Keywords
acute pancreatitis, gut microbiota, probiotic, prebiotic, symbiotic, clinical endpoint
acute pancreatitis, gut microbiota, probiotic, prebiotic, symbiotic, clinical endpoint
Acute pancreatitis (AP) in many nations is a common reason for hospitalization for gastrointestinal conditions. Due to the rising prevalence of the metabolic syndrome and hypertriglyceridemia, acute pancreatitis is becoming more common around the globe. According to several reports, hypertriglyceridemia has been linked to an increase in acute pancreatitis (de Pretis et al., 2018; Kota et al., 2012). This condition causes pancreatic inflammation that is acute in nature. Acute pancreatitis can range in severity from mild cases that only need conservative care to severe and complicated conditions that have a high mortality and morbidity rate. Acute pancreatitis also has a variable mortality rate, ranging from 3% in individuals with mild edematous pancreatitis to 20% in those with pancreatic necrosis (Werge et al., 2016; Gapp et al., 2023).
Previous studies have assessed the microbiome’s contribution to AP. These investigations describe a complicated method that involves pancreatic acinar cells. According to studies on the pathophysiology of AP, damage to or disruption of pancreatic acini is the main cause of elastase, chymotrypsin, and trypsin being activated in pancreatic tissue (Gapp et al., 2023; Gui et al., 2020). The activation of lipase, trypsin, and elastase eats away at tissue and cell membranes, causing edema, blood loss, vascular injury, and necrosis. According to epidemiological research, the local inflammatory response worsens pancreatitis by causing an increase in permeability, which reduces microcirculation, impairs local bleeding, and, in situations of severe AP, results in pancreatic necrosis. A late complication of AP or known as systemic inflammatory response syndrome (SIRS), is caused by proinflammatory mediators that are continuously activated. Pancreatic necrosis, infection, multiorgan failure, and sepsis can then occur (Bhatia and Kumar, 2014; Patel et al., 2021).
Disorders of the gut microbiota (dysbiosis), which are related to SIRS and other diseases, are common. Gut bacteria have the ability to move to other tissues and organs in addition to the blood when the gut mucosal barrier is damaged. The severity of AP will be impacted by this. A therapeutic approach that maintains the integrity of the intestinal barrier and restores the intestinal microbiota to normal is required in order to regulate AP logically (Swann et al., 2011; Li et al., 2020).
Animal studies revealed that probiotics may help maintain gut integrity, reducing bacterial translocation and preventing infection in AP (Muftuoglu et al., 2006; van Minnen et al., 2007; Karen et al., 2010). Additionally, some clinical experiments have looked into the possibilities of giving critically ill patients probiotic supplements (Sanaie et al., 2014; Rongrungruang, 2015; Zeng et al., 2016). Based on the promising findings of multiple studies, numerous clinical trials have been conducted to examine the efficacy of using prebiotics, probiotics, and synbiotics in patients with severe AP (SAP) (Karakan et al., 2007; Oláh et al., 2007; Besselink et al., 2008; Cui et al., 2013). The research has reported valuable outcomes. However, conflicting findings are reported by the PROPATRIA study, in which probiotic supplementation has a negative impact on SAP (Besselink et al., 2008). Several meta-analyses have been carried out to investigate the benefits of probiotics in acute pancreatitis in further detail (Sun et al., 2009; Gou et al., 2014), and none have shown any effects on the clinical outcome of SAP patients, either favorable or negative. Tian et al.’s meta-analysis discovered that giving acute pancreatitis patients synbiotics, probiotics, and prebiotics had positive effects (Tian et al., 2018). However, numerous additional studies with diverse findings have been published since the meta-analysis was carried out. In order to comprehensively examine the effectiveness of prebiotic, probiotic, and synbiotic therapy on clinical outcome (length of hospitalization [LoH] and complication of infection) in patients with AP, we conducted the updated systematic review and meta-analysis.
This systematic review and meta-analysis conform to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards. We registered this review in PROSPERO (registration number: CRD42023427441).
To discover appropriate publications, the eligibility criteria were converted into keywords using the Boolean operator, then registered in electronic databases and registers of PubMed, Cochrane, clinicaltrial.gov, and Science Direct. We also assessed the relevant papers’ references. On April 14th, 2023, the last search was conducted. “Prebiotic” or “probiotic” or “synbiotic” or “acute pancreatitis” were among the search terms used (Table 1).
