Keywords
Phage, pathogen reduction, environmental pathogens, antimicrobial resistance, water quality management, composting processes, biocontrol agents, agricultural sustainability
Escherichia coli is a critical priority pathogen due to its significant morbidity, mortality, and growing antimicrobial resistance, underscoring the urgent need for novel control strategies. This bacterium is frequently implicated in outbreaks associated with horticultural products, particularly those cultivated in organic farming systems. The aim of this study was to isolate and evaluate the potential of a bacteriophage as a biocontrol agent against E. coli in compost and agricultural irrigation water.
E. coli presence in compost samples (n=17) was determined through microbiological assays, and the bacterial identity was confirmed by PCR amplification of the phoA gene. Antimicrobial resistance profiles of the isolates were assessed using the disk diffusion method. Bacteriophage isolation was conducted from livestock fecal samples using a double-layer agar technique. The stability of the bacteriophage under varying pH levels and temperatures was evaluated, along with its replication dynamics. Additionally, the phage’s efficacy in reducing E. coli populations in compost and irrigation water was assessed. Genomic sequencing and bioinformatic analyses of the bacteriophage were conducted to characterize its genetic profile.
E. coli strains isolated exhibiting multidrug resistance were isolated from compost samples. The isolated bacteriophage, named Alux-21, exhibited stability at neutral pH and retained viability at both 4°C and 40°C over a six-month period. Importantly, the phage achieved a significant reduction of E. coli counts, exceeding 3.8 logs in compost and 3 logs in irrigation water, demonstrating its superior efficacy compared to previously reported phages in similar substrates. Genomic analysis confirmed the absence of virulence-associated, lysogeny, and antibiotic resistance genes.
The findings highlight Alux-21 as a sustainable biocontrol agent for E. coli in compost and irrigation water. Field validation will be crucial to establish its scalability and efficacy under real-world agricultural conditions.
Phage, pathogen reduction, environmental pathogens, antimicrobial resistance, water quality management, composting processes, biocontrol agents, agricultural sustainability
Antimicrobial resistance (AMR) in bacteria has emerged as one of the most critical threats to global public health, endangering millions of lives worldwide. It is estimated that antimicrobial-resistant bacteria account for approximately 13,600 deaths per day (Johnston et al., 2021; Murray et al., 2022). This crisis underscores the urgent need for new therapeutic approaches to effectively combat AMR (Xiao et al., 2023). Among the pathogens identified as critically important by the World Health Organization (WHO) is Escherichia coli, a bacterium characterized by its dual threat of causing severe infections and exhibiting increasing levels of antimicrobial resistance. This combination complicates treatment options, further exacerbating global morbidity and mortality rates (Ikuta et al., 2022; Jesudason, 2024).
Escherichia coli is a genetically diverse bacterium with significant public health implications due to its association with antimicrobial resistance and foodborne illnesses. Numerous epidemiological outbreaks have been linked to E. coli contamination in fresh produce, a problem worsened by the increasing consumption of organic foods (Heiman et al., 2015; Irvin et al., 2021; Tack et al., 2021; Waltenburg et al., 2022). In recent years, the global demand for organic produce has surged, promoting the adoption of organic farming practices (Bernabéu et al., 2023). While these practices have gained popularity worldwide, they often involve the use of organic soil amendments to improve soil fertility. However, inadequate quality control of these amendments may lead to contamination with foodborne pathogens, posing risks to soil, irrigation water, and produce (Murphy et al., 2024).
E. coli is a leading pathogen in organic food outbreaks, frequently linked to tomatoes, lettuce, and cucumbers (Coulombe et al., 2020; Irvin et al., 2021; Pires et al., 2023). Additionally, agricultural irrigation water also presents a critical route for E. coli entry into food production systems, particularly with crops consumed raw, where contamination can directly impact consumer safety. As such, effective management practices that enhance the microbiological quality of irrigation water are essential to mitigate food safety risks. These two vectors are significant pathways for introducing pathogenic bacteria into agricultural environments, creating substantial food safety and public health challenges. In this context, bacteriophages have emerged as promising biocontrol agents for mitigating E. coli contamination in agricultural settings. Their application offers a sustainable and targeted approach to enhance food safety (Álvarez et al., 2019; Soliman et al., 2023).
In organic farming, bacteriophages could play a pivotal role in reducing pathogen transmission, particularly when compost and irrigation water fail to meet microbiological quality standards. Despite their potential, few studies have investigated bacteriophage efficacy in compost or irrigation water, and no bacteriophage to date has been shown to effectively control E. coli in both environments (Heringa et al., 2010; Álvarez et al., 2019).
For biocontrol applications, bacteriophages must meet stringent criteria. They must demonstrate high lytic activity against bacterial cells and maintain stability under diverse environmental conditions such as varying temperatures, pH levels, and salinity, ensuring consistent performance (João et al., 2021). Moreover, it is crucial that bacteriophages lack genes associated with virulence, lysogeny, antibiotic resistance, or allergenicity (Suh et al., 2022). Scalability is also essential, requiring biological characteristics that enable large-scale propagation. Given these considerations, this study aimed to isolate and evaluate the potential of a bacteriophage as a biocontrol agent against E. coli in two critical agricultural environments: compost and irrigation water.
Seventeen compost samples were collected on July 3 and 5, 2023, from mature compost piles composed of a 10:4 (w/w) mixture of bovine manure and plant residues. These samples were selected to assess spatial variations and heterogeneity within the compost. The number of samples was determined based on the expected variability of Escherichia coli in compost and irrigation water, as informed by previous studies (Thomas et al., 2020). The compost piles, located at commercial composting facilities in Culiacán, Sinaloa, México, were approximately 5 meters in length and 2.5 meters in width. To guarantee representativeness, samples were collected from various points within each pile using a simple random sampling approach. Following collection, the samples were transported to the laboratory in refrigerated coolers.
For initial processing, 10 g of each sample were weighed and inoculated into 90 ml of buffered peptone water. This suspension was incubated at 37 °C for 24 hours. Following incubation, an aliquot of the culture was plated onto Petri plates containing CHROMagar™ E. coli medium (CHROMagar™ Microbiology, France) using a sterile inoculating loop. The plates were incubated at 37 °C for an additional 24 hours (Heratherm IMC18, Thermo Scientific™, USA). Colony morphology was then examined to identify characteristics typical of E. coli, including the presence of intense blue or pink colonies with smooth edges. Representative colonies were selected and plated onto fresh CHROMagar™ E. coli plates, followed by incubation at 37 °C for 24 hours. This re-isolation procedure was repeated five times to ensure the isolation of a pure strain (Werner et al., 2022).
For molecular identification, polymerase chain reaction (PCR) analysis was performed following the protocol of Li et al. (2020), with slight modifications. Amplification of the phoA gene was carried out using the GoTaq® PCR Core system (Promega, USA). The final reaction mixture contained 4.8 μl of 5× Green GoTaq buffer, 0.8 μl of dNTPs, 2.5 μl of MgCl2, 0.8 μl of each primer, 0.35 μl of Taq polymerase, and 100 ng of bacterial DNA template. PCR conditions included an initial denaturation at 94 °C for 7 minutes, followed by 30 cycles of denaturation at 94 °C for 30 seconds, annealing at 56 °C for 30 seconds, and extension at 72 °C for 30 seconds (Mastercycler Nexus, Eppendorf®, Germany). A final extension step was performed at 72 °C for 5 minutes. The resulting 622 bp gene fragment was visualized by electrophoresis on a 1.2% agarose gel at 60 V for 2 hours. The gel was stained with GelRed™ Nucleic Acid Gel Stain (Biotium, USA) and viewed under UV transillumination.
To assess the antimicrobial resistance profiles of E. coli strains, a panel of 12 antibiotics (Oxoid, USA) representing various antibiotic classes was used: carbapenems (imipenem), aminoglycosides (gentamicin, amikacin), penicillins (ampicillin, amoxicillin/clavulanic acid), fluoroquinolones (ciprofloxacin), quinolones (nalidixic acid), phenicols (chloramphenicol), tetracyclines (tetracycline, oxytetracycline), polymyxins (colistin), third-generation cephalosporins (cefoperazone), and sulfonamides (trimethoprim/sulfamethoxazole). Antimicrobial susceptibility testing was conducted using the disk diffusion method, following the guidelines of the Clinical and Laboratory Standards Institute (CLSI). For this procedure, bacterial cultures were resuspended in sterile 0.85% saline solution and adjusted to an optical density of approximately 0.1 at 600 nm. Subsequently, 200 μl of the bacterial suspension was spread onto Mueller-Hinton agar plates using a sterile swab to ensure an even inoculum distribution across the medium. Antibiotic-impregnated paper disks were then placed on the agar surface, and the plates were incubated at 37 °C for 24 hours. Following incubation, inhibition zone diameters were measured with a millimeter-calibrated Vernier caliper. The results were interpreted according to CLSI susceptibility criteria, classifying the strains as susceptible, intermediate, or resistant (CLSI, 2022).
