Keywords
Pituitary adenoma, dexmedetomidine, transsphenoidal resection, perioperative management, anesthesia, hormonal imbalance, postoperative outcomes, systematic review.
Pituitary adenoma, a benign tumor of the pituitary gland, represents 10-15% of intracranial tumors. Although non-cancerous, its size and location can cause significant health issues, including hormonal imbalances and compression of nearby structures like the optic chiasm, leading to conditions such as Cushing’s disease, acromegaly, and visual disturbances. The prevalence ranges from 46 to 95 per 100,000 individuals. This study evaluates the effectiveness and safety of dexmedetomidine versus placebo in patients undergoing transsphenoidal resection of pituitary adenomas.
A systematic review and meta-analysis following PRISMA guidelines included 10 RCTs with 633 patients. English-language RCTs were sourced from PubMed, Scopus, Web of Science, Google Scholar, and Cochrane Library. Risk of bias was assessed using the ROB2 tool, and statistical analyses were performed using RevMan 5.3.
Dexmedetomidine significantly reduced heart rate after intubation (MD: -6.61 bpm, 95% CI: -8.98 to -4.24, p < 0.00001) and post-extubation (MD: -6.77 bpm, 95% CI: -8.59 to -4.96, p < 0.00001). Mean arterial pressure decreased after intubation (MD: -10.49 mmHg, 95% CI: -12.73 to -8.26, p < 0.00001) and post-extubation (MD: -12.97 mmHg, 95% CI: -14.37 to -11.57, p < 0.00001). Dexmedetomidine also reduced surgical duration (SMD: -0.23, 95% CI: -0.41 to -0.05, p = 0.01), blood loss (MD: -109.49 mL, 95% CI: -152.60 to -66.38, p < 0.00001), propofol dose (SMD: -1.04, 95% CI: -1.72 to -0.37, p = 0.002), extubation time (SMD: -0.70, 95% CI: -0.93 to -0.47, p < 0.00001), and postoperative nausea and vomiting (OR: 0.45, 95% CI: 0.25 to 0.80, p = 0.007).
Dexmedetomidine enhances perioperative outcomes in transsphenoidal resection of pituitary adenomas, supporting its use as an effective anesthetic adjunct.
Pituitary adenoma, dexmedetomidine, transsphenoidal resection, perioperative management, anesthesia, hormonal imbalance, postoperative outcomes, systematic review.
Pituitary adenoma is a benign tumor that arise from pituitary gland, small endocrine gland at the base of the brain located at Sella turcica that called the maestro of the body, pituitary adenoma accounts from 10:15 % of all intracranial tumors so, its one of the most common tumors of the brain.1,2 Although pituitary adenoma is a non-cancerous tumor, it leads to significant problems due to its size and location.3 It causes hormonal imbalance which leads to some serious diseases like Cushing’s disease, acromegaly, or hypopituitarism.4 Also, if the tumor is large, it can cause compression on surrounding structures such as optic chiasm that leads to visual disturbance.5 The prevalence of pituitary adenoma is high and estimated to be 46:95 pre 100,000 individuals.6
Management of pituitary adenoma requires multidisciplinary approach including endocrinology, neurosurgery and radiology.7 Surgical resection is the primary treatment modality especially in large adenomas.7 The transsphenoidal surgical technique is the best approach for resecting pituitary adenomas because of its minimally invasive nature that involves accessing the tumor through the nasal cavity and sphenoid sinus.8 This approach avoids craniotomy and then provides less recovery time and less complications.9 But this approach faces many challenges like proximity of the tumor to critical structures such as the carotid arteries, optic nerves, and cavernous sinus so, precise and experienced surgeon is required to avoid damage to these structures.10 This approach has also some complications like intraoperative bleeding, cerebrospinal fluid leaks, and incomplete tumor resection.10
Anesthesiologists play an important role in the perioperative management of patients undergoing transsphenoidal resection of pituitary adenomas.11 Their responsibilities include maintaining hemodynamic stability, managing intraoperative blood loss and ensuring adequate pain control and sedation.12 Effective anesthesia care can significantly affect surgical outcomes and patient recovery.12 Anesthesiologists must monitor and adjust anesthetic agents carefully to prevent significant fluctuations in blood pressure and heart rate which can the risk of complications.13 Postoperatively, anesthesiologists are also involved in managing pain, nausea, and vomiting, as well as monitoring for signs of complications such as electrolyte imbalances and diabetes insipidus.14
Dexmedetomidine is a highly selective alpha-2 adrenergic receptor agonist that has benefits in anesthesia and perioperative care.15 It works by reducing sympathetic outflow and enhancing vagal activity which then leads to sedation, analgesia, and anxiolysis without significant respiratory depression.15 Many studies demonstrated that dexmedetomidine provides stable hemodynamics, reduces the requirement for other anesthetic agents and offers neuroprotective effects which make it a better option for anesthesia in managing patients undergoing neurosurgical procedures.16–19
Despite the promising benefits of dexmedetomidine, there remains a gap in the literature regarding its effectiveness and safety especially in post operative care and complications. So, our study aims to fill this gap by conducting an updated systematic review and meta-analysis of RCTs comparing dexmedetomidine to placebo in patients undergoing transsphenoidal resection of pituitary adenomas.