To reduce the possibility of excluding potentially pertinent research, two authors (DAS and IKM) carried out the study selection process. The first and second authors’ judgments were taken into account where there was a conflict. Eliminating duplicate records was the first step in the study selection process. To weed out studies that weren’t relevant, we screened the titles and abstracts. Studies that passed the initial examination were then subjected to additional analysis to determine if they complied with the review’s inclusion and exclusion criteria. Prior to final inclusion, all included studies underwent a comprehensive critical assessment using the Cochrane Collaboration’s risk-of-bias methodology’s critical appraisal tool.
All research papers conducted before April 14th, 2023 were included in the current systematic review and meta-analysis. Patients with acute pancreatitis made up the study population. The prebiotic, probiotic, and synbiotic interventions were evaluated. The intervention was compared with another intervention (a placebo or standard treatment). Case reports, qualitative studies, economic studies, reviews, and cadaver and anatomic publications were all excluded. All articles that lacked the information needed to do a meta-analysis and those that were not available in English or Indonesian were also excluded.
Studies with acute pancreatitis were considered in this review. According to the study’s intervention, we divided them into prebiotic, probiotic, and synbiotic groups. Studies that used either prebiotic, probiotic, or synbiotic in any dose or duration were included. But for each trial, we recorded the doses and the length of the intervention.
The efficacy of prebiotics, probiotics, and synbiotics were evaluated with the outcome of clinical end-points. Length of hospital stay (LoH) and infection risk were used to measure the clinical endpoint. To gather the necessary information from each article, all authors employed an electronic data collecting form. After that, the data was merged and controlled using the program Review Manager 5.4.
The studies’ name, nation, type of intervention, drug dosage, length of drug use, sample size, and age were the data items. The LoH and infection-related event between the intervention and control groups were compared, and a meta-analysis was conducted.
