial and fungal inocula. After 18 hours of incubation at 37°C, bacterial and fungal growth were assessed with resazurin. And MIC values were determined just before the appearance of pink coloration.
The thermal nociceptive response was assessed using a plantar test apparatus (UGO BASILE model 37370, Italy), following established protocol,20 with a few modifications. Mice were divided into four groups, each comprising six animals: Groups one received topical gel containing 1% PFEO, while groups tow received topical gel containing 4% PFEO applied to the right hind paw. Negative control groups were administered topical gel lacking PFEO, whereas positive control groups (standard) were treated with an anti-inflammatory and analgesic medication gel (diclofenac sodium topical gel: Voltarène® Emulgel 1%, GlaxoSmithKline, Brentford, UK.
The experimental setup was housed within a transparent plastic chamber with a glass floor. Prior to testing, animals underwent a five-minute acclimation period. A moving infrared heat source was directed at the right hind paw, and the time taken for paw withdrawal response was automatically recorded at 30-minute intervals to mitigate thermal sensitization and minimize behavioral disruptions.
In vitro anti-inflammatory activity was evaluated by measuring the denaturation of bovine serum albumin (BSA) according to the procedure described by Lekouaghet et al.21 In brief, the extract or standard (50 mg diclofenac) was mixed with a solution of BSA (0.2%) in Tris buffer (pH 6.8). The mixture was incubated at 37°C for 15 minutes, then heated in a water bath at 72°C for 5 minutes. The degree of protein precipitation was measured at 660 nm using a RoHS Uv-1800pc spectrophotometer (Macy, China), and results expressed as IC50 (mg/mL). The negative control contained 0.5 ml water and 0.5 ml BSA solution, while the control sample consisted of 0.5 ml extract and 0.5 ml Tris buffer solution (pH 6.8).
In vivo anti-inflammatory activity was demonstrated using a carrageenan-induced paw oedema model in mice (n = 6). Briefly, after application of different formulated topical gel doses (negative control), as well as gels containing 1% and 4% PFEO, the volume of the paw of mice was primarily measured before administration of 0.5% carrageenan in the left paw. Then, paw volume was recorded after topical treatment at intervals of 1, 2, 3, 4, 5 and 6 hours using a LE 7500 plethysmometer (Panlab, Spain). The standard reference used was Voltaren Emulgel 1%. The inhibition percentage was determined using the following equation:
Where
I% is the inhibition percentage of paw oedema.
Vt is the average paw volume after carrageenan injection.
V0 is the average paw volume before carrageenan injection
Elastase inhibition
Employing established protocols.22 we assayed porcine pancreatic elastase (3.33 mg/mL) with N-Succinyl-Ala-Ala-Ala-p-nitroanilide substrate (1.6 mM). PFEO samples (3 to 0.046 mg/mL) underwent pre-incubation, followed by substrate addition and a 20-minute reaction at 37°C. Absorbance at 400 nm was quantified using a microplate reader (FLUOstar Omega). Percentage inhibition (I%) was calculated using the equation (2) and IC50 values (mg/mL) were determined, benchmarked against epigallocatechin gallate (EGCG) as the positive control.
: The measured absorbance of enzyme activity in the presence of samples.
: The measured absorbance of enzyme activity in the absence of samples.
Collagenase inhibition
We employed the collagenase inhibition assay as per the method described in Ref. 23. Clostridium histolyticum collagenase enzyme (ChC-EC.3.4.23.3) at 0.8 unit/mL was incubated with PFEO ranging from 4 to 0.25 mg/mL for 15 minutes at 37°C. Substrate N-[3-(2-furyl)acryloyl]-Leu-Gly-Pro-Ala (FALGPA) was added, and absorbance was measured at 490 nm using a microplate reader (FLUOstar Omega). Percentage inhibition (I%) was calculated using the equation (2) and IC50 values were determined, with quercetin acting as the positive control.
Tyrosinase inhibition
Following the method outlined in Ref. 24, we evaluated tyrosinase inhibition utilizing l-3,4-dihydroxyphénylalanine (L-DOPA) as substrate. Extracts were mixed with fungal tyrosinase (330 U/mL) and incubated at 37°C. After 30 minutes, absorbance at 510 nm was assessed. IC50 values and inhibition percentage (I%) were calculated as per the provided formula. Quercetin acted as the positive control.
