Keywords
Fixed Drug Eruption, Ofloxacin, Dermatoses, Fluoroquinolone, Urinary Tract Infection
This article is included in the Datta Meghe Institute of Higher Education and Research collection.
Fixed Drug Eruption (FDE) is a rare form of drug-induced dermatoses that appears as well-defined, erythematous patches occurring within hours of medication’s administration with or without blistering and resolves with post-inflammatory residual hyperpigmentation.
A 50-year-old male patient visited the outpatient department with chief complaints of burning micturition, increased urine frequency, and high-grade fever with chills lasting three days. Blood and urine investigations indicated leukocytosis and remnants of pus cells, respectively, confirming the diagnosis of urinary tract infection intervened with a tablet ofloxacin. Two hours after drug consumption, intense itching was noticed over the body, which soon progressed to blackening discoloration; blisters developed with a burning sensation over the webs of the palm and on the arm, with hyperpigmentation of the lower lip. Furthermore, painful patches with serous discharge developed in the oral cavity and penile tip. He had encountered a similar episode seven years before, which confirmed the diagnosis of fixed drug eruption (FDE). Immediate treatment included injectable steroids, antihistamines, cephalosporin antibiotics, and intravenous fluids with discontinuation of the ofloxacin drug. He adhered well to the treatment and had a remarkable improvement after 72 hours with residual hyperpigmentation, following which, the patient was advised to avoid similar medicine in the future to prevent a recurrence.
This case report concludes that the adverse drug reactions should be considered mandatorily with antibiotic audits on a regular basis to ensure that the course of treatment is appropriate and adequate, and any inappropriate reaction should be reported immediately. The rationality of the treatment and inappropriate prescriptions must be reported.
Fixed Drug Eruption, Ofloxacin, Dermatoses, Fluoroquinolone, Urinary Tract Infection
Fixed drug eruption (FDE) or “eruption érythémato-pigmentée fixe” was initially reported and described by Bourns in 1889 and Brocq in 1894, respectively.1 FDE is a specific type of drug-induced dermatoses that emerges as clearly defined, erythematous (eryth) patches occurring with or without blisters within hours of the administration of the triggering medication and resolves with post inflammatory persisting hyperpigmentation.2 When exposed again to the same or a comparable class of medications, it typically occurs at the same area of the skin or mucous membrane.2
Ofloxacin (OF), a fluoroquinolone (FQ) of second generation, is quite efficient against a variety of bacterial infections with some adverse effects that include gastrointestinal disorders, skin reactions, and neurological reactions.3 They are commonly used antibiotics that can cause dermatological adverse drug reactions (ADRs) in about 1-2% of individuals.4 Rare hypersensitivity events due to OF include itching, skin rashes, urticaria, erythema and shock, and their frequency ranges from 0.4% to 2%.5
FDE can affect people of any age, however it most frequently affects individuals with documented median ages ranging from 35 to 60 years, and both men and women are equally susceptible to it.1 Recurrence of FDE is regulated by CD8+ memory T-cells, which are found in the basal layer of the epidermis of dormant FDE lesions.6 These cells drift upwards in the epidermis within 24 hours of ingesting the triggering medicine,6 generate cytokines such interferon-gamma and TNF-alpha (1), acquire the features of a natural killer cell, and express the cytotoxic chemicals granzyme B and perforin along with cell surface molecule CD56.6 This natural killer feature that the cells acquired during FDE subsides once the acute stage of the condition has passed and they remain dormant in the epidermis in the region of prior eruption for a long time.6 Bullous FDE, however, is not frequently recorded. Thus, this case report emphasizes a rare instance of FDE brought on by the administration of the drug OF.
The case report was amended according to the CARE guidelines.
A 50-year-old South-Asian male patient presented at the Acharya Vinoba Bhave Rural Hospital, Sawangi, India, with the main complaints of burning micturition, increased frequency of urination, nausea, vomiting, pain in low back and high-grade fever with chills lasting three days at the medicine outpatient department (OPD). The patient described a past history of urinary tract infection (UTI) seven years before for which he had taken unknown medicine that he was not able to recollect that caused him intense itching along with blackening of skin of upper and lower limbs and gluteal region along with development of blisters.