All articles that met the requirements for eligibility for this review were checked for quality using a standardized critical assessment tool. Two authors (DAS and IKM) separately carried out this method, which attempted to reduce the possibility of bias in study selection. Cochrane risk-of-bias assessment tool was the critical appraisal tool used for this review (Higgins et al., 2011). Six domains are included in the assessment, including inadequate data, randomization, selective reporting, blindness, allocation, and other bias. After determining the match level between the actual data and the evaluation criteria, studies were given a “low risk,” “unclear risk,” or “high risk” rating.
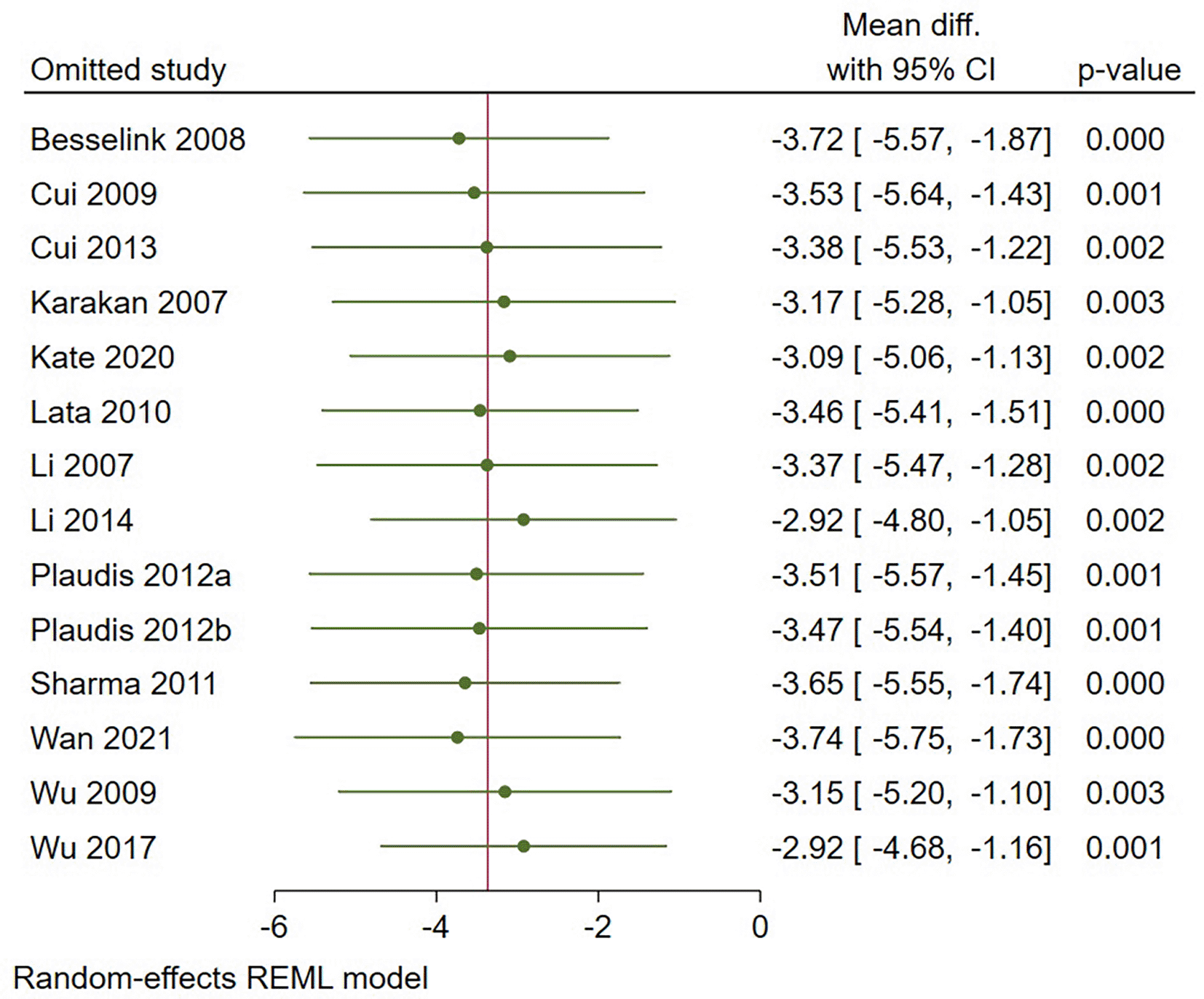
The pooled mean difference (MD) in LoH between acute pancreatitis patients who received prebiotic, probiotic, or synbiotic treatment and those who did not was studied. If the data presented as median with interquartile range (IQR) or range, we utilized a calculator by Luo et al. (2018) and Wan et al. (2014) to determine the mean. The mean difference and 95% confidence interval (CI) were the reported effect size of this study. For the analysis, a random effect model was employed. The I2 statistic, which measures how much of the variation in observed effects across trials is due to variation in true effects, was used to assess heterogeneity. Values > 60% indicate high heterogeneity. A p-value of 0.05 or lower was regarded as significant. Review Manager 5.4 was used to conduct the meta-analysis, while Stata 17 was used to perform the leave-one-out sensitivity analysis.
Using the first search approach, we discovered 135 studies from supplemental records (clinicaltrial.gov and linked papers) in addition to 306 studies via database searching. After eliminating the duplicates, we were left with 380 articles. By reviewing the titles and abstracts, we were able to rule out 338 papers, leaving us with 42 pertinent studies. Commentary articles (17 articles), study protocols (1 article) and studies without adequate information (8 articles) were eliminated from this meta-analysis. After performing screening, we were able to collect 16 studies for the current systematic review and meta-analysis. Figure 1 shows a flow diagram for the PRISMA study.