: L-DOPA, tyrosinase and phosphate buffer.
: Includes L-DOPA and phosphate buffer.
: Extract, L-DOPA and tyrosinase.
: Extract and L-DOPA.
The chemical composition analysis of P. graveolens (Geraniaceae) flowers was conducted using gas chromatography–mass spectrometry (GC-MS). The findings are presented in Table 1, and chromatogram in Figure 2 revealing the identification of twenty chemical constituents in total. Among them, six major constituents were identified, with Geraniol (22.83%) being the most abundant, followed by β-citronellol (19.51%), γ-Eudesmol (15.36%), and geranyl tiglate (9.38%). Additionally, fourteen minor constituents were identified, including linalool (3.81%) and neryl formate (1.31%). The chemical composition of P. graveolens essential oils is subject to several influencing factors, such as origin, climatic conditions, and soil physicochemical properties.25 Additionally, Juárez, Z., et al26 identified 16 compounds, showing similarities to our results, albeit with a higher content of geraniol as the major compound (31.02%). Other predominant terpene compounds included β-citronellol (18.26%), (-)-aristolene (13.19%), and c-eudesmol (10.22%). Similarly, Ben Slima, A., et al.27 analyzed P. graveolens from Tunisia, revealing 18 constituents comprising 89.04% of the total essential oil. The most abundant components (>4%) were β-citronellol (29.3%), geraniol (10.53%), linalool (10.42%), and citronellal formate (9.54%). In another study, Al-Mijalli, S.H., et al.24 investigated P. graveolens harvested in Northwest Morocco at three phenological stages. They observed variations in primary components across growth stages, with notable concentrations of menthol, isogeraniol, eremophilene, and menthene at different stages, consistent with findings from P. graveolens cultivated in Tunisia.28 Notably, the highest content of β-citronellol (30.61%) was observed in the post-flowering stage, while the lowest was in the vegetative stage (21.93%).
The presence of microorganisms like Staphylococcus aureus, Streptococcus pyogenes, and yeasts such as Malassezia species can directly contribute to skin inflammation. Studies have identified strains of Staphylococcus epidermidis, Malassezia spp and candidas spp, in a large proportion of individuals with atopic dermatitis.29–31
PFEO efficacy against various bacterial strains, a fungus, and a yeast was assessed, including Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus anthracis, Staphylococcus epidermidis, MRSA, Streptococcus pyogenes, Candida albicans, and Malassezia spp. The agar well diffusion method was employed, and the results are detailed in Table 2. Inhibition zone measurements, reported in millimeters, ranged from sensitive (9–14 mm) to highly sensitive (15–19 mm) based on established criteria.32 Notably, Malassezia furfur exhibited the highest sensitivity, evidenced by a larger inhibition zone and the lowest MIC values (28.67 ± 1.15mm, MIC = 0.25 ± 0.00μL /mL) followed by Staphylococcus aureus (23 ± 1.00 mm, MIC = 0.33 ± 0.14). Streptococcus pyogenes (19.33 ± 1.53 mm, MIC = 0.67 ± 0.29 μL/mL), Candida albicans (18.83 ± 0.76 mm, MIC = 1.00 ± 0.87 μL /mL), MRSA (17 ± 1.00 mm, MIC = 2.67 ± 1.15 μL/mL), Pseudomonas aeruginosa (16.33 ± 0.58 mm, MIC = 3.00 ± 4.33 μL /mL) and Staphylococcus epidermidis (16.67 ± 1.15 mm, MIC = 10.67 ± 4.62 μL/mL) also demonstrated sensitivity. Statistical analysis revealed no significant difference (p < 0.05) between the inhibitory effects of PFEO and the antibiotic ampicillin against Pseudomonas aeruginosa (MIC = 2.16 ± 1.75 mg/mL), Staphylococcus epidermidis (MIC = 6.66 ± 2.31 mg/mL), Staphylococcus aureus (MIC = 2.08 ± 1.87 mg/mL), and MRSA (MIC = 4.66 ± 3.05 mg/mL). However, ampicillin showed no activity against Bacillus anthracis and Streptococcus pyogenes, whereas PFEO demonstrated significant efficacy. Furthermore, no significant difference (p < 0.05) was observed between fluconazole and PFEO in their effectiveness against Candida albicans and Malassezia spp. The presence of oxygenated monoterpenes, such as β-citronellol and geraniol, predominantly contribute to this activity. τ-Muurolol and α-cadinol demonstrated strong antifungal activity against several pathogens, with IC50 values ranging from 18.3 to 80.6 μg/mL.33 The complexity of the essential oil mixture makes it challenging to attribute the antimicrobial activity to a single compound.34 Synergistic effects between multiple constituents may underlie this activity.