On examination, the patient was coherent, well-oriented and stable with blood pressure 110/70 mmHg, for investigations the patient’s blood and urine sample were sent for laboratory investigation, which revealed increased C-reactive protein, raised blood creatinine, leukocytosis with neutrophilia and increased number of pus cells that confirmed the diagnosis of UTI caused by the bacteria Escherichia coli. As the bacteria was found sensitive to the drug OF it was prescribed to the patient along with the advice of consumption of plenty of water. The first dose of OF was taken by the patient on the same evening.
At midnight, after 2 hours of drug consumption, intense itching started all over the body, especially involving upper and lower limbs and gluteal region, which soon progressed to blackening of skin on these parts. Additionally, physical examination revealed development of five blisters with burning sensation measuring 2–2.5 cm in diameter that were well circumscribed having an erythematous base over webs of palm (Figure 1) and on arm with negative Nikolsky sign7 along with hyperpigmentation of the lower lip. Furthermore, the patient developed painful erosions with serous discharge formation in oral cavity (Figure 2), penile tip, and prepuce (Figure 3). Based on the above presentation following the administration of the drug OF, the patient was referred to dermatology OPD where a diagnosis of fixed drug eruption was confirmed.
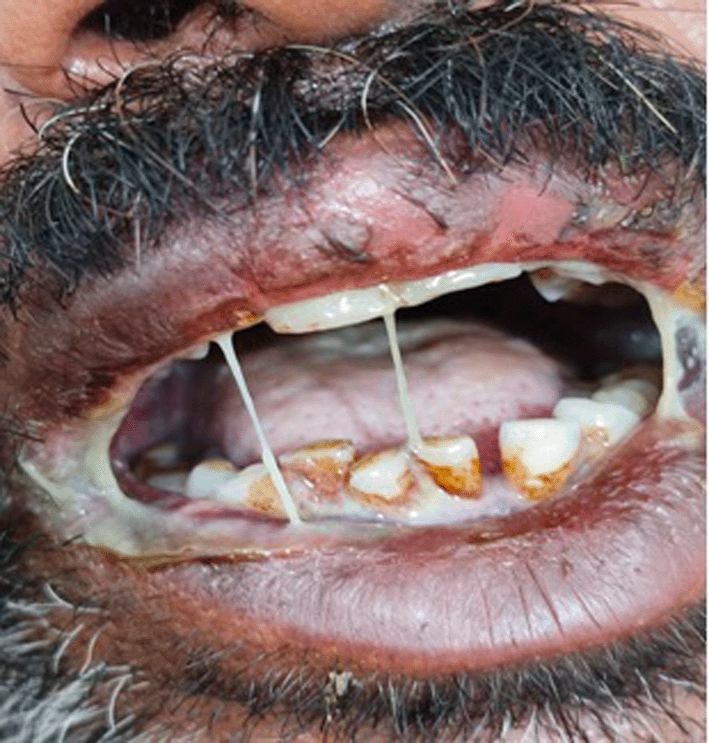
Before commencing medical intervention, the objective of the treatment was described to the patient and written informed consent was obtained. Following which immediate medical treatment was started with injectable steroids, injection ceftriaxone and levocetirizine, intravenous fluids, and topical betamethasone butyrate propionate ointment along with discontinuation of OF.
The patient adhered well to the above mentioned treatment as the outcomes of the intervention demonstrated subsiding of itching after 3 to 4 hours, oral and penile ulceration healed in 48 hours and overall condition of the patient improved after 72 hours with the presence of residual hyperpigmentation. Patient was advised to avoid similar medicine in future to avoid recurrence.