Bacteriophage isolation was performed using fecal samples collected on August 7 and 8, 2023, from livestock housed at the “La Posta Zootecnia” animal production unit of the Faculty of Agronomy, Autonomous University of Sinaloa. Ten grams of each fecal sample were transferred into 90 ml of sterile purified water and mixed thoroughly by inversion. Subsequently, 35 ml of the mixture was transferred to centrifuge tubes and centrifuged at 3,500 × g for 10 minutes (Sorvall™ 17R, Thermo Scientific™, USA). The supernatant was collected in a sterile tube and subjected to two additional rounds of centrifugation under the same conditions. The final supernatant was filtered through a 0.45 μm pore-size nitrocellulose membrane, and the filtrate was collected for further processing.
Bacteriophage isolation was conducted using the double-layer agar technique. A 1 ml aliquot of an exponential-phase E. coli culture, grown in tryptic soy broth (TSB, MCD LAB, México) was added to 3 ml of molten 0.4% TSB-agarose, maintained at 50 °C. This mixture was poured onto tryptic soy agar (TSA, MCD LAB, México) plates. Once solidified, four 5 μl drops of the filtrate were placed onto the surface of the solidified medium. After drying, plates were incubated at 37 °C for 18–24 hours. Following incubation, plates were inspected for lytic zones, indicating bacteriophage activity against E. coli (Kosznik-Kwaśnicka et al., 2020).
Lytic zones were carefully excised using a sterile plastic Pasteur pipette and individually transferred to microcentrifuge tubes containing 500 μl of sterile purified water, followed by gentle mixing by inversion. This suspension was centrifuged at 8,500 × g for 5 minutes, and the supernatant was transferred to a new sterile microcentrifuge tube. To increase the bacteriophage concentration, a second round of the double-layer agar technique was performed. Specifically, 1 ml of bacterial culture and 100 μl of the recovered supernatant were added to 3 ml of 0.4% TSB-agarose and poured onto TSA plates. Plates were incubated at 37 °C for 18–24 hours. After incubation, plates were examined for lytic plaques, from which the largest and clearest plaques were excised using a sterile plastic Pasteur pipette and transferred to a microcentrifuge tube containing 500 μl of sterile purified water. This isolation and purification process, involving lytic plaque excision and the double-layer agar technique, was repeated five times to ensure the isolation of a single bacteriophage type (Topka et al., 2019).
To increase the concentration of the bacteriophage, a double-layer agar technique was employed as previously described. After the incubation period, 5 ml of sterile water was added to each Petri dish, and the plates were left at room temperature for 2 hours. The soft agar layer was then carefully scraped off using a sterile bacteriological loop and transferred to a sterile 50 ml conical tube. The collected suspension underwent centrifugation at 3,500 × g for 10 minutes at room temperature, after which the supernatant was transferred to a sterile tube for subsequent processing. This centrifugation process was repeated three additional times to ensure maximum removal of culture medium residues and bacterial debris.
Following the initial centrifugation steps, the final supernatant was passed through a 0.22 μm sterile syringe filter with a polyethersulfone membrane (GVS Filter Technology, USA), and the resulting filtrate was transferred to a polypropylene tube for further concentration by ultracentrifugation at 40,000 × g for 2 hours at 4 °C (MSE PRO MA1084-1, USA). After the centrifugation, the supernatant was discarded, and the resulting pellet was resuspended in 10 ml of sterile water.
The bacteriophage suspension was subsequently purified through dialysis using a Slide-A-Lyzer device with a molecular weight cutoff (MWCO) of 20,000 (Thermo Fisher, USA), following the protocol outlined by Le Guellec et al. (2023). Lastly, the concentration of the bacteriophage was determined via serial tenfold dilutions and the double-layer agar technique to quantify the phage titers accurately.
To assess the stability of the bacteriophage across a range of pH values, a series of sterile saline solutions (0.85%) were prepared, adjusted to pH 3, 5, 7, 9, and 11 (± 0.05) using either 5 M HCl or 5 M NaOH. A purified bacteriophage suspension was added to each pH-adjusted solution at a 99:1 ratio, achieving a final concentration of 1 × 106 PFU/ml. These suspensions were then incubated at 37 °C for 1 hour. The viability of the bacteriophage was subsequently evaluated through serial tenfold dilutions and the double-layer agar method, as described by Bagińska et al. (2024). All experiments were conducted in triplicate to ensure reproducibility.
Based on the results of the pH stability tests, the bacteriophage was diluted in sterile water adjusted to the pH at which optimal stability was observed, yielding a final concentration of approximately 1 × 108 PFU/ml. This suspension was divided into two aliquots: one was stored at 4 °C, and the other at 40 °C. Bacteriophage concentration in each sample was monitored monthly for a duration of 6 months using serial tenfold dilutions and the double-layer agar technique. Each assay was performed in triplicate to confirm consistency and reliability of the results.
To investigate the replication dynamics of the bacteriophage, a single colony of E. coli was inoculated into 100 ml of TSB and incubated in a water bath at 37 °C with constant agitation at 80 rpm (Shel Lab, USA). Once the bacterial culture reached an optical density (OD) of 0.1 at 600 nm, corresponding to an approximate concentration of 0.8 × 108 CFU/ml, the bacteriophage was added at a multiplicity of infection (MOI) of 0.1. Aliquots were collected from the culture every five minutes and transferred into microcentrifuge tubes.
To assess intracellular phage replication, 100 μl of chloroform was added to one of the tubes, while the other tube was left untreated. The addition of chloroform lyses the bacterial cells, releasing intracellular phage particles. Both treated and untreated samples were then centrifuged at 10,000 × g for 1 minute, and the bacteriophage concentration in the supernatant was quantified using serial tenfold dilutions followed by the double-layer agar method, as outlined by Kropinski (2018). All bacteriophage concentrations were determined in triplicate to ensure accuracy and reproducibility. as outlined by
A single colony of E. coli was inoculated into TSB and incubated for 24 hours at 37 °C. Subsequently, 1 ml of this culture was transferred into four flasks, each containing 200 ml of TSB, and incubated at 37 °C with agitation at 80 rpm. The optical density of the bacterial culture was monitored at a wavelength of 600 nm (OD600).
When the optical density reached 0.5 (~2 × 108 CFU/ml), purified bacteriophage suspension was added to three of the flasks at different multiplicities of infection (MOI): 0.1, 0.01, and 0.001, respectively. The fourth flask served as a control to monitor E. coli growth in the absence of bacteriophage. All flasks were incubated under the same conditions as previously described, and the optical density of the cultures was measured hourly over a period of 6 hours, following the protocol outlined by Khunti et al. (2023).
This experimental design allowed for the assessment of the bacteriolytic effect of the bacteriophage at varying MOIs, providing insights into its efficacy against E. coli. All measurements were performed in triplicate to ensure reliability and accuracy of the results.
Compost was prepared using a mixture of bovine manure and vegetable residues in a ratio of 10:4, obtained after 90 days of composting at a facility in Culiacán, Sinaloa, México. The compost was sterilized at 121 °C for 20 minutes under pressure, with this process repeated three times to ensure sterility. Following sterilization, the moisture content was determined using the drying method and adjusted with sterile water to achieve a final moisture level of 30% (Heringa et al., 2010). For the experiment, 100 g of compost was placed in each of three sterile, sealable plastic bags. The following treatments were applied: in the first bag, 1 ml of sterile water was added as absolute control; in the second bag, 1 ml of a purified E. coli suspension was introduced to reach a final concentration of 1 × 106 CFU/g (bacterial control). This concentration was selected based on previous studies reporting elevated levels of E. coli in various biofertilizers (Black et al., 2014; Miller et al., 2013). In the third bag, 1 ml of the same bacterial suspension was added, achieving the same concentration as the bacterial control. After a 1-hour incubation period to allow for acclimatization, 500 μl of a purified bacteriophage suspension was introduced to the third bag, resulting in a final concentration of 1 × 104 PFU/g (phage treatment).