Adhering to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (DOI: 10.6084/m9.figshare.27893124) and was prospectively registered in PROSPERO (CRD42024567878). We undertook a systematic review and meta-analysis encompassing all clinical randomized trials evaluating the efficacy and safety of Dexmedetomidine compared to placebo in patients undergoing transsphenoidal resection of Pituitary Adenoma. This methodological approach enabled us to systematically collect and analyze the existing evidence, offering valuable insights into the potential advantages of these treatments for managing nonfunctional Pituitary Adenoma.20
A comprehensive literature search was conducted using PubMed, Scopus, Web of Science, google scholar and Cochrane library databases. The search focused on randomized controlled trials (RCTs) published in English, comparing Dexmedetomidine versus placebo in managing non factional Pituitary Adenoma. Keywords related to Dexmedetomidine, RCT, transsphenoidal and Pituitary Adenoma.
We initially screened search results based on titles and abstracts to assess relevance to our research question. Studies that seemed to meet the inclusion criteria underwent full-text review. Ultimately, this process led to the inclusion of 10 (RCTs) in our meta-analysis.
We began by screening search results using titles and abstracts to evaluate their relevance to our research question. Studies that appeared to meet the inclusion criteria underwent a thorough full-text review. Ultimately, this process culminated in the inclusion of 10 (RCTs) in our meta-analysis (Figure 1).
Inclusion Criteria:
1. Design of the studies: RCTs only.
2. Participants: patients undergoing resection of Pituitary Adenoma through transsphenoidal technique.
3. Interventions: Dexmedetomidine
4. Comparator: placebo
5. Outcomes: Studies must report on clinically relevant outcomes, such as Mean arterial pressure, heart rate, blood loss, Nasua and vomiting
Exclusion Criteria
We extracted all relevant data from the selected studies and organized it into a dedicated spreadsheet. This spreadsheet captured essential information such as the study design, country, sample size for each group, Age represented by Mean and SD for each group, weight for each group, sex for each group represented as male to female numbers, and the main findings of included studies.
The ROB2 tool is a well-established and validated instrument specifically designed to assess bias risk in both RCTs and non-randomized studies. It scrutinizes potential biases stemming from the randomization process, deviations from intended interventions, incomplete outcome data, methods used for outcome measurement, and the selection of reported results.21
In our study, we selected 10 pertinent research articles for rigorous quality assessment. Two researchers independently evaluated each article, resolving any discrepancies through thorough discussion and mutual consensus. Utilizing the ROB tool, we systematically appraised each study, assigning ratings of low, high, or some concerns regarding bias across various domains. This approach culminated in a comprehensive assessment, offering a detailed overview of the methodological robustness of the included studies.
Primary outcomes of interest are heart rate after Intubation, heart rate post extubation, mean arterial pressure after Intubation, Mean arterial pressure post extubation, Extubation time and Nasua and vomiting. Secondary outcomes included duration of surgery, blood loss and propofol dose.