| Strains | Method | Antibacterial activity | Positive Controls |
|---|---|---|---|
| Pseudomonas aeruginosa | Inhibition zones (mm) | 16.33 ± 0.58 A | 17.66 ± 1.53 1 A |
| MIC (μL/mL) | 3.00 ± 4.33 A | 2.16 ± 1.75 1 A | |
| Staphylococcus aureus | Inhibition zones (mm) | 23.00 ± 1.00 A | 16.00 ± 2.65 1 B |
| MIC (μL/mL) | 0.33 ± 0.14 A | 2.08 ± 1.87 1 A | |
| Staphylococcus epidermidis | Inhibition zones (mm) | 16.67 ± 1.15 A | 17.00 ± 1.00 1 A |
| MIC (μL/mL) | 10.67 ± 4.62 A | 6.66 ± 2.31 1 A | |
| Bacillus anthracis | Inhibition zones (mm) | 10.40 ± 0.53 | - |
| MIC (μL/mL) | 5.33 ± 2.31 | ||
| MRSA | Inhibition zones (mm) | 17.00 ± 1.00 A | 13.66 ± 2.08 1 B |
| MIC (μL/mL) | 2.67 ± 1.15A | 4.66 ± 3.05 1 A | |
| Streptococcus pyogenes | Inhibition zones (mm) | 19.33 ± 1.53 | - |
| MIC (μL/mL) | 0.67 ± 0.29 | ||
| Candidas albicans | Inhibition zones (mm) | 18.83 ± 0.76 A | 24.66 ± 4.16 2 A |
| MIC (μL/mL) | 1.00 ± 0.87 A | 0.012 ± 0.005 2 A | |
| Malassezia spp | Inhibition zones (mm) | 28.67 ± 1.15 A | 18.33 ± 1.52 2 A |
| MIC (μL/mL) | 0.25 ± 0.00 A | 0.54 ± 0.43 2 A |
Several researchers have investigated the antimicrobial properties of P. graveolens essential oil compounds. Previously, Boukhris et al.35 explored this activity across different phenologicl stages, revealing bactericidal efficacy with MIC values ranging from 0.15 to 2.5 μg/mL. Notably, others have exhibited potent activity against clinical S. aureus strains,36 with MIC values ranging from 0.25 to 2.5 μL/mL. Remarkably, 47 out of 70 clinical S. aureus strains displayed sensitivity to Pelargonium graveolens essential oil concentrations of 1.00 μL/mL or lower. Similarly, it was evaluated against various Malassezia strains,37 demonstrating inhibition zones from 10 to > 50 mm in diameter, particularly effective against M. obtusa, notably in Zataria multiflora. Furthermore, P. graveolens essential oil was assessed against Candida spp. The most potent oil, sourced from South Africa (Laszlo), displayed MIC values ranging from 128 to 256 μg/mL across all isolates. Among Candida species, C. parapsilosis exhibited the highest sensitivity (MIC: 128 μg/mL), followed by C. tropicalis (MIC: 256 μg/mL). However, oils from Brazil and South Africa (Verbena) demonstrated limited efficacy, with a MIC of 512 μg/mL against most isolates.38 Certain components of essential oils disrupt membrane-associated enzyme proteins, thereby inhibiting their production.39 Essential oils also inhibit DNA, RNA, protein, and polysaccharide