The contents of the bags were manually mixed, and the compost was kept under environmental conditions characteristic of agricultural systems in Culiacán, Sinaloa for 48 hours. Ambient temperatures ranged from 19 to 31 °C, while relative humidity levels were regularly between 55% and 60%. These parameters were specifically used to closely replicate the agricultural environments characteristic of the region, thus increasing the ecological relevance and practical applicability of the experimental findings. At the conclusion of the incubation period, 10 g of compost was sampled from each treatment, mixed with 90 ml of sterile water, and transferred to new sealable bags, which were vigorously agitated for 5 minutes. Decimal dilutions were then performed, and bacterial concentration was quantified using the spread plate method on CHROMagar™ E. coli (Heringa, 2008). This experimental design enabled the assessment of the bacteriophage’s effectiveness in reducing E. coli levels in compost, contributing to understanding the potential of bacteriophages as biological control agents in agricultural practices.
For the irrigation water samples, five distinct locations were selected for analysis, strategically selected in areas of intensive agricultural use within the Culiacán Valley, México. These locations were specifically selected because of their proximity to high-risk crops, such as tomatoes and cucumbers. The sampling was conducted between September 4 and 6, 2023, using a stratified convenience sampling strategy, ensuring that the samples are representative of typical agricultural settings.
Following standard sampling protocols, agricultural irrigation water samples were collected in 1-liter sterile polypropylene bottles from various locations within the Culiacán Valley, Sinaloa, México. The samples were transported to the laboratory under refrigerated conditions, where pH (Thermo Scientific Orion Star A111, USA), electrical conductivity (Hanna Instruments Hi98130, USA), and organic matter content were determined using the loss-on-ignition method. Each water sample underwent sterilization three consecutive times at 121 °C under pressure for 15 minutes.
Subsequently, samples were selected based on common values observed in the analyzed data. Specifically, samples with a pH of approximately 7.14, an electrical conductivity of 0.20 mS, and an average organic matter content of 0.08% were chosen for the biological control assays. These parameters reflect average conditions of irrigation water in agricultural fields of the Culiacán Valley, ensuring that the experiments closely mimic real-world agricultural environments, thereby enhancing the practical relevance of the findings. In two sterile conical tubes, 35 ml of sterile irrigation water was added, followed by the inoculation of a purified E. coli suspension to achieve a final concentration of approximately 1 × 106 CFU/ml. This concentration was selected to simulate elevated contamination levels in irrigation waters within agricultural regions, which pose a high risk of introducing pathogens into the food supply system, thereby providing a realistic context for evaluating the bacteriophage’s efficacy as a biological control agent (Shaw et al., 2016; Yin et al., 2020).
Additionally, one of the tubes received a bacteriophage inoculation at a final concentration of 1 × 104 PFU/ml. The contents of the tubes were gently mixed by inversion and incubated at room temperature for 24 hours. Following incubation, bacterial concentrations in each treatment were quantified using serial tenfold dilutions and the plate count method on selective CHROMagar™ E. coli medium (Álvarez et al., 2019). All assays were performed in triplicate to ensure reproducibility and reliability of the results. This experimental framework allows for a comprehensive assessment of the bacteriophage’s potential as a biological control agent against E. coli in irrigation water, contributing to improved agricultural practices and food safety measures.
The DNA of the bacteriophage was extracted following the SDS-proteinase K protocol described by Sambrook and Russell (2006). The extracted DNA was utilized to construct a genomic library with a 300-bp insert using the TruSeq DNA Nano kit (Illumina, USA), employing random fragmentation according to the manufacturer’s instructions. The quality of the libraries was assessed using a Qubit 2.0 fluorometer (Thermo Fisher Scientific) and the Bioanalyzer 2100 (Agilent Technologies, CA, USA). Subsequently, the libraries were diluted and sequenced on the Illumina MiSeq platform using the MiSeq v3 reagent kit, resulting in approximately five million paired-end reads of 150-bp.
Assembly and bioinformatics analysis were performed in accordance with the recommendations by Philipson et al. (2018) and Turner et al. (2021). The Seqtk Toolkit was employed for random sampling of between 20,000 and 50,000 reads to achieve a coverage depth of approximately 100× post-assembly. The quality of the reads was evaluated with FastQC v0.74, while adapters and low-quality sequences (Phred index < 30) were discarded using the Trimmomatic v0.39 read preprocessing tool. De novo assembly of the reads was conducted using SPAdes v3.15.5, with k-mers set at 33, 55, 77, and 99.
To identify open reading frames (ORFs), the tools Glimmer v3.02, GeneMark v2.5, GeneMark.hmm v2.0, and GeneMarkS v 4.28 were utilized, and their functions were predicted through searches in the National Center for Biotechnology Information (NCBI) database. Additionally, automated genome annotation was carried out using PhageScope v1.3, Prodigal v1.0.1, and eggNOG-mapper v 2.1.12 platforms. Independent Rho factor transcription terminators and promoters were identified using the PhagePromoter v0.1.0, FindTerm, and ARNold servers, respectively. The presence of tRNA genes was evaluated using ARAGORN and tRNAscan-SE.
To characterize the lifestyle of the bacteriophage, the BACPHLIP, PhageAI, and PHACTS tools were employed. Potential genes associated with the synthesis of virulence factors were identified through the VFDB (www.mgc.ac.cn/VFs/search_VFs.html, accessed 6 November 2024) and VirulentPred (http://203.92.44.117/virulent/submit.html, accessed 6 November 2024) platforms. Finally, the presence of antibiotic resistance genes in the bacteriophage genome was analyzed using the CARD (https://card.mcmaster.ca, accessed 8 November 2024) and AMRFinderPlus (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA313047, accessed 8 November 2024) platforms.
This comprehensive approach to genomic sequencing and bioinformatics analysis facilitates a deeper understanding of the bacteriophage’s genetic characteristics, potential virulence factors, and antibiotic resistance profiles, contributing valuable insights for future applications in biocontrol and therapeutic strategies.
To assess bacteriophage stability across varying pH levels and storage temperatures, the data were first subjected to normality testing using the Shapiro-Wilk test and to homogeneity of variances using Levene’s test. A one-way ANOVA was conducted to evaluate pH stability, while a two-way repeated measures ANOVA was applied to analyze storage stability, incorporating both temperature and time as factors. Tukey’s post hoc test was used for pairwise comparisons between groups, with statistical significance set at p < 0.05.
For the biological control assay in compost, normality and homogeneity of variance were confirmed prior to conducting a one-way ANOVA to compare E. coli concentrations across the different treatments (absolute control, bacterial control, and phage treatment). Tukey’s test was applied for pairwise comparisons between treatments.
In the biological control efficacy test in irrigation water, normality and variance homogeneity were verified using the Shapiro-Wilk test and Levene’s test. A two-sample t-test was then employed to compare E. coli concentrations between treated and untreated samples after 24 hours. All statistical analyses were performed using Minitab® 18, and results are expressed as mean ± standard deviation.
Among the 17 compost samples derived from cattle manure and plant residues, the presence of Escherichia coli was identified in five samples using CHROMagar™ E. coli culture medium, with subsequent confirmation achieved through molecular identification via amplification of the phoA gene. Typically, the presence of mesophilic bacteria such as E. coli in compost piles is uncommon, as the temperatures during composting processes often exceed 50 °C, even under suboptimal conditions, limiting the survival of these bacteria (Thomas et al., 2020). However, some studies suggest that pathogenic bacteria can survive in the upper layers of compost piles, where lower temperatures prevail, or when ambient temperatures are lower (Werner et al., 2022; Zhang et al., 2019). In this study, sampling was conducted on the surface of static compost piles, which may have facilitated the presence of E. coli in the analyzed samples.
It is well-documented that E. coli is frequently isolated from bovine feces, posing a significant health risk, as certain strains have the potential to cause both intestinal and extraintestinal infections in humans (Martínez-Vázquez et al., 2021; Massé et al., 2021). Consequently, monitoring the presence of such pathogens in composts that utilize manure as a raw material is recommended. The presence of E. coli in compost used as organic fertilizer represents a potential public health risk, as it could serve as a vehicle for the introduction of this pathogen into the food production chain (Liu et al., 2021). Therefore, it is crucial to implement effective strategies to reduce the burden of pathogenic bacteria in such biofertilizers, thereby strengthening food safety frameworks and protecting consumer health.
In summary, the detection of E. coli in compost samples underscores the necessity for continuous surveillance and control measures to mitigate the risk associated with the application of manure-derived composts in agriculture. This is particularly relevant given the increasing interest in organic farming practices, where the use of compost as a soil amendment can inadvertently introduce pathogens into the food supply. Future research should focus on developing and optimizing composting processes that effectively eliminate pathogenic organisms while maintaining the beneficial properties of compost as a soil amendment.
The antimicrobial sensitivity of five E. coli strains isolated from compost samples was evaluated against a panel of antibiotics commonly used in clinical treatments and livestock production.