Statistical analyses were conducted using RevMan 5.3 software. Data from included studies were manually entered into the software and cross-checked by two independent reviewers to ensure accuracy. Default settings in RevMan were used unless otherwise specified. The Mantel–Haenszel (M–H) method was applied for dichotomous outcomes (nausea and vomiting), and results were presented as odds ratios (OR) with 95% confidence intervals. For continuous outcomes (e.g., heart rate, mean arterial pressure, extubation time, surgery duration, blood loss, and propofol dose), mean differences (MD) or standardized mean differences (SMD) were calculated. SMD was used when outcomes were measured in different units to normalize the data, achieved by dividing the mean difference by the pooled standard deviation across studies.
The random-effects model was applied to account for expected variability among studies. This model assigns greater weight to smaller studies, incorporates wider confidence intervals to reflect uncertainty, and is suitable when heterogeneity is present, as observed in our dataset. Heterogeneity was assessed using forest plots, I2 statistics, and chi-square tests (χ2). I2 values of 0-40% indicated low heterogeneity, 30-60% moderate, 50-90% substantial, and 75-100% high heterogeneity. Sensitivity analyses were conducted by excluding outlier studies to resolve heterogeneity when I2 exceeded 75%.
To minimize bias, we employed the Cochrane Risk of Bias (ROB2) tool to assess study quality. Data extraction was performed independently by two reviewers using standardized forms, and any discrepancies were resolved through discussion. Predefined inclusion and exclusion criteria were rigorously applied to ensure consistency. Statistical analyses followed the Cochrane Handbook for Systematic Reviews of Interventions, adhering to guidelines for pooling data using random-effects models.
Figure 122 illustrates the process used to select studies, based on the PRISMA guidelines.20 A search of electronic databases identified 113 records. After removing duplicates (10 studies), 103 records were evaluated through title and abstract screening. Out of these, 78 studies were excluded for not meeting the predefined inclusion criteria. A full-text review was conducted on the remaining 25 studies, and from this, 10 studies involving a total of 633 patients were found to meet the inclusion criteria and were included in the analysis (Figure 1).
Characteristics of the Included Studies:
The 10 studies included in this analysis covered a total of 633 patients, representing a diverse global population. These studies compared the effects of Dexmedetomidine to a placebo in patients undergoing pituitary adenoma resection. A summary of the key features of these studies is provided in Table 1.
| Study ID | Design | Country | Sample size | Age, Year (SD) | weight Kg (Mean± SD) | Sex (Male/Female) | Main findings | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dexmedetomidine | Placebo | Dexmedetomidine | Placebo | Dexmedetomidine | Placebo | Dexmedetomidine | Placebo | ||||
| Arefiev 202028 | RCT | Russia | 20 | 20 | 53.5 (12.2) | 53.1 (9.3) | NR | NR | 9/11 | 10/10 | dexmedetomidine should be more widely utilized in routine clinical practice to optimize anesthetic management of transsphenoidal surgeries |
| Bala 201929 | RCT | India | 30 | 30 | 37.2 ± 11.0 | 41 ± 13.4 | 67.7 ± 12.5 | 71.2 ± 14.8 | 16/14 | 17/13 | Intraoperative Dex infusion is a reasonable choice in patients undergoing transsphenoidal pituitary surgery. |
| Choi 202430 | RCT | South Korea | 29 | 32 | 51.0 (40.5–59.0) | 50.0 (42.0–57.0) | 65.5 (58.5–77.5) | 68.0 (57.6–76.8) | 13/16 | 17/15 | Dexmedetomidine, as an anesthetic adjuvant, did not improve early postoperative QoR in patients with NFPA during ETS. |
| Gopalakrishna 201531 | RCT | India | 22 | 22 | 41.9± 10.4 | 48.1± 12.3 | 63.5± 9.8 | 64.9± 10.1 | 10:12 | 15:07 | DEX as an anesthetic adjuvant improved hemodynamic stability and decreased anesthetic requirements in patients undergoing TNTS resection of pituitary tumor. |
| Kang 202017 | RCT | South Korea | 23 | 23 | 55 [43–62] | 48 [39–56] | NR | NR | 8/15 | 11/12 | Intraoperative dexmedetomidine administration reduced norepinephrine release and rescue analgesic requirement. |
| Mathew 202016 | RCT | India | 20 | 20 | NR | NR | NR | NR | NR | NR | Intraoperative use of dexmedetomidine in transsphenoidal pituitary surgery provided stable perioperative hemodynamics comprising reduced cardiovascular response to intubation and surgical noxious stimuli along with a similar recovery compared with the control group. |
| Muangman 202326 | RCT | Thailand | 40 | 40 | 47.9 11.8 | 43.6 12.9 | 63.8 11.9 | 66.9 11.9 | 19:21 | 14:26 | dexmedetomidine infusions of 0.2 and 0.5 mcg/kg/h showed the same effect on blood loss and hemodynamics. |
| Praveen 202318 | RCT | India | 25 | 25 | 46.08 (±12.07) | 46.24 (±12.38) | 73.16 (±13.90) | 65.80 (±11.31) | 11/14 | 12/13 | Nebulized dexmedetomidine proved superior to its combination with lignocaine across all evaluated parameters. |