synthesis in fungal and bacterial cells.40 Terpenoids, for instance, can impact membrane-catalyzed enzyme activities.41 However, the mode of action varies depending on the microorganism type, with Gram-negative bacteria like Pseudomonas aeruginosa exhibiting intrinsic resistance due to their hydrophilic outer membrane.42 The growth rate of Gram-positive bacteria such as S. aureus is reduced even by low concentrations of sesquiterpene T-cadinol, likely due to interaction with primary energy metabolism. In fact, essential oils exhibiting lipophilic properties, tend to be more effective when they interact with cytomembranes. Their low aqueous solubility prevents their toxicity in the cytomembranes.43
Monoterpenols such as geraniol and β-citronellol, which are abundant in the tested PFEO, have been investigated in prior studies for their ability to disrupt the lipopolysaccharide outer layer and induce disintegration of the outer membrane.44,45
The results of the topical analgesic effect of PFEO are depicted in Figure 3. The PFEO demonstrates moderate and dose-dependent analgesic effects. The plantar test was conducted every 30 minutes up to 120 minutes after topical administration. All tested preparations exhibited analgesic activity as early as 30 minutes post-topical application. Data analysis revealed the analgesic effect of all studied preparations compared to the control group. The gel prepared with PFEO (4% w/w) exhibited potent analgesic effects after 60 minutes, with minimal effect observed throughout the 30 minute observation period. The effect of PFEO on antinociceptive activity was less pronounced at a concentration of 1%. However, the analgesic effect of this preparation was superior to that of the reference gel (negative control) after 30 minutes. Voltarène® significantly increased pain tolerance time. The Voltarène® gel showed a similar antinociceptive effect to the preparation containing 4% PFEO. Nociceptive pain arises from tissue damage or inflammation, such as cuts, burns, bruises, sprains, or arthritis. It typically manifests as throbbing, aching, or sharp sensations and can occur in localized or widespread areas.46 These effects may be attributable to the involvement of both peripheral and central inhibitory mechanisms induced by the volatile compounds.47 β-Citronellol that found a major compounds in PFEO (19.51%), have been previously demonstrated to have anti-hyperalgesic effects in Swiss mice models without affecting motor coordination, reducing