Resistance to ampicillin was observed in all strains except Eco-3, which showed intermediate susceptibility to this antibiotic. Despite this exception, Eco-3 was identified as multidrug resistant due to its resistance to multiple antibiotic classes, including aminoglycosides (gentamicin and amikacin), tetracyclines (tetracycline and oxytetracycline), and sulfonamides (trimethoprim/sulfamethoxazole). In addition, Eco-3 exhibited intermediate susceptibility to ciprofloxacin and cefoperazone, highlighting its complex resistance profile.
Strains Eco-1 and Eco-4 displayed resistance to nalidixic acid, trimethoprim/sulfamethoxazole, gentamicin, and amikacin, further emphasizing a concerning trend of resistance to key therapeutic antibiotics. Eco-2 and Eco-5 exhibited resistance to chloramphenicol. Among all tested antibiotics, colistin exhibited antibacterial activity, effectively inhibiting all five strains, which highlights its critical role in combating resistant isolates.
The detection of resistance to tetracyclines, particularly in strain Eco-3, is alarming due to the widespread use of these antibiotics in livestock production. This finding suggests that the resistance patterns observed may be influenced by selective pressures associated with agricultural practices, where antibiotics are commonly employed for disease prevention and growth enhancement. The variability in resistance profiles among the studied strains underscores the potential role of compost as a reservoir for antibiotic-resistant bacteria, with significant implications for public health and agricultural sustainability.
These findings underscore the critical importance of rational antibiotic use in agricultural systems, consistent with the One Health approach, to limit the emergence and dissemination of multidrug resistant bacteria (Galarde-López et al., 2024; González-Aguilar et al., 2022). The resistance patterns documented in this study emphasize the broader impact of antibiotic use in livestock production, raising concerns about the potential transfer of resistant strains into the food chain and adjacent environments. Bacteriophage strategies may aid in reducing reliance on antibiotics in agriculture, thereby reducing the risks posed by antibiotic resistance in agricultural and clinical settings.
Seven bacteriophages were isolated from livestock fecal samples. Among these, one was selected for detailed characterization based on its ability to produce the clearest and most well-defined lysis plaques, as well as its demonstrated broad-spectrum lytic activity against all five E. coli strains identified in this investigation. This phage was designated Alux-21, a name derived from the indigenous Mayan language, meaning “the protector”. The lysis plaques produced by Alux-21 are completely clear, measuring between 2.5 and 3.5 mm in diameter, with a translucent halo surrounding them. This type of lysis plaque is typically associated with efficient viral adsorption to the host bacterium, a short replication cycle, and a large burst size (Mangieri et al., 2020; Nascimento et al., 2022; Valencia-Toxqui & Ramsey, 2024). Furthermore, the presence of a translucent halo around the lysis plaques suggests that phage Alux-21 possesses the capability to degrade bacterial surface polysaccharides through the action of depolymerases (Vukotic et al., 2020). These enzymes enhance the lytic activity of phages, increasing their efficacy as biological control agents by facilitating the degradation of biofilms (Volozhantsev et al., 2020). Thus, phage Alux-21 may exhibit preliminary biological characteristics favorable for its potential use as a biological control agent against E. coli.
The replication dynamic of phage Alux-21 was evaluated using a one-step growth curve experiment. The results revealed an 89% decrease in the number of detectable viral particles within the first 5 minutes post-infection ( Figure 1), indicating that Alux-21 rapidly adsorbs to bacterial cells compared to other coliphages (Malik et al., 2021; Yao et al., 2023; Yazdi et al., 2020). The eclipse phase, corresponding to the period during which the phage replicates its DNA and assembles new viral particles within the host cell, lasted approximately eight minutes after adsorption. The latency phase, defined as the period from adsorption until the release of virions, lasted approximately 13 minutes. The burst size phase began around 20 minutes post-adsorption, resulting in the release of approximately 120 ± 5 virions.
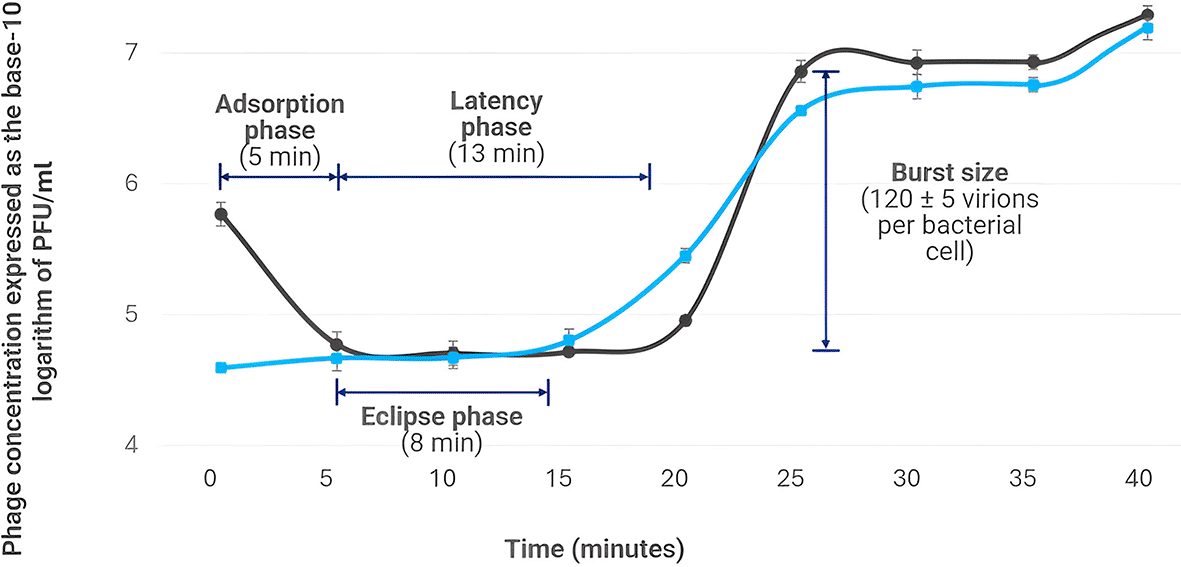
The graph highlights key phases of the replication cycle, including a rapid 89% reduction in detectable viral particles within the first five minutes post-infection, indicating efficient adsorption. The eclipse phase concludes at eight minutes, followed by the burst phase at 20 minutes, releasing an average of 120 ± 5 virions per infected cell. The green line represents intracellular virion formation (chloroform-treated), while the black line indicates extracellular virions (untreated).
These characteristics are crucial for assessing the potential of phage Alux-21 as a biological control agent, as strictly lytic phages typically have substantially shorter latency times and higher adsorption rates, implying a greater capacity to infect and lyse bacterial cells within a reduced timeframe (Eriksen et al., 2023). Notably, the latency period and burst size are key parameters to consider when selecting phages with potential as biological control agents, as those with short latency periods and large burst sizes are believed to inactivate target bacteria more efficiently (Zhang et al., 2023). In this context, the replication characteristics of Alux-21 are favorable when compared to other phages with potential for controlling E. coli, such as vB_EcoM-Sa45lw and vB_EcoStr-FJ63A, which exhibit burst sizes of 80 and 11 virions per cell, and latency periods of 27 and 30 minutes, respectively (Liao et al., 2022; Xiao et al., 2023).
The replication cycle of Alux-21 aligns with that of other coliphages, characterized by short replication cycles and high intracellular replication efficiency, enhancing its bacteriolytic action on the host bacterium (Alexyuk et al., 2022). Additionally, the burst size of Alux-21 suggests a high capacity for disseminating virions to infect additional bacterial cells, a desirable characteristic in phages intended for the biological control of pathogenic bacteria (Mizuno et al., 2020; Nawaz et al., 2024).
One of the most critical criteria for selecting bacteriophages for the control of pathogenic bacteria is their stability during storage, as well as under specific application conditions (Duyvejonck et al., 2021). In this study, we assessed the stability of Alux-21 across different pH values to determine the optimal conditions for maintaining its viability. Furthermore, the results provide insights into the effects of various pH levels, representative of diverse agricultural environments, on bacteriophage concentration.
The findings indicate that phage Alux-21 exhibits the greatest stability at a neutral pH of 7, as a significantly higher viral concentration was observed compared to other pH levels ( Figure 2). This suggests that neutral conditions are most favorable for preserving the integrity and infectivity of Alux-21. This finding is consistent with previous studies, which have reported that various bacteriophages tend to maintain stability in conditions close to neutrality, a factor that is relevant for both storage and application in the biological control of pathogenic bacteria (Shahin et al., 2021; Xiao et al., 2022).