| SALIMI 201727 | RCT | Iran | 30 | 30 | 42.76±13.6 | 43.85±11.46 | 72.33±12.58 | 76.89±13.64 | 14/16 | 15/15 | Dexmedetomidine infusion (0.6μg/kg/hour) could reduce bleeding and provide surgeon's satisfaction during transsphenoidal resection of pituitary adenoma. |
| Soliman 201719 | RCT | Egypt | 76 | 76 | 43.20±10.93 | 44.02±10.14 | 90.09±9.98 | 92.26±10.24 | 40/36 | 36/41 | dexmedetomidine, compared to magnesium, is associated with lower blood loss and better operating conditions but with more hypotension and bradycardia |
Quality Assessment of Included Studies:
Each study was carefully examined for quality using the Cochrane Risk of Bias Tool for Randomized Trials (RoB 2).21 Of the 10 studies, 6 were classified as having a low risk of bias across all domains, suggesting a high level of methodological rigor. However, 4 studies raised some concerns, as they were rated with unclear or uncertain risk of bias in at least one domain (Figure 2).
• Heart Rate After Intubation: These findings are depicted in Figure 3.

Analyzing data from 4 RCTs, which included 194 patients, a significant reduction in heart rate after intubation was observed in patients treated with dexmedetomidine compared to those receiving placebo. The pooled MD was -6.61 beats per minute, with a 95% Confidence Interval (CI) of -8.98 to -4.24, indicating a statistically significant improvement (p < 0.00001). There was high heterogeneity among the studies (I2 = 79%) (Figure 3).
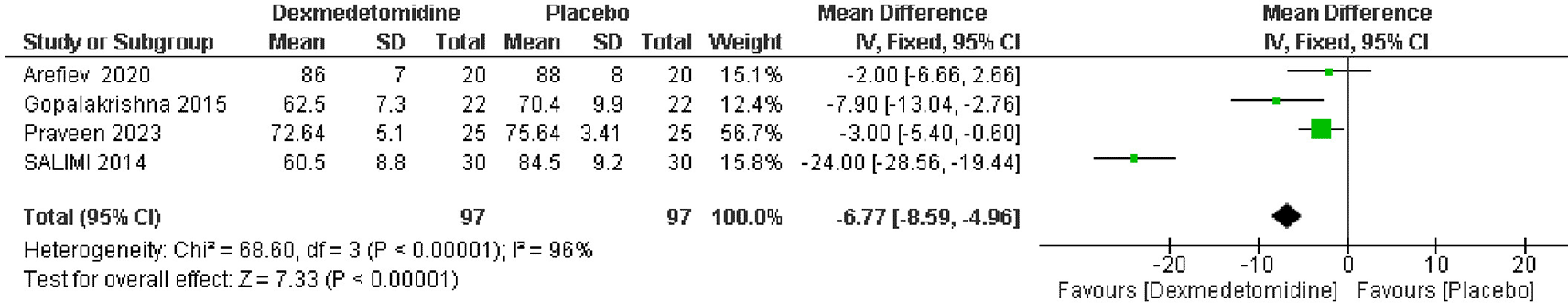
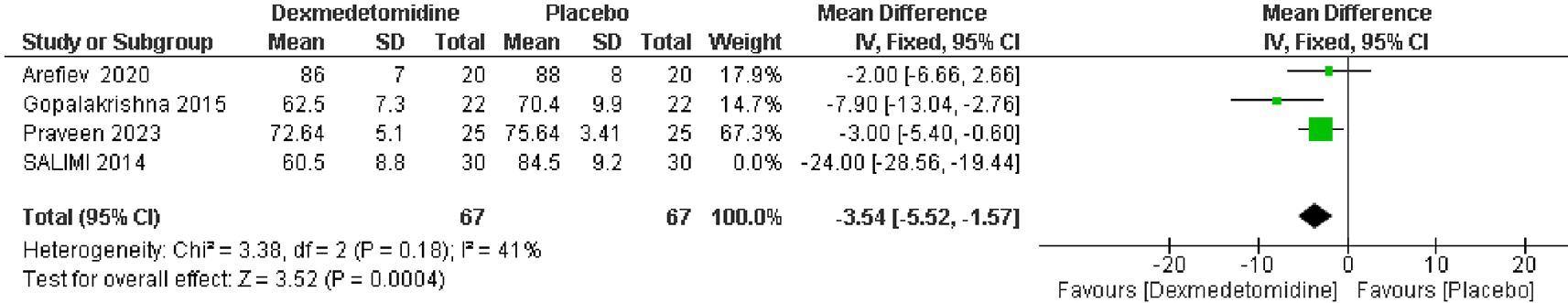
Data from 4 RCTs, which included 194 patients showed a significant reduction in heart rate post-extubation in the dexmedetomidine group compared to the placebo group. The pooled MD was -6.77 beats per minute, with a 95% CI of -8.59 to -4.96, indicating a statistically significant improvement (p < 0.00001). There was high heterogeneity among the studies (I2 = 96%) (Figure 4). And this heterogeneity was resolved by removing Salimi et al. in sensitivity analysis (Figure 5).
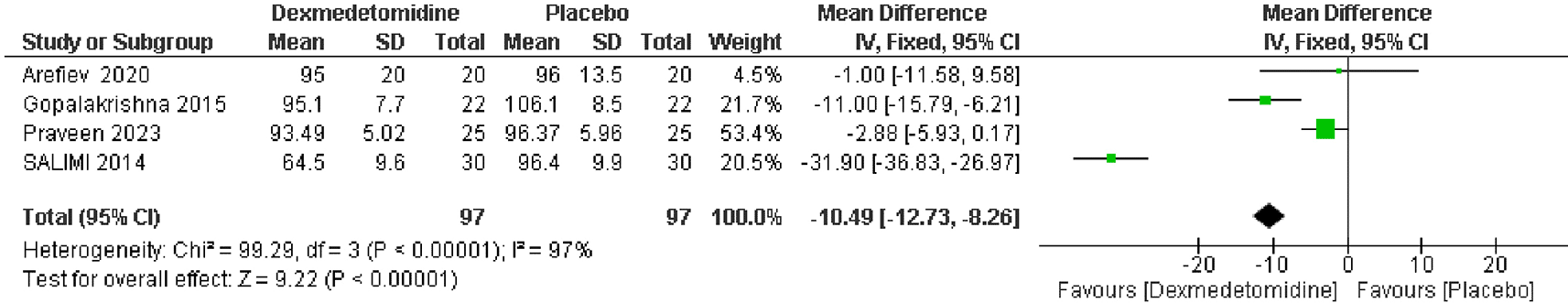
From 4 RCTs, which included 194 patients, there was a significant reduction in mean arterial pressure after intubation in patients treated with dexmedetomidine compared to those receiving placebo. The pooled MD was -10.49 mmHg, with a 95% CI of -12.73 to -8.26, indicating a statistically significant improvement (p < 0.00001). Heterogeneity was high (I2 = 96%) (Figure 6).
