Keywords
xylanase, glycosyl hydrolase 11, GH11, pH, conformational change, structure, flexibility
Glycosyl hydrolase 11 (GH11) xylanase is utilized in various in industrial applications such as baking, fruit juice production, pulp processing, and animal feed. Thermophilic GH11 from Thermoanaerobacterium saccharolyticum (TsaGH11) exhibits maximum activity at acid pH with high catalytic efficiency toward beechwood xylan. TsaGH11 activity is pH dependent, exhibiting relative low hydrolase activity at basic pH. However, the effect of a basic pH environment on the structure of TsaGH11 correlated with enzyme activity remains unknown. To understand pH-dependent activity changes, the crystal structure of TsaGH11 at basic pH was determined and compared with that of TsaGH11 at acid pH.
TsaGH11 was crystallized at basic pH of 8.5, and the crystal structure was determined at 1.95 Å resolution. The structure, flexibility, and water molecules of TsaGH11 at pH 8.5 and pH 4.3 were compared.
The open and closed conformations of TsaGH11 at pH 8.5 are reported. Subtle movements of the side chains of amino acids involved in the substrate-binding cleft and catalytic residues were observed. The overall temperature factor of TsaGH11 at pH 8.5 was higher than that at pH 4.6. The position of water molecules near the catalytic residues in TsaGH11 exhibited variations in different pH environments.
The structural comparison of TsaGH11 at basic and acidic pH offers valuable insights into the pH-dependent functionality of TsaGH11, enhancing our understanding of these structural alterations.
xylanase, glycosyl hydrolase 11, GH11, pH, conformational change, structure, flexibility
Endo-xylanases (EC 3.2.1.8) cleaves the β-1,4 glycosidic bonds in the xylan backbone, converting the polymeric xylan into xylose or xylooligosaccharides.1 In the carbohydrate-active enzyme database (CAZy), various glycoside hydrolase (GH) families (GH 5, 7, 8, 9, 10, 11, 12, 16, 26, 30, 43, 44, 51, and 62) are classified into xylanase.2,3 Each of these xylanase families exhibits distinct amino acid sequence, amino acid length, functional domains or enzymatic activity.4 Among them, GH11 has been considered a “true xylanase” because it exhibits high specificity and selectivity toward xylan substrates compared with other endo-xylanase GH families.1,5,6 GH11 xylanases is utilized as valuable biocatalysts in various industrial fields such as pulp, food, feed, textile, pharmaceutical, and biorefinery industries.7,8 The optimal activity conditions for xylanases vary based on environmental factors such as pH, temperature, salt, and metal ions.9,10 The industrial application field or scope of xylanase changes depending on its unique optimal pH activity.11 For example, in baking and fruit juice clarification, which require high activity at acidic pH, acidophilic xylanases are preferred,7,12 whereas in the pulp industry, where basic pH is desired, alkaliphilic xylanases are preferred.9
Hemicellulose-degrading thermophilic anaerobe Thermoanaerobacterium saccharolyticum prefers growing in high temperatures (30°C–66°C) and acidic conditions (pH 3.85–6.35).13 This strain acts as a biological catalyst for producing ethanol from cellulosic biomass.13–15 T. saccharolyticum ferments various carbohydrates, including glucose, cellobiose, xylan, xylose, starch, arabinose, mannose, and galactose.16 The enzymatic and structural characterization of GH11 from T. saccharolyticum (TsaGH11) has been previously reported.6 TsaGH11 exhibits maximum xylanase activity at pH 5.0 and 70°C, with specific activities of 5,622.0 and 3,959.3 U/mg for beechwood and oat spelt xylans, respectively. TsaGH11 exhibits superior catalytic performance, with kcat values of 34015.3 s−1, for beechwood xylan. Optimal enzyme activity of TsaGH11 was observed at pH 5.0, with relatively high activity at pH 4–6. However, hydrolase activity of TsaGH11 decreased to 60% at pH > 7.0. Activity variations are also observed depending on the buffering environment.6 For example, at pH 8, the Tris solution exhibits approximately 50% activity, whereas sodium phosphate solution shows <20% activity, and beyond pH 9, the relative enzyme activity drops to <20%.6 Consequently, TsaGH11 displays high activity in an acidic pH range but exhibits decreased activity in a basic pH environment, but the structural analysis of how pH can affect the activity of TsaGH11 still remains unknown.
To investigate the structural changes in TsaGH11 in response to pH, an extended crystallographic experiment of TsaGH11 was performed, and the crystal structure of TsaGH11 at pH 8.5 (TsaGH11pH8.5) was subsequently determined at 1.95 Å resolution. The crystal structure features of TsaGH11pH8.5 were described and compared with those of TsaGH11 at pH 4.6 (TsaGH11pH4.6). These structural analyses contribute to our understanding of the pH-dependent activity of TsaGH11.
Following chemicals were used in this study: Luria-Berani broth medium (LPS solution, Catalog No. LB-05, Daejeon, Republic of Korea,), ampicillin (LPS solution, Catalog No. AMP25), Isopropyl β-D-1-thiogalactopyranoside (LPS solution, Cat No. IPTG025), Tris (Sigma-Aldrich, St. Louis, MI, USA, Catalog No. T15760), NaCl (Sigma-Aldrich, Catalog No. S9888), Ammonium acetate (Sigma-Aldrich, Catalog No. A7262), Glycerol (Sigma-Aldrich, Catalog No. G5516).