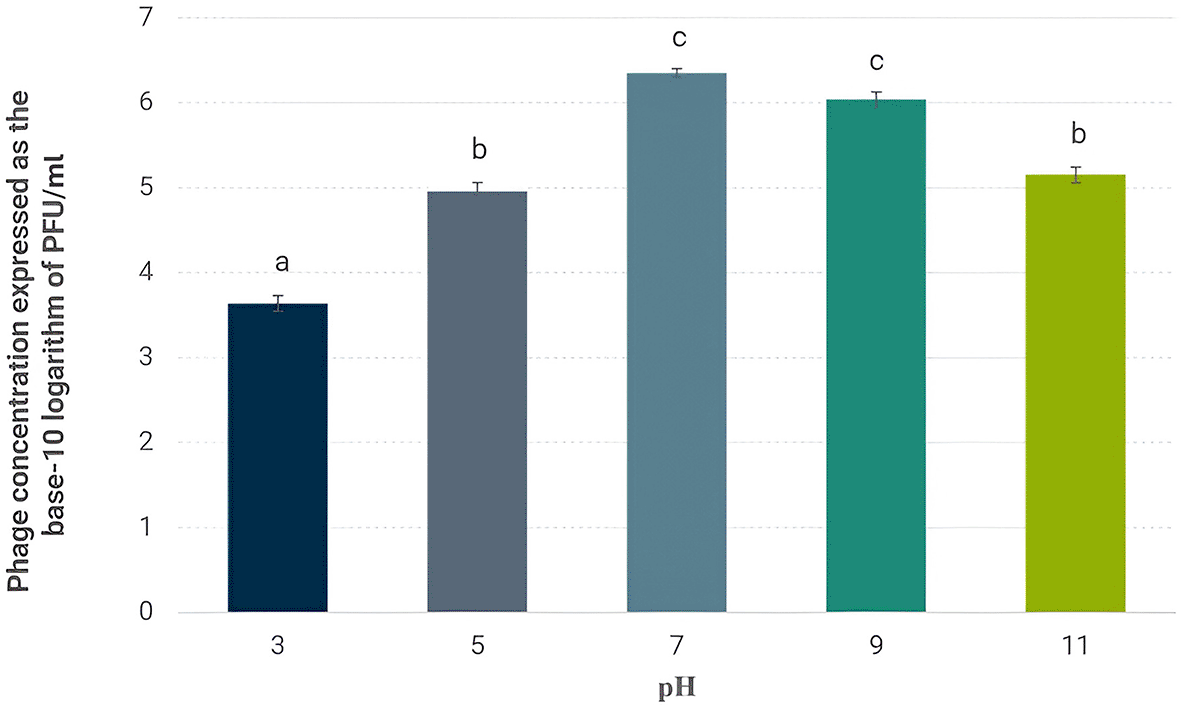
The figure illustrates the concentration of viable Alux-21 phage particles following a one-hour exposure to pH values of 3, 5, 7, 9, and 11. Error bars indicate the standard deviation of the measured concentrations, reflecting variability among experimental replicates.
In contrast, both acidic (pH 3) and alkaline (pH 11) conditions resulted in a notable reduction in bacteriophage concentration. These extreme pH levels are associated with adverse effects on viral structure, likely due to protein denaturation or capsid damage, thereby reducing the phage’s ability to effectively infect host bacteria (Majewska et al., 2023; Sada & Tessema, 2024). Thus, these extreme conditions could compromise the viability and functionality of Alux-21. It is important to note that the optimal pH of composts for maximizing their efficacy typically ranges around 7.5 (Pezzolla et al., 2021; Sayara et al., 2020). Meanwhile, the recommended pH range for agricultural irrigation water is between 6.5 and 7, ensuring suitable conditions for a wide diversity of crops (Guimarães et al., 2021). Therefore, this suggests that phage Alux-21 could maintain its viability in such environments without the need for additional formulations.
These results underscore the importance of maintaining bacteriophage suspension in conditions close to neutral pH to ensure stability during storage and application. Extreme pH values, such as 3 and 11, should be avoided, as they compromise phage viability, limiting its use in environments where prolonged activity is required. In summary, this assay establishes that a pH of 7 is the most suitable for prolonging the viability of phage Alux-21.
Once pH 7 was identified as the optimal condition for extending the viability of Alux-21, its stability during storage at temperatures of 4 °C and 40 °C was evaluated ( Figure 3). After six months, the concentration of phage Alux-21 stored at 4 °C showed no significant reduction, suggesting that cool storage is effective for maintaining its long-term viability. This result aligns with other studies reporting that low temperatures favor the preservation of the structural and functional integrity of bacteriophages, minimizing the degradation of their protein components (Kim et al., 2024; Xiao et al., 2022).
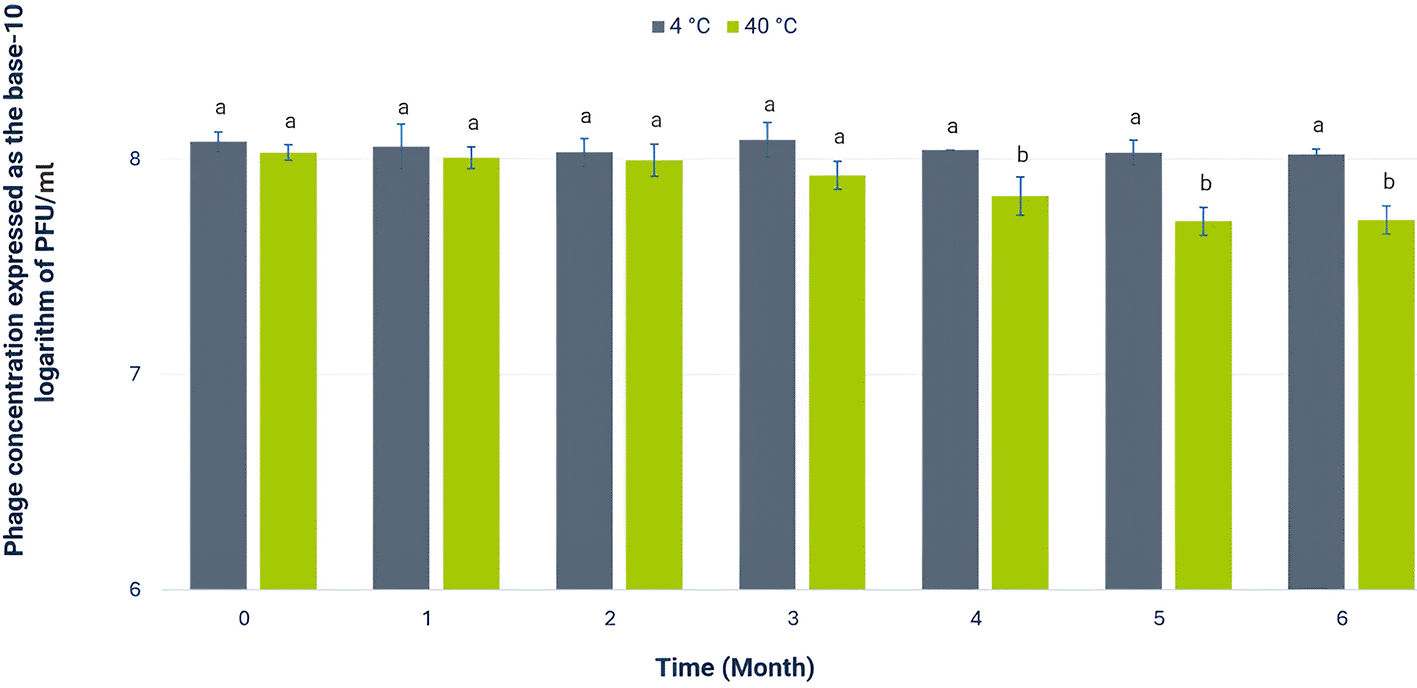
The bar plot illustrates the concentration of bacteriophage Alux-21 stored at two different temperatures, 4 °C (black bars) and 40 °C (green bars), monitored over a six-month period. At 4 °C, the phage concentration remained stable, with no significant reduction observed, indicating the phage’s long-term viability under refrigeration. Conversely, storage at 40 °C resulted in a moderate decline in concentration, corresponding to a decrease of approximately 0.5 log units, suggesting partial degradation under elevated temperature conditions. Error bars indicate the standard deviation of the measured concentrations, highlighting the variability among experimental replicates.