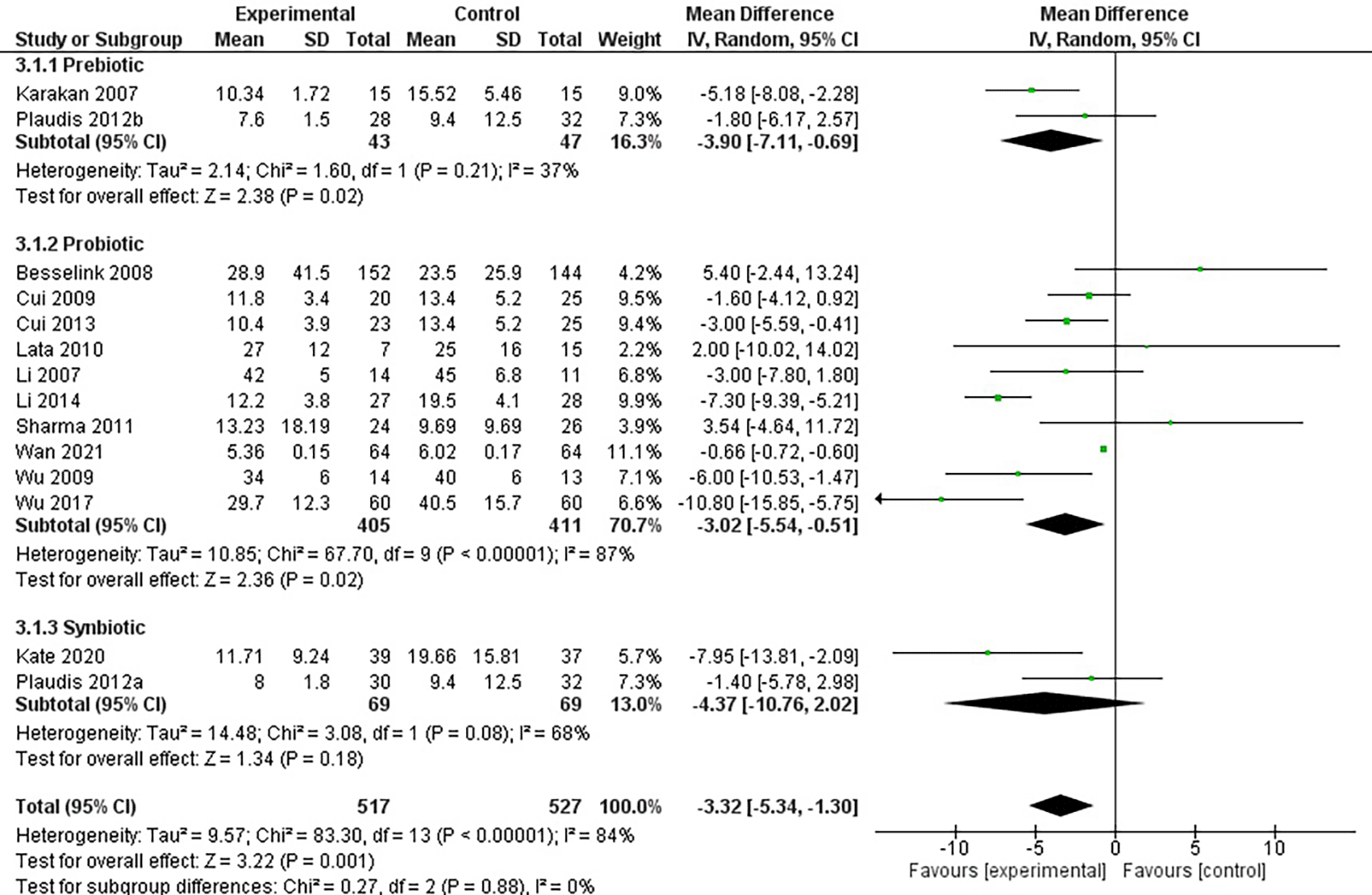
We included 16 studies in our systematic review and meta-analysis. A total of 13 studies were included for the analysis of LoH, while seven studies were included for analysis of complications of infection. In a study by Plaudis et al., they stratified the patients into three groups (synbio, fibre, and control). Therefore, in this systematic review and meta-analysis, we divided study by Plaudis et al. (2012) into two, i.e. Plaudis 2012a (for the synbiotic group) and Plaudis 2012b (for the prebiotic group). Table 2 displays the studies’ features and an overview of the results.