inflammatory mediators, and activating the descending pain pathway. Moreover, Linalool; identified in PFEO (3.6%), was studied for its impact on the peripheral somatic sensory system. It was found to concentration-dependently block sciatic nerve excitability and inhibit action potential generation in dorsal root ganglion neurons.48 This indicates that linalool may possess local anesthetic properties by acting on voltage-dependent Na+ channels, suggesting its potential as a pharmacotherapeutic agent targeting these channels.
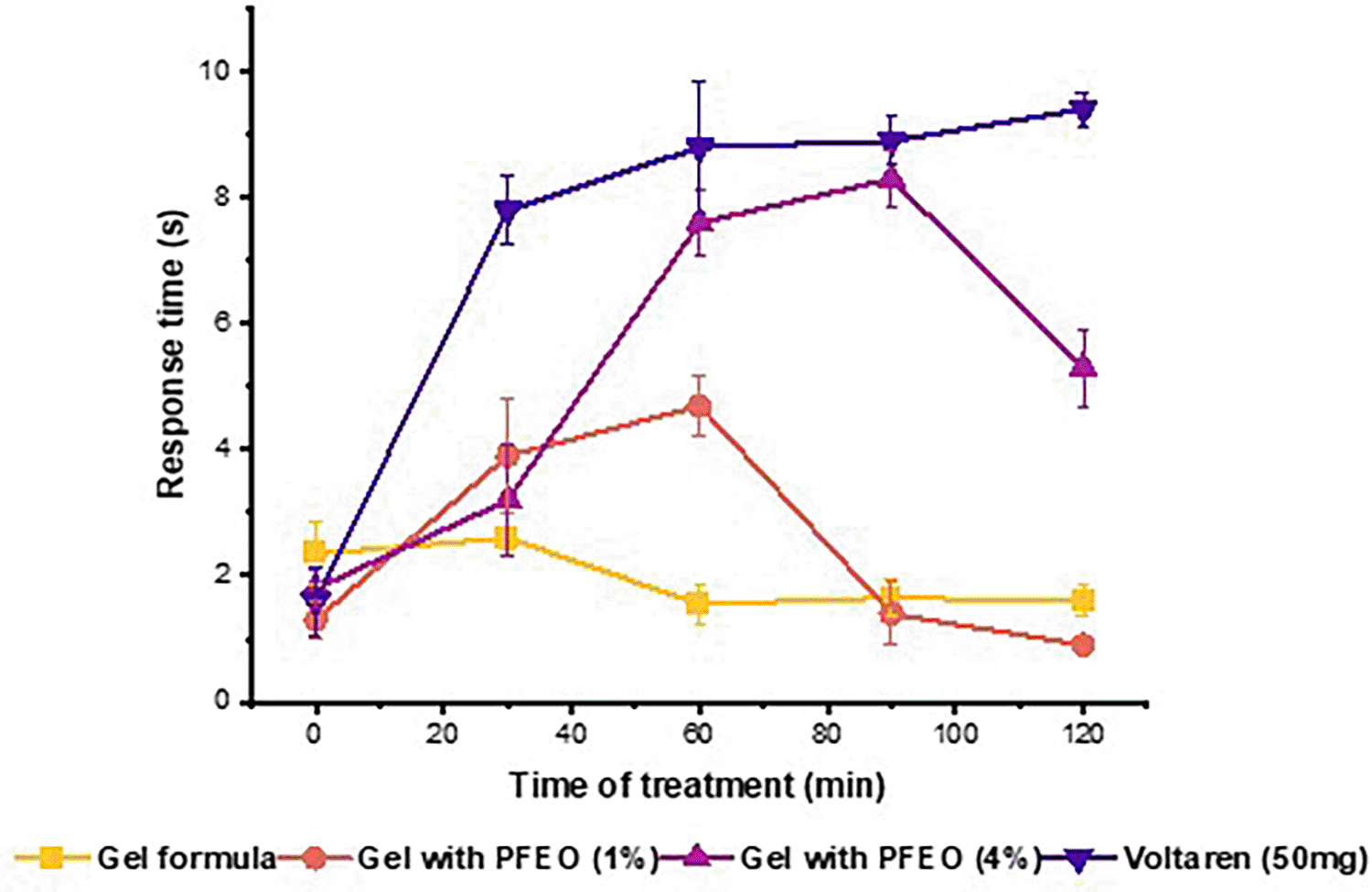
Thermal nociceptive response in mice treated with topical gels containing 1% and 4% PFEO and Diclofenac (Voltarène® Emulgel 1%) assessed by plantar test. All values are given as means ± standard error (n = 5).
Sakurada et al examined the effects of linalool and linalyl acetate, in a mouse capsaicin pain model. Injections of linalool, or linalyl acetate into the paw reduced signs of capsaicin-induced pain in a dose-dependent manner, and revealing that the pain relief induced by linalool injections was countered by naloxone hydrochloride and naloxone methiodide, suggesting involvement of peripheral opioid mechanisms.46 These findings are consistent with those obtained previously on capsaicin-induced pain behaviour and support the suggestion of a localised antinociceptive effect of PFEO on cutaneous nociceptors.
In vitro anti-inflammatory activity of PFEO was assessed using the protein denaturation method of Mulvihill et al.49 Protein denaturation breaks crucial bonds such as electrostatic, hydrogen, hydrophobic and disulphide bonds, which are integral in maintaining the three-dimensional structure of proteins. Structural alteration of proteins is linked to the initiation of the inflammatory response, leading to the production of autoantigens, triggering inflammatory reactions.50 The aim of this method is to explore the potential of PFEOs to attenuate inflammation induced by protein denaturation. However, no scientific investigations have yet explored the in vitro anti-inflammatory effects of PFEO using this method.
The results indicate that the inhibition of BSA denaturation by PFEO varies from 86.48% ± 1.00 at a concentration of 20 μL/ml to 57.20 ± 0.11% at a concentration of 0.312 μL/ml ( Figure 4). The inhibitory effect of PFEO is comparable to that of diclofenac (50 mg), the standard anti-inflammatory drug, which achieved inhibitory percentages of 91 ± 0.014% and 31.5 ± 0.004% at equivalent concentrations.