In contrast, the temperature of 40 °C was selected for evaluation because, in the northwest region of México where the phage was isolated, ambient temperatures can reach this level during the summer months. Assessing the stability of phage Alux-21 at 40 °C allows us to determine whether it can remain active without the need for additional stabilizers, potentially reducing costs and enhancing its applicability in agricultural systems that experience these environmental temperatures. Storage at 40 °C resulted in a moderate reduction in the concentration of Alux-21 after six months, with a decrease of only 0.5 log units. This behavior contrasts with findings for certain coliphages, which have shown drastic reductions in concentration at similar temperatures, often leading to complete inactivation within a matter of days (Kim et al., 2024).
The stability of Alux-21 at 40 °C represents a significant advantage, as many biological agents, including bacteriophages, typically require special formulations—such as microencapsulation or refrigerated storage conditions—to maintain their viability. These requirements can significantly increase production, transportation, and storage costs, thereby limiting their accessibility and potential application as biological control agents against pathogenic bacteria (Kering et al., 2020). The stability observed in Alux-21 at 40 °C could mitigate these costs, allowing for broader and more efficient use across diverse agricultural environments.
The bacteriolytic capacity of phage Alux-21 against E. coli under in vitro conditions was assessed, revealing that, compared to the control without phage inoculation, treatment with Alux-21 reduced the concentration of E. coli starting at two hours post-inoculation at a multiplicity of infection (MOI) of 0.001 ( Figure 4). This reduction was even more pronounced at an MOI of 0.01, and the bacteriolytic effect intensified from 90 minutes onward when applying an MOI of 0.1. At all assessed MOIs, bacterial concentrations declined to near-zero levels after three hours.
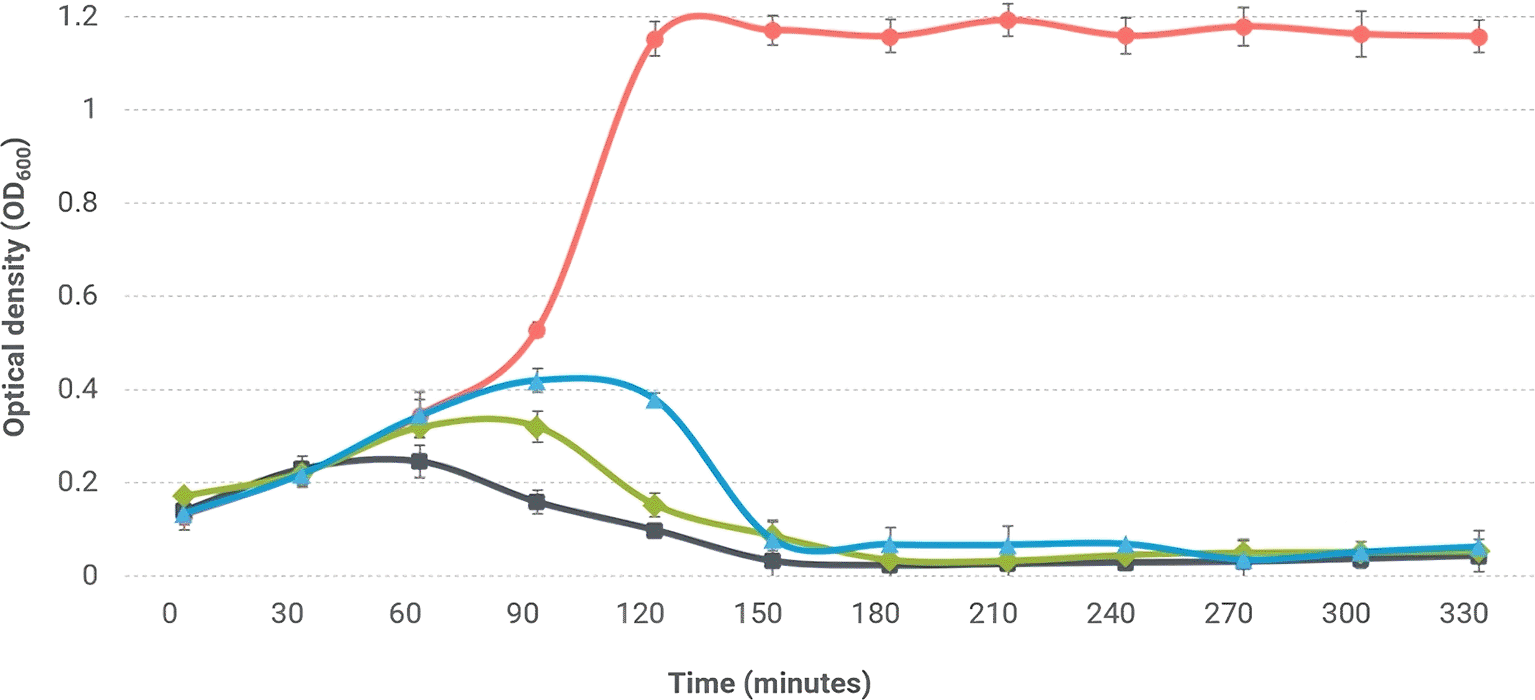
The figure depicts the lytic activity of bacteriophage Alux-21 against E. coli in tryptic soy broth (TSB). The red line represents the growth curve of E. coli in the absence of bacteriophage treatment, serving as the control. In contrast, the black, green, and blue lines illustrate the bacterial growth curves under treatment with Alux-21 at multiplicities of infection (MOI) of 0.1, 0.01, and 0.001, respectively. A significant reduction in bacterial growth is observed across all tested MOI levels, highlighting the remarkable efficacy of Alux-21 in suppressing E. coli proliferation, even at the lowest MOI.
Previous studies have documented that the reduction of bacterial populations induced by bacteriophages is dose-dependent, indicating that higher phage concentrations lead to greater decreases in bacterial counts (Abdelrahman et al., 2022). Notably, phage Alux-21 exhibited the capability to significantly diminish bacterial concentrations even at a low MOI of 0.001, contrasting with other coliphages that typically require an MOI of 1 or 10 to achieve similar reductions (Abdelrahman et al., 2022; Zhao & Meng, 2023).
Additionally, it is worth highlighting that in some coliphages, the use of low MOIs, such as 0.01, results in an initial decrease in bacterial concentration; however, resistant bacterial cells may begin to emerge after approximately six hours (Kuek et al., 2023). This phenomenon was not observed with Alux-21 during the same experimental timeframe, marking a positive characteristic of this phage as a biological control agent.
These findings underscore the potential of Alux-21 to rapidly reduce E. coli populations, which is promising for applications requiring swift and effective action against this pathogen. Particularly, the effect observed at an MOI of 0.001 within two hours post-inoculation is highly relevant in scenarios where bacteriophage concentrations may be limited due to environmental conditions.
The capacity of phage Alux-21 to reduce the concentration of E. coli in compost was evaluated. In the treatment without the application of bacteriophage, the bacterial concentration reached 7.8 × 106 CFU/g after 48 hours of incubation. This finding indicates that the characteristics of the compost used in this experiment create a favorable environment for the growth of E. coli under the evaluated experimental conditions ( Figure 5A). Previous studies have reported that compost can promote the survival and growth of E. coli, with increases exceeding four logarithmic units compared to the initial concentration, presenting a potential risk for the proliferation and dissemination of the pathogen in agricultural environments (Kim et al., 2009; Miller et al., 2013; Palmer et al., 2010).