OF is a FQ prescribed to treat diseases of the prostate, skin, urinary system, and lungs caused due to bacteria. Anaphylactic responses and incidences of shock related to hypersensitivity have been documented during post-marketing surveillance.8 The terminology FDE indicates formation of eryth patches as a consequence of administration of a medication. Based on the location of the lesion and their clinical characteristics, various morphological forms of FDE, such as pigmenting, non-pigmenting, and bullous FDE, are known.3 According to a systematic review, antibiotics are responsible for 28% of ADRs, and the estimated prevalence rate of ADRs caused by ofloxacin is 4.27%.9
The basic presentation of the OF-related FDE seen in this case was intense itching all over the body. In particular, it involved upper and lower limbs and gluteal region, which soon progressed to blackening of skin on these parts. Development of blisters with burning sensation were well circumscribed with an erythematous base over webs of palm and on arm along with hyperpigmentation of the lower lip, and painful erosions with serous discharge formation in oral cavity, penile tip and prepuce. Development of blisters made the condition of this patient more severe. These related findings were published in a study that characterized generalized bullous FDE as standard FDE lesions occur at three of six different anatomic regions with blisters covering and accounting for not less than 10% surface area of the body. It was referred as Stevens-Johnson syndrome, which was basically differentiated on the basis of morphological characteristics of the eruption and the site of onset.10
The pathophysiological process consists of cell-mediated cytotoxic reaction, which is antibody-dependent, and CD8+ effector or memory T cells play a critical role in reactivating the reaction when exposed to the triggering medication again, however the precise mechanism is still unknown.3 However, the evidence indicates that these cells are primarily responsible for the occurrence of localized tissue destruction in FDE, and arousal of these cells triggers skin lesion.1,3 OF and other quinolones can cause both the IgE-mediated as well as delayed form of hypersensitivity reaction.2,11
In this case the patient was diagnosed with UTI for which OF was prescribed that resulted in FDE. Similarly, a previous review demonstrated various ADRs of OF that involved angioedema when given in case of diarrhea,9 cutaneous vasculitis in diabetic foot and UTI, intense erythema with sub-corneal pustulation, fever, and neutrophil leukocytosis in bronchitis and pharyngitis.9 The ADRs improved when OF that was being administered was discontinued, as there was no other cause that might have independently caused the reaction, and it was therefore, confirmed to be an FDE. Treatment for these reactions should primarily focus on cessation of the triggering drug and consumption of topical corticosteroids or antihistamines, and cephalosporin antibiotics.3 In this case, injectable steroids, injection ceftriaxone, and levocetirizine and intravenous fluids, topical betamethasone butyrate propionate ointment along with discontinuation of OF was commenced. When the patient returned for follow-up one month later, the lesions had subsided and the patient had responded well to the treatment.
The primary take away lessons involve strict avoidance of the culprit drug, which can protect the patient from recurrence, for diagnosis of fixed drug reactions the patient can be rechallenged by oral or patch test with the offending drug causing eruptions repeatedly at the identical skin site (that is fixed), and a note can be written on the discharge card with mention of offending drug molecule along with patient education for future prescription by other doctors.
This case report highlights fixed drug eruption caused due to ofloxacin drug during treatment of UTI and concludes that all hospitals should be mandated to track ADRs along with conduction of antibiotic audits on a regular basis to ensure that the course of treatment is appropriate and adequate, and any inappropriate prescription should be reported. A regular antibiotic audit must be done to check the rationality of the treatment and in case of inappropriate prescribing, errors must be reported. Additionally, ofloxacin must be included in the differential diagnosis of FDE and healthcare providers need to be made aware of illogical drug combinations.
I had this tendency of increased urination for 15 years. But this time it was with burning micturition and fever. I visited a nearby hospital. They admitted me and initiated with some medication. After two hours of consuming my first dosage, I felt itching everywhere on my body. Then I noticed weird black stains over my lower lip and palms, so I immediately called the medical staff on duty who immediately took my concern into consideration and called for further help. Again, I got prescribed with some other medications and after three days, I was relieved a bit with the symptoms.
Written informed consent for publication of clinical details and images was obtained from the patient.
All data underlying the results are available as part of the article and no additional source data are required.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Allergic contact dermatitis, fixed drug eruption
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 11 Mar 24 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)