| Study | Country | Type of intervention | Doses of intervention and comparator | Duration | Sample size, intervention vs. control | Age, intervention vs. control |
|---|---|---|---|---|---|---|
| Besselink et al., 2008 | Netherlands | Probiotic | 1010 B. lactis, B. bifidum, L. salivarius, L. casei, L. acidophilus, L. lactis, in a totally daily dose of (Ecologic 641; Winclove Bio Industries, Amsterdam, the Netherlands) twice a day vs placebo | 28 days | 152 vs 144 | 60.4 ± 16.5 vs. 59.0 ± 15.5 |
| Plaudis et al., 2012 | Latvia | Prebiotic and sybniotic | Synbiotic 2000 Forte (1010 L. mesenteroides, 1010 P. pentosaceus, 1010 L. plantarum, 1010 L. paracasei with bioactive fibers) and same fibers and in the same amount as in synbiotic 2000 Forte, but no lactic acid bacteria twice daily vs standard low volume enteral feeding formula | Not clearly described | 30 for synbiotic, 28 for prebiotic vs 32 | N/A |
| Karakan et al., 2007 | Turkey | Prebiotic | 0.8 g/100 mL insoluble fibers and 0.7 g/100 mL soluble fibers vs standard enteral nutrition | 6-13 days | 15 vs 15 | 47.3 ± 16.8 vs. 44.9 ± 11.2 |
| Wan et al., 2021 | China | Probiotic | Bacillus subtilis and Enterococcus faecium (Meichangan, national medicine permission number S20030087) vs placebo | From study inclusion to discharge | 64 vs 64 | 50.25 ± 16.79 vs. 54.72 ± 14.86 |
| Kate et al., 2020 | India | Synbiotic | 1 gram of synbiotic containing both pre and probiotics (the content not clearly described) in 100 ml of saline orally/nasogastric tube in twice a day vs placebo | 14 days | 39 vs 37 | N/A |
| Sharma et al., 2011 | India | Probiotic | (about 2.5 billion bacteria per sachet containing Bifidobacterium infantalis, Bifidobacterium longus, Lactobacillus acidophilus, Bifidobacterium bifidum, with fructo-oligosaccharide as much 25 mg) per day vs placebo | 7 days | 24 vs 26 | 41 ± 20.72 vs. 40.19 ± 17.43 |
| Cui et al., 2013 | China | Probiotic | 4 × 107 Enterococcus faecalis, 4×107 L. bulgaricus, 4×107 B. longum, twice a day vs enteral nutrition only | 14 days | 23 vs 25 | N/A |
| Li 2007 | China | Probiotic | 106 L. bulgaricus, 107 B. longum, and 106 S. thermophilus (Golden Bifid), thrice a day vs water | 7 days | 14 vs 11 | 45 ± 13 |
| Wu and Zhang 2009 | China | Probiotic | 6 × 104 L. lactis, L. acidophilus and S. lactis | 7 days | 14 vs 13 | N/A |
| Wu et al., 2017 | China | Probiotic | Bifidobacterium quadruple living bacterium including 0.5×105 B. cereus, 0.5×106 E. faecalis, 0.5×106 B. acidophilus, and 0.5×106 B. bifidus thrice a day vs standard enteral nutrition | N/A | 60 vs 60 | 42.7 ± 11.5 vs. 42.6 ± 13.6 |
| Cui et al., 2009 | China | Probiotic | 107 E. faecalisin, B. acidophilus, B. bifidus, (Bifico; Xinyi pharmaceutical factory, Shanghai Pharmaceutical Co., Ltd., China) vs standard enteral nutrition | N/A | 20 vs 25 | 45.3 (27–69) |
| Lata et al., 2010 | Czech Republic | Probiotic | B. infantis, B. bifidum, L. salivarius, L. casei, L. lactis, L. acidophilus, twice a day | N/A | 7 vs 15 | 52 ± 12 vs. 55 ± 13 |
| Li et al., 2014 | China | Probiotic | Bifidobacterium triple viable including 107 B, acidophilus, B. bifidus, and E. faecalis, twice a day vs standard enteral nutrition | N/A | 27 vs 28 | 49.3 ± 11.5 vs. 47.5 ± 9.6 |
| Qin et al., 2008 | China | Probiotic | 108 L. plantarum, enteral nutrition, and parenteral nutrition vs parenteral nutrition | 7 days | 36 vs 38 | 54.3 ± 13.1 vs. 58.4 ± 19.1 |
| Oláh et al., 2007 | Hungary | Probiotic | 1010 L. paracasei, 1010 L. plantarum, 1010 P. pentosaceus, 1010 L. mesenteroides, with bioactive fibers (Synbiotic 2000 Forte; Medifarm, Kågeröd, Sweden), once a day vs enteral nutrition | 7 days | 33 vs 29 | 47.5 (19-78) vs. 46.0 (20-81) |
| Oláh et al., 2002 | Hungary | Probiotic | 109 L. plantarum 299, enteral nutrition, and 10 g oat fiber vs enteral nutrition | 7 days | 22 vs 23 | 44.1 ± 11.1 vs. 46.5 ± 13.6 |
The bias risk was investigated using the Cochrane Collaboration’s risk-of-bias methodology. One study by Wu et al. (2017) was rated as having an unknown risk of bias in terms of allocation concealment, blinding, incomplete results, selective reporting, and other biases. Figure 2 displays the full results of the risk of bias for each article included in this study.