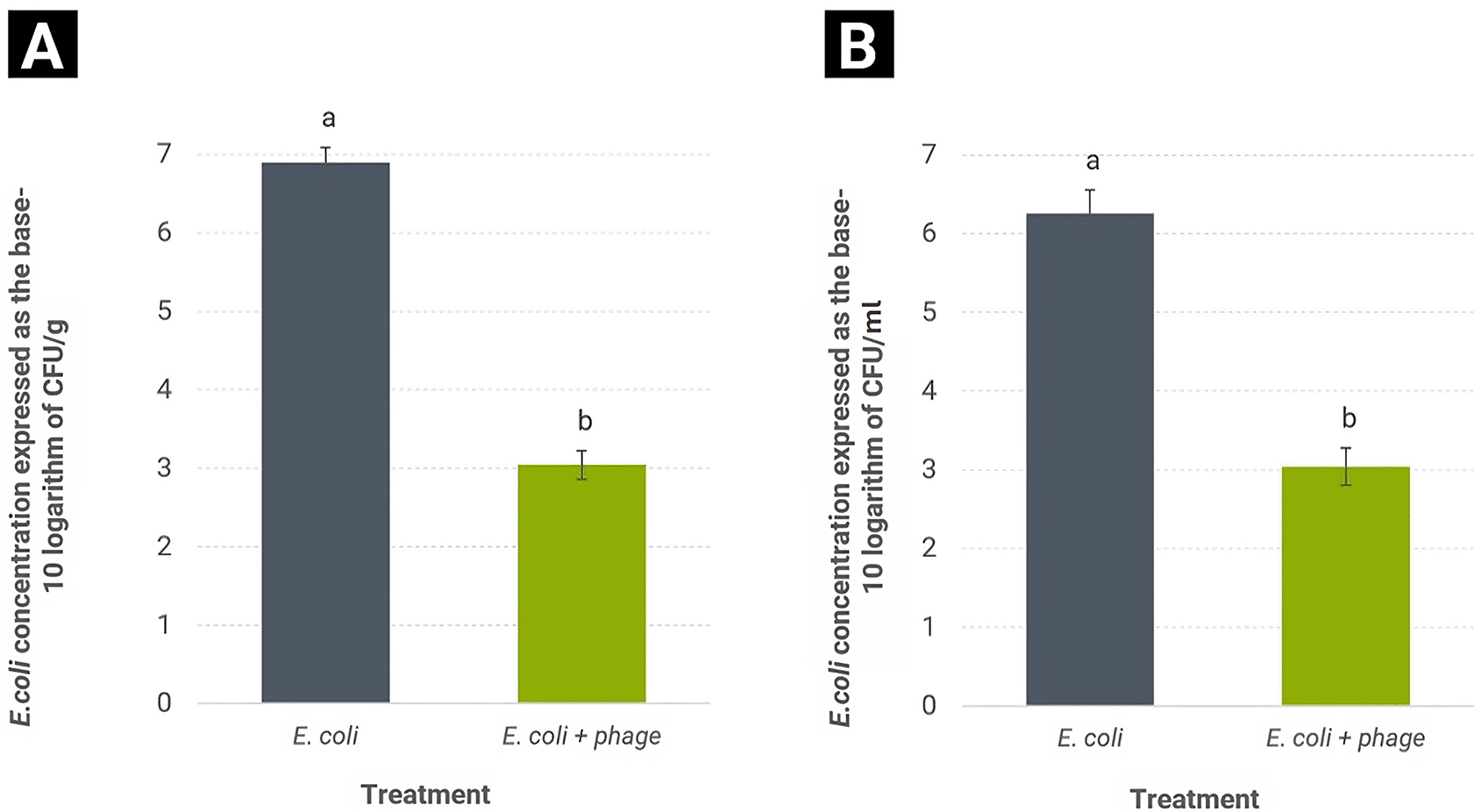
(A) Bar graph illustrating the concentration of Escherichia coli in compost during biological control assays. The black bar represents the bacterial concentration in untreated compost, showing an average value of 7.8 × 106 CFU/g. In contrast, the blue bar represents the E. coli concentration in compost treated with the Alux-21 bacteriophage, with an average value of 1.1 × 103 CFU/g. (B) Bar chart depicting the results of the biological control of E. coli in agricultural irrigation water. The black bar represents the concentration of E. coli in the untreated sample, which reached 1.8 × 106 CFU/ml. In contrast, the blue bar indicates samples treated with the Alux-21 bacteriophage, which exhibited a significant reduction in bacterial concentration, with a final count of 1.5 × 103 CFU/ml. Distinct letters above the bars denote statistically significant differences (p < 0.05). The variability in the data is represented by the standard deviation.
In contrast, the treatment in which E. coli suspension was inoculated followed by the application of phage Alux-21 demonstrated a significant reduction in bacterial concentration, registering a count of 1.1 × 103 CFU/g of compost. This represents a reduction of over 3.8 logarithmic units in bacterial concentration compared to the treatment without phage. These results align with findings from other studies that have documented the capacity of bacteriophages to control pathogenic bacteria in compost, achieving significant declines in bacterial concentrations in such organic substrates, thereby highlighting the potential of bacteriophages as biological control agents (Heringa et al., 2010; Ullah et al., 2017).
These results suggest that phage Alux-21 has potential for the biological control of E. coli in compost, achieving a significant reduction in bacterial load within a short incubation period. This is particularly relevant in organic agriculture, where the use of compost is common, and several outbreaks of E. coli have been associated with this type of biofertilizer (Chen et al., 2018; Islam et al., 2004; King et al., 2012; Sharma & Reynnells, 2016). The incorporation of phage Alux-21 in the final stage of composting could mitigate the risk of introducing E. coli into the organic food production chain, contributing to strengthening food safety schemes in sustainable agricultural production systems, as suggested by some authors for other phages (Otawa et al., 2012; Ullah et al., 2017). This aspect is particularly significant given the growing demand for organic products and the increasingly stringent requirements for compliance with food safety standards.
However, it is important to emphasize that while the results regarding the capacity of Alux-21 to control E. coli in compost are promising, its viability in practical applications must be assessed under various environmental conditions. Factors such as pH, temperature, compost composition, and the presence of other microorganisms may affect the bacteriolytic activity of the phage (Fister et al., 2016; Heringa et al., 2010; Wang et al., 2020). Future studies should focus on evaluating the effectiveness of Alux-21 under these variables. Additionally, the emergence of phage-resistant strains is a critical aspect that could limit the long-term efficacy of phage Alux-21. To mitigate this risk, it is advisable to continue the isolation and characterization of additional bacteriophages, with the aim of developing a phage cocktail to reduce phage resistance.
The evaluation of phage Alux-21 for controlling E. coli in irrigation water samples yielded promising results. Following the incubation period, the concentration of E. coli in the treatment without phage reached 1.8 × 106 CFU/ml, suggesting that the conditions of the irrigation water used in the trial facilitate moderate growth of E. coli ( Figure 5B). In contrast, samples treated with Alux-21 exhibited a significant reduction in bacterial concentration, with a final count of 1.5 × 103 CFU/ml. This represents a reduction of over three logarithmic units in bacterial load compared to the control.
These findings indicate that Alux-21 has potential as a biological control agent for reducing E. coli concentrations in agricultural irrigation water. This could represent a future strategy to improve the microbiological quality of irrigation water, as has been suggested for other bacteriophages (Álvarez et al., 2019; Soliman et al., 2023). This is particularly relevant in the organic food production sector, where the application of chemical treatments is restricted, and biological control offers an ecologically sustainable alternative. Moreover, in many agricultural regions experiencing prolonged droughts, sustainable practices are essential for conservation, and the use of bacteriophages could be efficiently integrated into these systems.
However, despite these encouraging results, further research is necessary to evaluate the stability of Alux-21 under the varying physicochemical conditions that may be encountered in irrigation water. Additionally, it is crucial to conduct evaluations under field conditions to ensure the viability and effectiveness of this approach in real agricultural production scenarios, thereby confirming its applicability.
The genomic analysis revealed that phage Alux-21 possesses a double-stranded DNA genome with a linear topology, spanning 42,729 base pairs (bp) and exhibiting a GC content of 54.31%. The genome includes 60 open reading frames (ORFs), organized into functional modules responsible for DNA processing, host cell lysis, genetic material packaging, and morphogenesis ( Figure 6).