Atopic dermatitis is associated with dysregulated inflammatory responses, arising from compromised skin barrier function, marked by deficiencies in key proteins such as filaggrin and claudin. This impairment leads to heightened water loss, increased allergen penetration, and elevated inflammation. Additional factors including elevated IL-1 release and alkaline pH resulting from soap usage exacerbate inflammation and barrier disruption. Therapeutic interventions targeting barrier restoration, such as topical delivery of filaggrin or synthetic compounds, offer potential relief from atopic dermatitis symptoms.51 Sesquiterpenes as Germacrene D, δ-cadinene, α-cadinene, τ-Cadinol and τ-muurolol in essential oils have demonstrated efficacy in reducing inflammation markers and targeting various signaling pathways involved in inflammatory processes.52,53
PFEO’s therapeutical effect on carrageenan-induced oedema was examined at different concentrations, and the results are presented in the Figure 5. The data show that topical application of the PFEO-based gel at concentrations of 1% and 4% exhibits significant anti-inflammatory activity, comparable to the control treatment of the formulated gel. Indeed, after one hour, 1% PFEO gel showed an inflammation inhibition of approximately 9.22 ± 4.16%. Meanwhile, the 4% PFEO gel showed a more pronounced reduction in inflammation, reaching an average value of 26.76 ± 8.21% (p < 0.05). The gel formulation free of PFEO showed limited efficacy, indicating that the active phytochemicals in PFEO are responsible for the reported anti-inflammatory effects. The standard Voltaren Emulgel 1% achieved a substantial inflammation inhibition of 14.05 ± 2.00%. The findings shows that while PFEO gel, particularly at higher concentrations, offers promising results, it remains slightly inferior to the synthetic anti-inflammatory Voltaren Emulgel 1% in terms of immediate efficacy. However, between 2 and 3 hours of treatment, a significant difference was observed between the formulated gel, the 1% PFEO gel and the 4% PFEO gel compared with Voltaren (p < 0.05).

Anti-Inflammatory efficacy of Topical Gels Containing 1% and 4% PFEO and Diclofenac (Voltarène® Emulgel 1%) in Carrageenan-Induced Paw Edema Model in Mice: Percentage Inhibition Over 6 Hours. All values are given as means ± standard error (n = 5). Analysis by ANOVA followed by Tukey's test. Values with different letters in the same time of treatment are significantly different (p < 0.05).
Following 4 and 6 hours of treatment, the group receiving the 4% PFEO gel showed significantly enhanced anti-inflammatory activity compared with the formulated gel and the 1% PFEO gel (p<0.05). Notably, the 4% PFEO gel inhibited inflammation after 4 hours by 56.39 ± 8.17% and after 5 hours of application by 75.41 ± 7.22%. The Voltaren gel achieved maximum inhibition after 6 hours (I% = 92.60 ± 7.47%), as did the 4% PFEO gel (I% = 80.78 ± 9.31%) and the 1% PFEO gel (I% = 53.50 ± 2.51%). In a comparison between 1% and 4% PFEO formulations, it is clear that the higher concentration produces a greater anti-inflammatory effect. This dose-dependent response suggests that the efficacy of PFEO is closely linked to the concentration of the active compounds. Esters and sesquiterpenes, such as citronellol acetate, bornyl acetate and (-)-germacrene D, are likely to enhance the penetration of PFEO through the skin, improving its bioavailability and therapeutic efficacy. Besides increasing their lipophilicity, the esterification of these alcohols also enhances their anti-inflammatory effects by facilitating deeper penetration into tissues.54 The findings suggested that PFEO exerted its effects via a modulation of early and late inflammatory responses. PFEO components, such as geraniol and β-citronellol, have been reported to inhibit lipopolysaccharide (LPS)-induced nitric oxide (NO) and prostaglandin E2 (PGE2) production,55 reducing both the early histamine-induced response and prostaglandin-related inflammation at later stages. Collectively, the mechanism of action of these compounds involves the inhibition of key enzymes and mediators in the inflammatory pathway, such as cyclooxygenase (COX) enzymes and nitric oxide.56 For instance, (+)-β-linalool and geraniol have been found to modulate the release of pro-inflammatory cytokines and reduce oxidative stress,57 both key factors in the progression of inflammation, reinforcing the overall efficacy of PFEO. Although Voltaren remains highly effective, PFEO has considerable potential as a natural alternative, particularly at higher concentrations.