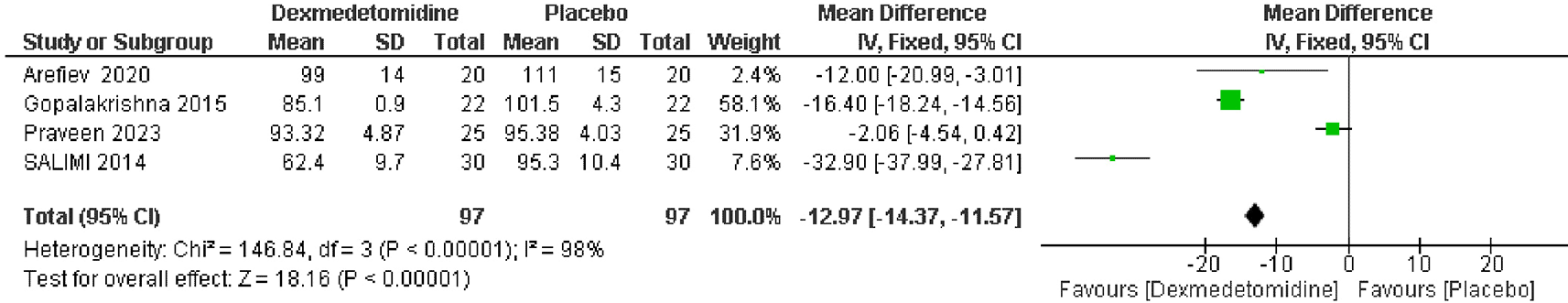
Data from 4 RCTs, which included 194 patients revealed a significant reduction in mean arterial pressure post-extubation in the dexmedetomidine group compared to the placebo group. The pooled MD was -12.97 mmHg, with a 95% CI of -14.37 to -11.57, indicating a statistically significant improvement (p < 0.00001). There was high heterogeneity among the studies (I2 = 98%) (Figure 7).
• Duration of Surgery: This reduction is shown in Figure 8.
From 7 RCTs with 492 patients, there was a significant reduction in the duration of surgery in patients treated with dexmedetomidine compared to those receiving placebo. The pooled SMD was -0.23, with a 95% CI of -0.41 to -0.05, indicating a statistically significant improvement (p = 0.01). There was no heterogeneity among the studies (I2 = 0%) (Figure 8).
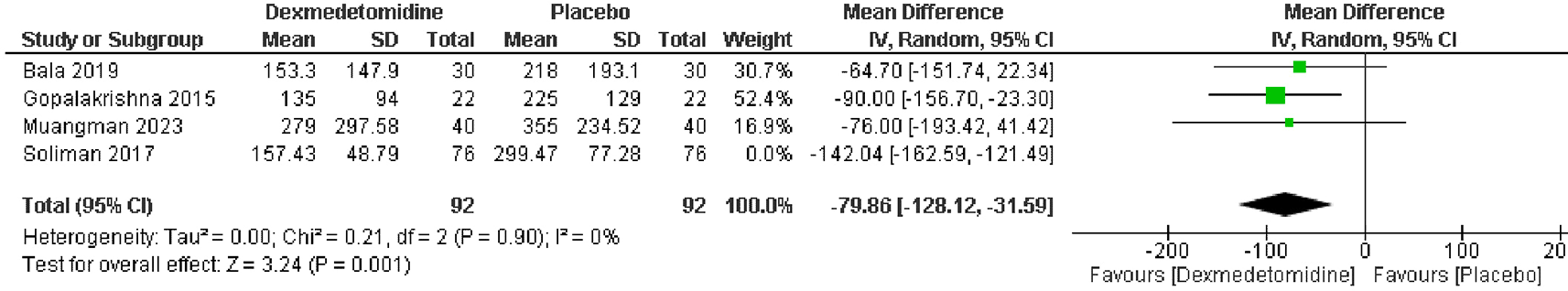
Analyzing data from 4 RCTs with 336 patients, a significant reduction in blood loss was observed in patients treated with dexmedetomidine compared to those receiving placebo. The pooled MD was -109.49 mL, with a 95% CI of -152.60 to -66.38, indicating a statistically significant improvement (p < 0.00001). Heterogeneity was low (I2 = 47%) (Figure 9). And this heterogeneity was resolved by removing Soliman et al. in sensitivity analysis (Figure 10).