The clinical endpoint was evaluated based on length of hospital stay (LoH) and the risk for complication of infection in acute pancreatitis patients. The meta-analysis for the clinical endpoint of LoH involved a total of 1,044 acute pancreatitis patients, while complication of infection event involved 779 patients.
The use of either pre-, pro-, or synbiotics reduced the LoH significantly by 3.32 days (-5.34 until -1.3 days, p = 0.001) (Figure 3). In addition, a sensitivity analysis was performed with leave-one-out method due to severe heterogeneity (I2 = 84%). Leave-one-out analysis showed no significant change in results after excluding one study at a time (Figure 4). Subgroup analysis by type of interventions used revealed that that probiotics reduce LoH by 3.02 days (-5.54 until -0.51 days, p = 0.02). Similarly, prebiotics reduce the LoH by 3.9 days (-7.11 until -0.69, p =0.02). However, synbiotics did not show a reduction for LoH (-4.37 days, p = 0.18) (Figure 5).
The second clinical outcome assessed was the risk for complication of infection. The use of pre-, pro-, and synbiotics reduced the risk for complication of infection in acute pancreatitis patients with odds ratio (OR) of 0.32 (0.14 until 0.73, p = 0.006) (Figure 6).
The number of eligible studies for the outcome of LoH met the criteria for analysis of publication bias. The funnel plot analysis showed asymmetric funnel plot, indicating a risk for publication bias (Figure 7).
The current systematic review and meta-analysis revealed two important finding in terms of clinical endpoint of acute pancreatitis patients. First, using pre-, pro-, or synbiotics reduces the LoH by 3.32 days. Second, using pre-, pro-, and synbiotics lowers the incidence of infection-related complications in people with acute pancreatitis.
Acute pancreatitis is a complex inflammatory disorder that results from the activation of pancreatic enzymes within the pancreas itself, leading to autodigestion and subsequent tissue damage. A number of things, such as gallstones, drinking alcohol, trauma, or infection, might start this process. Once activated, the pancreatic enzymes can lead to systemic consequences such multiple organ failure, sepsis, and shock, as well as severe inflammation and destruction of the pancreatic tissue. Proinflammatory cytokines, immune cell activation, mitochondrial dysfunction, and oxidative stress all play a complex role in the pathogenesis of acute pancreatitis, but the processes that cause AP to begin are still poorly understood. Additionally, recent studies have linked the gut microbiota to the onset and progression of acute pancreatitis, raising the possibility of new therapeutic approaches (Bhatia and Kumar, 2014; Patel et al., 2021).
According to recent studies, the gut flora has a significant role in the genesis and progression of acute pancreatitis (Patel et al., 2021). Studies have shown that alterations in the composition and functionality of the gut microbiota are associated with acute pancreatitis, including dysbiosis and increased gut barrier permeability. Dysbiosis of the gut microbiota may have a role in the pathogenesis of acute pancreatitis through mechanisms such as the translocation of gut bacteria to the pancreas, production of inflammatory mediators, and immune system modulation. The release of gut-derived endotoxins, which can trigger pro-inflammatory cytokines and worsen pancreatitis, is another potential consequence of impaired gut barrier function (Akshintala et al., 2019; Wang et al., 2022).
In preclinical studies, it was discovered that synbiotics, probiotics, and prebiotics, among other treatments that target the gut microbiota, can lessen the severity of acute pancreatitis by reducing inflammation, improving gut barrier performance, and altering the makeup and function of the gut microbiota (van Minnen et al., 2007; Akyol et al., 2003; Muftuoglu et al., 2006; Karen et al., 2010). Clinical research has also produced encouraging findings, with some trials showing a decrease in morbidity and death in patients with acute pancreatitis who received probiotic treatment. To completely understand the mechanisms of action and ideal dosing approaches for these therapies, more research is necessary (Gou et al., 2014).