The antioxidant potential of PFEO was evaluated through DPPH, FRAP, ABTS, and TAC assays. Results are summarized in Table 3.
| Antioxidants activity | IC50 (mg/mL) | |
|---|---|---|
| PFEO | Positive Controls | |
| DPPH | 0.216 ± 0.019 A | 0.140 ± 0.0101 A |
| FRAP | 0.053 ± 0.031 A | 0.420 ± 0.130 1 B |
| TAC | 0.047 ± 0.014 A | 0.053 ± 0.230 1 A |
| ABTS | 0.094 ± 0.012 A | 0.004 ± 0.0031 B |
PFEO displayed modest ABTS reduction activity and highest scavenging potency in the DPPH, TAC, and FRAP assays, as evidenced by IC50 values of 0.094 ± 0.012 mg/mL for ABTS, 0.014 ± 0.183 mg/mL for TAC, 0.216 ± 0.019 mg/mL for DPPH, and 0.053 ± 0.014 mg/mL for FRAP. As a reference, ascorbic acid exhibited superior anti-radical effects compared to PFEO in ABTS (IC50 = 0.004 ± 0.003 mg/mL) and FRAP (IC50 = 0.420 ± 0.130 mg/mL) (p > 0.05). Nevertheless, statistically no significant difference between PFEO and positive control, the ascorbic acid (p < 0.05) in TAC (IC50 = 0.053 ± 0.23 mg/mL) and DPPH (IC50 = 0.14 ± 0.01 mg/mL) asssays, as detailed in Table 3. The highest antioxidant activity of PFEO may be attributed, in part, to its composition rich in geraniol, beta-citronellol, naphthalenemethanol, and geranyl tiglate. Due to the complex nature of essential oils, various protocols have been devised to assess their free radical scavenging potential. The recent emphasis on essential oils (EOs) arises from their potential to alleviate oxidative stress, essential for prolonging shelf life in cosmetic and pharmaceutical formulations. Antioxidants operate by providing hydrogen atoms to stabilize radicals generated during lipid oxidation, employing diverse mechanisms such as radical scavenging, electron or hydrogen atom donation, enzyme activity regulation, and metal ion chelation.58 Mitochondria, the primary origin of reactive oxygen species (ROS), plays a pivotal role in diseases associated with oxidative stress.59 Phenolic compounds present in antioxidant-rich extracts like essential oils regulate mitochondrial biogenesis, facilitating the elimination of impaired mitochondria and the generation of new ones to maintain cellular balance.60 EOs, abundant in phenolic acids and flavonoids, demonstrate notable antioxidant activity, attributed to constituents like linalool, 1,8-cineole, geranial, neral, and citronellal contribute to their antioxidant efficacy owing to their phenolic structure.61
In alignment with the sampling site of this study,24 Al-Mijalli et al explored the antioxidant potential of Pelargonium graveolens essential oils (PGEOs) obtained from aerial parts across different growth stages in Taounate, Morocco. Their findings illustrated an increasing antioxidant effect from dormancy to full flowering, peaking during full flowering (IC50: DPPH = 0.083 mg/mL, FRAP = 0.116 mg/mL, ABTS = 0.132 mg/mL). Notably, our study observed slightly higher activity in DPPH and FRAP assays but comparable results in the ABTS assay, possibly attributable to variations in the parts of plant utilized. Additionally, consistent with our results, Lohani et al.62 reported comparable scavenging activity in PGEOs from India, with an IC50 value of 0.018 mg/mL. In contrast, investigation of PGEOs from Germany exhibited lower antioxidant activity (IC50 = 0.802 mg/mL).63 Lastly, PGEO leaves from Palestinian demonstrated potent anti-DPPH radical activity (IC50 = 0.004 ± 0.45 mg/mL), approximately twice the potency of the PFEO.64
The reparative potential of PFEO on skin tissues in vitro, was assessed, targeting enzymes such as tyrosinase, elastase, and collagenase. Results outlined in Table 4 demonstrate substantial inhibitory effects of PFEO against tyrosinase (IC50 = 0.042 ± 0.026 mg/mL), elastase (IC50 = 0.080 ± 0.018 mg/mL), and collagenase (IC50 = 0.121 ± 0.018 mg/mL). These inhibitory properties were compared (p < 0.05) to those of kojic acid (IC50 = 0.044 ± 0.020 mg/ml for tyrosinase and epigallocatechin for elastase (IC50 = 0.032 ± 0.012 mg/mL) and collagenase inhibition (IC50 = 0.064 ± 0.038 mg/ml). A prior study evaluated cream formulations containing P. graveolens EO for their collagenase and elastase inhibition potential. Increasing the concentration from 1 to 0.1 mg/ml resulted in heightened anticollagenase activity, reaching a maximum inhibition of 28.4% at 1 mg/ml. Similarly, the highest antielastase inhibition was recorded at 108.2% at a concentration of 1 mg/mL.65 Additionally, the aerial parts of P. graveolens EO from Morocco, evaluated at the full flowering stage, exhibited promising IC50 value against tyrosinase (0.124 ± 0.07 mg/mL).66 The enzyme inhibitory effects observed in our study are likely attributable to the presence of geraniol and beta-citronellol, which have been previously identified as potential inhibitors of these enzymes.67,68 The findings imply the therapeutic potential of PFEO for promoting skin regeneration, particularly in conditions like atopic dermatitis, targeting collagenase, tyrosinase and elastase enzymes.