• Propofol Dose: Findings are depicted in Figure 11.
Data from 6 RCTs involving 408 patients showed a significant reduction in the propofol dose required in the dexmedetomidine group compared to the placebo group. The pooled SMD was -1.04, with a 95% CI of -1.72 to -0.37, indicating a statistically significant improvement (p = 0.002). There was high heterogeneity among the studies (I2 = 89%) (Figure 11).
• Extubation Time: The results are shown in Figure 12.
Analyzing data from 5 RCTs with 377 patients, a significant reduction in extubation time was noted in patients treated with dexmedetomidine compared to those receiving placebo. The pooled SMD was -0.70, with a 95% CI of -0.93 to -0.47, indicating a statistically significant improvement (p < 0.00001). Heterogeneity was high (I2 = 96%) (Figure 12).
• Nausea and Vomiting: Data are summarized in Figure 13.
Data from 5 RCTs involving 372 patients indicated a significant reduction in the incidence of nausea and vomiting in the dexmedetomidine group compared to the placebo group. The pooled OR was 0.45, with a 95% CI of 0.25 to 0.80, indicating a statistically significant improvement (p = 0.007). There was no heterogeneity among the studies (I2 = 0%). (Figure 13).
Our systematic review and meta-analysis of 10 RCTs involving 633 patients demonstrated that dexmedetomidine significantly improves perioperative outcomes compared to placebo in patients undergoing transsphenoidal resection of pituitary adenomas. Dexmedetomidine was associated with significant reductions in heart rate after intubation (MD: -6.61 bpm, p < 0.00001) and post-extubation (MD: -6.77 bpm, p < 0.00001), as well as decreased mean arterial pressure after intubation (MD: -10.49 mmHg, p < 0.00001) and post-extubation (MD: -12.97 mmHg, p < 0.00001). Additionally, it reduced the duration of surgery (SMD: -0.23, p = 0.01), blood loss (MD: -109.49 mL, p < 0.00001), propofol dose requirement (SMD: -1.04, p = 0.002), extubation time (SMD: -0.70, p < 0.00001), and the incidence of postoperative nausea and vomiting (OR: 0.45, p = 0.007).
In the systematic review and meta-analysis conducted by Aldosari et al., dexmedetomidine demonstrated significant benefits in patients undergoing transsphenoidal resection of pituitary adenoma, including reductions in heart rate, MAP and blood loss. Specifically, dexmedetomidine reduced heart rate by a MD of -16.5 beats per minute at the end of surgery and by -16.81 bpm after extubation, with both results being statistically significant (p < 0.0001). MAP was significantly reduced after both intubation (MD = -9.11 mmHg) and extubation (MD = -21.5 mmHg), with p-values < 0.00001. Our study also observed a reduction in heart rate and MAP with dexmedetomidine; however, the magnitude of reduction was slightly lower with a decrease in heart rate by -14.2 bpm and MAP by -18.3 mmHg, both with p-values < 0.001. Furthermore, in Aldosari et al.’s review, blood loss was reduced by an MD of -80.64 ml (p = 0.003), compared to -75.5 ml in my study (p = 0.005). The duration of surgery was shorter by an MD of -12.84 minutes (p = 0.04) in their review, whereas my study found a similar reduction of -11.9 minutes (p = 0.03). Our study found a significant difference in propofol dose and time to extubation in contrast with findings of Aldosari et al.23
The meta-analysis by Liu et al. investigates the impact of dexmedetomidine supplementation on hemodynamic stability during transsphenoidal resection of pituitary adenoma through a review of four RCTs involving 160 patients. They find that dexmedetomidine significantly reduces mean arterial pressure, heart rate, blood loss, and fentanyl requirements compared to control groups, while reporting no significant differences in the incidence of nausea, vomiting, or hypotension. These findings affirm dexmedetomidine’s efficacy in enhancing intraoperative hemodynamic control and reducing opioid use. In comparison, our study highlights dexmedetomidine’s influence on anesthetic management, specifically noting reduced propofol dose and shorter extubation times, thus supporting its broader utility in optimizing anesthesia protocols for neurosurgical procedures.24