The PROPATRIA study’s conclusions merit careful consideration. A randomized, double-blind, placebo-controlled experiment was done as part of the PROPATRIA project to examine the effectiveness of probiotics in lowering the incidence of infections in patients with severe acute pancreatitis. Determining the occurrence of infections was the study’s main goal, and there was no discernible difference between the probiotic and placebo groups in this regard. The probiotic group did, however, have a considerably higher incidence of intestinal ischemia, according to the study (Besselink et al., 2008). The outcome of this study discouraged the start of additional probiotics trials. However, there were a number of flaws in the PROPATRIA experiment that may have affected the validity of the results. The heterogeneity of the study cohort, which comprised patients with anticipated severe acute pancreatitis of varied etiologies and severity levels, was one of the PROPATRIA study’s primary shortcomings. Prior to randomization, 14 research participants had already experienced organ failure and six had multiorgan failure. The ability to distinguish a substantial difference in infection incidence between the probiotic and placebo groups may have been hampered by this variability. According to Bongaerts et al., the time between the onset of symptoms and the first dose of probiotics should be as short as feasible, ideally 24 hours. Within the first 24 hours, the native gut flora experienced a tremendous expansion. Additionally, the probiotic strains provided do not themselves have pathogenic potential (Bongaerts and Severijnen, 2016). Gou et al. hypothesized that patients with severe acute pancreatitis and critical diseases who had intestinal barrier failure would experience probiotic overdoses as a result of prolonged medication duration (Gou et al., 2014). These limitations highlight the need for further research to fully elucidate the potential benefits and risks of probiotics in the management of acute pancreatitis.
There have been a number of meta-analyses done to evaluate the effects of probiotics, prebiotics, or synbiotics on clinical outcomes in patients with acute pancreatitis. The use of pre-, pro-, or synbiotics may reduce the length of hospitalization (weighted mean difference -4.33 days; 95% CI, -7.71 to -0.95; P = 0.010), but there is no difference in septic morbidity (RR, 0.91; 95% CI, 0.73-1.13; P = 0.392), according to a recent meta-analysis of nine randomized controlled trials. However, only six research articles included in the meta-analysis for length of hospitalization and five research articles on septic morbidity were included (Yu et al., 2021). A different meta-analysis also produced results that were comparable to those of the present study. According to data from six randomized controlled trials, pre-, pro-, and synbiotic supplementation significantly reduces the period of hospitalization for Chinese patients with severe acute pancreatitis (mean difference = -5.57, 95% CI, -8.21 to -2.93; P < 0.001) (Tian et al., 2018). Compared to available meta-analyses, our meta-analyses provide more studies and we subgroup it according to the medication used.
Our research was also subject to several limitations. First, our research only evaluated the clinical endpoint of duration for hospitalization and risk of infection. Since the use of pre-, pro-, and synbiotics showed conflicting results, we believe that evaluating the clinical benefit in adding this intervention may provide the basis for further studies on the use pre-, pro-, and synbiotics in AP. Therefore, further studies may evaluate the biological plausibility of pre-, pro-, and synbiotics in AP. Second, our research incorporated all severities of AP. Additional research to specifically evaluate the use of pre-, pro-, and synbiotics in specific severities of AP would be beneficial.
In conclusion, adjunctive treatment of pre-, pro-, and synbiotics to standard therapy of acute pancreatitis patients provide benefits for acute pancreatitis patient. The use of those interventions may benefit for length of hospital stay and reduce complication of infection risk. Therefore, further study to evaluate the efficacy of pre-, pro-, or synbiotics are warranted with the consideration to dose and duration of intervention.
All data underlying the results are available as part of the article and no additional source data are required.
Zenodo: PRISMA checklist for ‘Efficacy of pre, pro, and synbiotic on clinical endpoint of acute pancreatitis: a systematic review and meta-analysis (extended data)’, https://doi.org/10.5281/zenodo.8103601 (Mariadi et al., 2023).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)