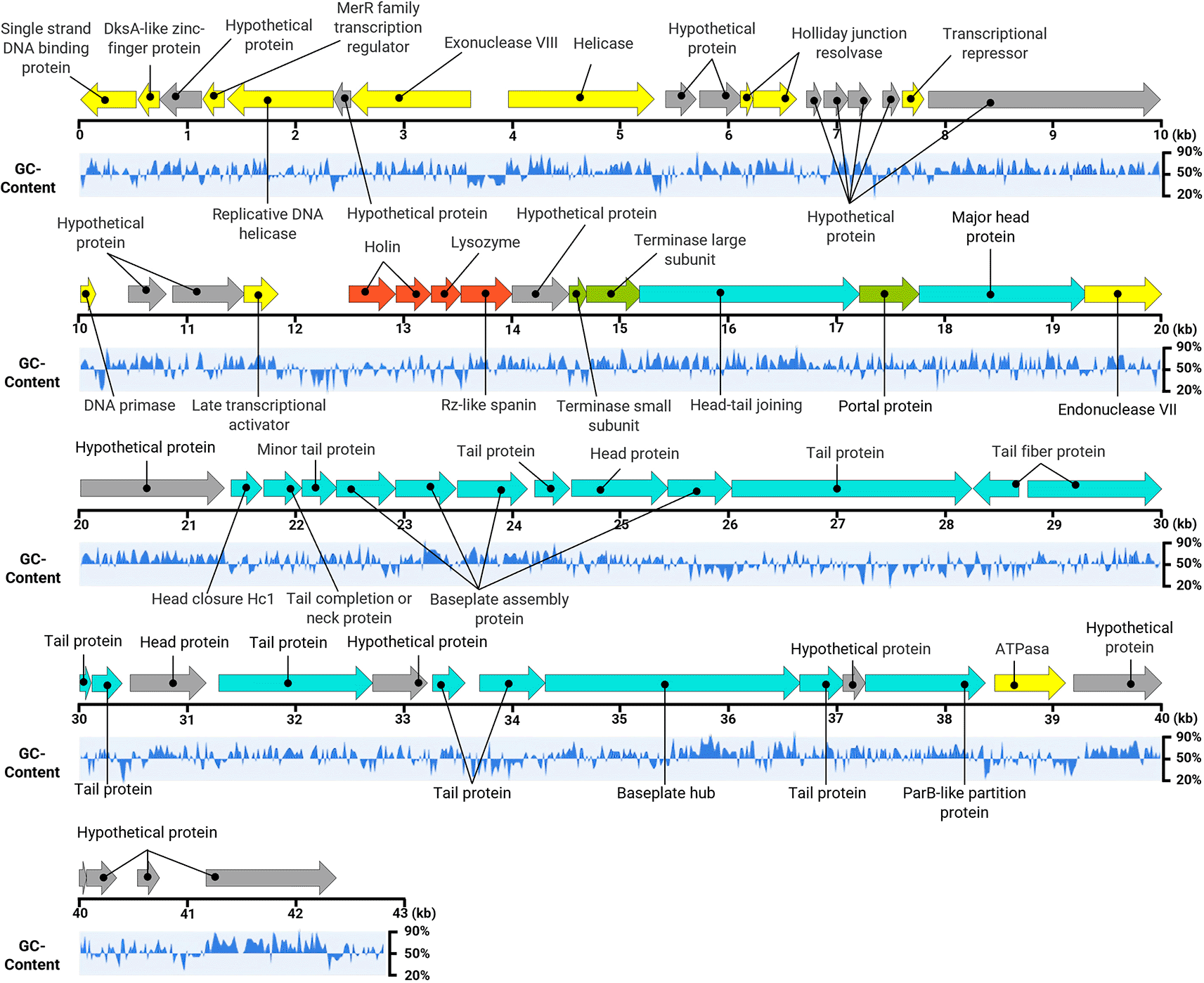
The figure illustrates the entire genome map of bacteriophage Alux-21, with individual genes depicted as arrows to indicate their respective locations and transcriptional orientations. Each gene is color-coded according to its predicted function, enabling a clear distinction between structural (blue), replication-associated (yellow), DNA packaging (green), lysis (red) and hypothetical protein (gray) and auxiliary genes. The analysis revealed no evidence of genes encoding virulence-associated factors, lysogeny-related mechanisms, or proteins with allergenic potential. This genomic characterization underscores the suitability of Alux-21 for applications in biocontrol, as the absence of such undesirable elements minimizes potential risks associated with its application.
The DNA replication module comprises ORFs encoding critical proteins for genome replication and transcription, including a single-stranded DNA-binding protein (ORF 1), a DksA-like zinc-finger protein (ORF 2), a MerR family transcription regulator (ORF 4), DNA helicases (ORFs 5 and 8), an exonuclease (ORF 7), holliday junction resolvases (ORF 11 and ORF 12), DNA primase (ORF 19), and a late transcriptional activator (ORF 22). These proteins play critical roles in the replication and transcription of the Alux-21 genome, ensuring efficient viral propagation within the host.
In terms of host cell lysis, the genome of Alux-21 encodes the proteins holins (ORFs 23 and 24), lysozyme (ORF 25), and an Rz-like spanin (ORF 26). Together, these proteins facilitate host cell membrane pore formation, cell wall degradation, and the subsequent release of virions. This lytic gene cluster is critical for the phage’s lytic life cycle, allowing the destruction of the bacterial host at the end of viral replication.
For the packaging of genetic material, ORFs were identified that encode the terminase small subunit (ORF 28), terminase large subunit (ORF 29), and portal protein (ORF 31), all of which are essential for encapsulating viral DNA within the capsid during assembly. Additionally, the morphogenesis-related ORFs encode structural proteins essential for capsid formation (ORFs 32, 35, 42, and 48) and tail formation (ORFs 36, 37, 41, 44, 45, 46, 47, 49, 51, 52, and 54), as well as components of the baseplate assembly (ORFs 38, 39, 40, 43, and 53). These structural components are indispensable for virion assembly and phage infectivity. The genome sequence of Alux-21 has been comprehensively annotated and archived in the NCBI GenBank database under accession number PQ589898. Additionally, the sequencing data are available in the Sequence Read Archive (SRA) under project ID PRJNA1191041.
Notably, the genomic analysis did not detect any genetic elements associated with virulence factors, antibiotic resistance, host genome integration, or allergens. The absence of these elements is strongly recommended for phages intended for biological control and/or therapeutic applications (Garvey, 2022; Li et al., 2020). These findings indicate that Alux-21 is a strictly lytic phage, devoid of proteins that could pose potential biosecurity risks, positioning it as a safe candidate for biocontrol applications from a genomic standpoint.
This study demonstrated that the isolated bacteriophage, designated as Alux-21, possesses substantial potential as a biological control agent against E. coli. The findings indicate that this phage exhibits significant lytic activity not only under laboratory culture conditions but also in compost and agricultural irrigation water, effectively reducing E. coli concentrations. This pathogen is of major concern for public health due to its high morbidity and mortality rates and its significant antimicrobial resistance.
Moreover, phage Alux-21 maintains stability across a range of pH levels and remains viable for extended periods at both 4 °C and 40 °C, underscoring its suitability for agricultural applications without the need for protective formulations. Additionally, the genome of Alux-21 lacks genes associated with virulence factors, antimicrobial resistance, lysogeny, or proteins with potential allergenic properties, characteristics that make it ideal for phages intended for biological control applications.
These findings position Alux-21 as a viable, sustainable solution for reducing E. coli contamination in agricultural systems, particularly in compost and irrigation water, where chemical treatments are impractical or restricted. Field trials and further validation will be crucial to advancing its adoption in real-world scenarios. This progress could have significant implications for enhancing food safety and public health. However, while the results obtained under experimental conditions are promising, it is essential to progress towards field studies that evaluate its efficacy in real-world agricultural settings and assess its interactions with other microorganisms present in these ecosystems. Future research will be imperative to validate its application as a robust and sustainable biological control solution.
Luis Amarillas (LA), Mitzi Estrada-Acosta (ME-A), and Luis Lightbourn-Rojas (LL-R) were responsible for the conceptualization of the study, the methodological development, and data analysis. LL-R secured the necessary funding for this research, while LA, ME-A, Ruben León-Chan (RL-C), Jorge Padilla (JP) and Enrique López-Avendaño (EL-A) performed the experiments and data collection. Research planning and execution were overseen and coordinated by ME-A, Antonio González-Balcázar (AG-B) and LL-R, with LA, RL-C, and AG-B ensuring the reproducibility and accuracy of the results. LA was responsible for visualization preparation, and LA, R-LC, JP, EL-A, AG-B, ME-A, and LL-R collaborated on drafting and revising the manuscript. All authors reviewed and approved the final manuscript and assume full responsibility for the veracity and integrity of the work.
GenBank of NCBI: Sequence Read Archive (SRA) of raw reads from the sequencing of the Alux-21 phage genome. Access number PRJNA1191041; https://www.ncbi.nlm.nih.gov/sra/PRJNA1191041 (Amarillas et al., 2023).
Zenodo: Supplemental Files, https://zenodo.org/records/14233172, 10.5281/zenodo.14233172 (Amarillas et al., 2023).
Under the license CC BY 4.0 Attribution 4.0 International
This project contains the following extended data:
Supplemental Table 1: This table provides a comprehensive summary of the open reading frames (ORFs) identified in the genome of the bacteriophage. It includes detailed information on their genomic positions, predicted coding regions, and the putative functions of the encoded proteins, as inferred from annotation analyses.
Supplemental Table 2: This table presents the experimental results of the one-step growth curve analysis of the bacteriophage, as well as its stability under varying pH conditions and during storage. Additionally, it includes data on the phage’s efficacy as a biocontrol agent against E. coli in compost and irrigation water samples.
We extend our sincere gratitude to Fedra Padilla-Lafarga, Jesús Antonio Núñez, and Zendy Mariela Martínez for their invaluable technical support and contributions throughout the experimental phases of this study. Their expertise and unwavering commitment were instrumental to the successful advancement and completion of this research.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Phage therapy and application. Antimicrobial resistance.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Environmental and food microbiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 16 Dec 24 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)