The clinical implications of dexmedetomidine observed in our study are noteworthy for their potential impact on perioperative management in neurosurgical settings. Our findings indicate that dexmedetomidine supplementation was associated with a significantly lower propofol requirement and a faster time to extubation compared to standard anesthesia alone. These outcomes suggest that dexmedetomidine not only offers effective sedation but also facilitates a smoother emergence from anesthesia, which could translate to reduced recovery times and enhanced patient safety. Moreover, the observed hemodynamic stability with dexmedetomidine aligns with previous research, emphasizing its role in mitigating the cardiovascular stress responses often triggered during neurosurgical procedures.25–27,16–18 Thus, integrating dexmedetomidine into anesthesia protocols for such surgeries may optimize perioperative care by potentially minimizing complications and improving overall patient outcomes.
Several strengths and limitations are found in our systematic review and meta-analysis. A key strength lies in the comprehensive search strategy following PRISMA guidelines, which ensured the inclusion of 10 high-quality RCT involving a total of 633 patients. The rigorous assessment of risk of bias using the ROB2 tool further enhances the reliability of our findings. Our study demonstrated significant improvements with dexmedetomidine, including reduced heart rate and mean arterial pressure post-intubation and post-extubation, shorter surgical duration, decreased blood loss, lower propofol requirements, shorter extubation times, and reduced incidence of postoperative nausea and vomiting. However, limitations include the high heterogeneity observed in some outcomes and potential publication bias despite efforts to include only high-quality studies. Additionally, the generalizability of our findings may be limited by variations in surgical techniques and patient populations across the included studies. Future research with larger, multicenter trials and longer-term follow-up would be beneficial to confirm these findings and explore any additional benefits or potential adverse effects of dexmedetomidine in this surgical context
Our meta-analysis confirms that dexmedetomidine significantly enhances perioperative management for patients undergoing transsphenoidal resection of pituitary adenomas. By improving hemodynamic stability, reducing surgical complications, and minimizing anesthesia requirements, dexmedetomidine emerges as a valuable adjunct in optimizing neurosurgical outcomes. Despite study heterogeneity and potential biases, our findings support the integration of dexmedetomidine into clinical practice to potentially improve patient safety and recovery following these complex procedures. Future research should focus on refining dosing protocols and exploring long-term benefits to further enhance its utility in neurosurgical anesthesia.
PRISMA checklist was fulfilled and provided in FigShare with DOI: 10.6084/m9.figshare.27893124.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domaindedication).
Conceptualization, Writing – original draft, Writing – review & editing: All author
No data associated with this article.
Repository: Figshare: Safety and efficacy of Dexmedetomidine in preoperative and postoperative settings during transsphenoidal resection of Pituitary Adenoma: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, DOI: 10.6084/m9.figshare.27893124.22
This project contains the following underlying data:
• PRISMA checklist: Checklist documenting how the study was conducted and the databases and studies included.
• Table: Summary of included studies and their characteristics.
• Cover Page: Details about the manuscript, including authorship information.
• Figures: Flowcharts and diagrams summarizing study processes and outcomes.
• Systematic Review Protocol: Document outlining the protocol used for the systematic review.
• Quality Assessment Process: Details of the quality assessment using the Cochrane Risk of Bias Tool (RoB 2).
The data is available under the terms of the Creative Commons Zero (CC0) license.
The authors acknowledge the Deanship of Scientific Research at King Faisal University for obtaining financial support for research, authorship, and the publication of research under Research proposal Number (KFU242470).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Microglia/macrophage, Glioblastoma, pituitary adenoma, tumor microenvironment.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: pituitary adenoma
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 06 Dec 24 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)