| Target metabolic enzymes | IC50 (mg/mL) | |
|---|---|---|
| PFEO | Positive Controls | |
| Tyrosinase | 0.042 ± 0.026 A | 0.044 ± 0.020 1 A |
| Elastase | 0.080 ± 0.018 A | 0.032 ± 0.012 2 A |
| Collagenase | 0.121 ± 0.018 A | 0.064 ± 0.038 2 A |
This study explored the therapeutic potential of Pelargonium graveolens (Geraniaceae) essential oil in the management of atopic dermatitis through a multidimensional approach. The analysis revealed that this essential oil possesses antimicrobial, antioxidant, and anti-inflammatory properties, in addition to exerting inhibitory effects on key enzymes involved in skin repair. The results showed that Pelargonium graveolens essential oil exhibits inhibitory effect against several bacterial and fungal strains, as well as moderate antioxidant activity. Furthermore, it demonstrated inhibitory effects on enzymes involved in collagen and elastin degradation, suggesting its potential in promoting skin regeneration. Additionally, in vivo tests confirmed its analgesic efficacy, thereby alleviating pain associated with atopic dermatitis. These findings suggest that PFEO could be a promising therapeutic option for managing symptoms of atopic dermatitis.
All animal procedures throughout the experiments were conducted in strict compliance with ethical standards and regulations to ensure the welfare of the mice. The study was considered and approved by the Institutional Ethics Committee for the Care and Use of Laboratory Animals of the Faculty of Sciences Dhar El Mehraz of the University Sidi Mohamed Ben Abdallah in Fez, Morocco (Approval NO-07/2024/LBEAS). Ethical guidelines for the handling and use of animals were established by the Institutional Ethics Committee in accordance with European Community Directive EEC/86/EEC (Union, 1986).
Animal sacrifice statement: All experiments on animals were carried out in accordance with ethical standards in accordance with European Community Directive EEC/86/EEC (Union, 1986). All measures were taken to minimize animal suffering during the study. The analgesic and anti-inflammatory effects of the topical gel containing Pelargonium graveolens flower essential oil were evaluated without the use of additional analgesics. Animals were subjected to humane euthanasia at the end of the experiments, following established protocols to ensure their well-being and minimize discomfort.
The project contains the following underlying data: Raw data for peer review & calculations. https://doi.org/10.6084/m9.figshare.27257685.v169
• Antioxidant, antibacterial, and enzyme inhibitory effects - raw data.xlsx. (Raw data on antioxidant activity, enzyme inhibition, and antimicrobial assays, along with calculated values such as IC50 and enzyme inhibition rates).
• In vivo analgesic effect of PFEO - raw data.xlsx. (Raw data on latency time measurements in mice for evaluating the analgesic effect of PFEO).
• In vivo anti-inflammatory effect of PFEO - raw data.xlsx. (Contains raw data on dermal volume measurements in mice and calculations related to the anti-inflammatory activity of PFEO).
• GC-MS volatile compounds analysis of PFEO - raw data.pdf. (Detailed report on the volatile compounds identified in Pelargonium graveolens essential oil via GC-MS analysis).
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Figshare: ARRIVE 2.0 checklists for Assessment of Pelargonium graveolens flower essential oil: Antimicrobial, antioxidant, enzyme inhibition and in vivo topical analgesic and anti-inflammatory efficacy as treatment for atopic dermatitis. https://doi.org/10.6084/m9.figshare.27264441.v1.70
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The authors acknowledge Prof. Hamid Khamar of the Botany Department of the Institute Scientific de Rabat, Morocco, for authenticating the plants. Thanks, are also due to the pharmaceutical industry Zenith Pharma in Casablanca, for its cooperation in supplying some of the reagents used in this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)