The preparation of purified protein was similar to that in a previous report.6 In brief, vector DNA containing the TsaGH11 gene with an N-terminal hexahistidine tag was transformed into Escherichia coli BL21(DE3). Cells were grown in LB broth medium supplemented with ampicillin (50 μg/mL) at 37°C. When the culture optical density at 600 nm reached 0.6–0.8, protein expression were induced by addition of 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) and then incubated at 18°C while shacking for overnight. After harvesting the cells and disrupting them via sonication, the supernatant was loaded onto a Ni-NTA affinity resin (Qiagen, Hilden, Germany) in a column. The resin was washed with 50 mM Tris-HCl (pH 8.0), 200 mM NaCl, and 20 mM imidazole, and then proteins were eluted with 50 mM Tris-HCl (pH 8.0), 200 mM NaCl, and 300 mM imidazole. To cleave the N-terminal hexahistidine tag, elution fractions were pooled and incubated with thrombin at 22°C overnight. Samples were concentrated using Centricon (Sartorius, 10-kDa cutoff) and loaded onto a Sephacryl S-100 10/300 column (GE Healthcare, Chicago, IL, USA) in 10 mM Tris-HCl (pH 8.0) and 200 mM NaCl. The purified protein was concentrated to ~20 mg/mL using a Centricon for crystallization. Protein concentrations were determined using a NanoDrop 1000 spectrophotometer (ThermoFisher).
Crystallization screen of TsaGH11 was initially performed using the sitting-drop vapor diffusion method at 22°C. Purified TsaGH11 solutions (500 nL) were mixed with equal volume of crystallization solution from commercially available crystallization kits (Hampton Research, Aliso Viejo, CA, USA) on 96 × 2-well MRC nanoplates (Innovadyne). Microcrystals were obtained under four conditions with the following crystallization solutions: (i) Salt RX H10: 0.1 M Tris-HCl, pH 8.5, and 4.0 M ammonium acetate; (ii) Crystal screen B9: 0.2 M magnesium acetate, 0.1 M sodium cacodylate, pH 6.5, and 30% (v/v) 2-methyl-2,4-pentanediol; (iii) Index A11: 0.1 M HEPES, pH 7.5, and 3.0 M sodium chloride; and (iv) Index A12: 0.1 M Tris-HCl, pH 8.5, and 3.0 M sodium chloride. To obtain large crystals, crystal optimization experiments were performed using the hanging-drop vapor diffusion method at 22°C. Suitable TsaGH11 crystals for X-ray diffraction experiment were obtained under condition (i) by scaling up to mix 2 μL of the protein solution with 2 μL of the reservoir solution in 24-well VDX crystallization plates (Hampton Research).
The X-ray diffraction data were collected on beamline 11C at Pohang Light Source II (PLS-II, Pohang, Republic of Korea).17 The TsaGH11 crystal was immersed in a cryoprotectant solution consisting of a reservoir solution supplemented with 20% (v/v) glycerol for 10 s. The TsaGH11 crystal was mounted on the goniometer under a liquid nitrogen stream at 100 K. Diffraction data were recorded using a Pilatus 6M detector. Diffraction images were indexed, integrated, and scaled using the HKL2000 program.18
The electron density map of TsaGH11 structure was obtained via molecular replacement method using MOLREP (version 11.2.08).19 Crystal structure of TsaGH11 at pH 4.6 (PDB code: 8IH0)6 was used as search model. Manual model building was performed using COOT (version 0.9.6).20 Structure refinement was performed using phenix.refine in PHENIX.21 Water molecules were automatically added during the refinement using the default parameters in PHENIX.21 The final coordinates of TsaGH11pH8.5 was validated using MolProbity.22 Structural figures were generated using PyMOL (version 2.4.1.; http://pymol.org/2). The structure factor and coordinates were deposited at the Protein Data Bank under access code 8X1D (https://www.rcsb.org/structure/8X1D).
Differences in the secondary structures of TsaGH11pH8.6 and TsaGH11pH4.6 were analyzed using 2StrucCompare23 with the Dictionary of Secondary Structure of Proteins (DSSP)24 and the STRuctural IDEntification (STRIDE)25 method. The B-factor and normalized B-factor were analyzed using PHENIX.21
Protein conformation depends on environmental factors such as pH, temperature, and interactions.26,27 Although TsaGH11 exhibits high activity at acidic pH, this activity diminishes at basic pH,6 and the crystal structure of TsaGH11 at an acidic pH (pH 4.6, which is close to that at optimal activity) has been determined.6,28 To comprehend the change in TsaGH11 activity influenced by pH from a structural perspective, an extended crystallization assessment was conducted to obtain the crystal structure of TsaGH11 at basic pH. Microcrystals of TsaGH11 were obtained from four new crystallization solutions: (i) 0.1 M Tris-HCl, pH 8.5, and 4.0 M ammonium acetate; (ii) 0.1 M Tris-HCl, pH 8.5, and 3.0 M sodium chloride; (iii) 0.1 M HEPES, pH 7.5, 3.0 M sodium chloride; and (iv) 0.2 M magnesium acetate tetrahydrate, 0.1 M sodium cacodylate, pH 6.5, and 30% (v/v) 2-methyl-2,4-pentanediol. These crystallization conditions at three different pH values (6.5, 7.5, and 8.5) were distinct from those previously reported (pH 4.6) (Figure 1a). Among them, the crystallization conditions (i) of TsaGH11 grown at pH 8.5 differ only in pH from those previously reported for TsaGH11pH4.6 (0.1 M sodium acetate, pH 4.6, and 4.0 M ammonium acetate), which showed the same crystal morphology as TsaGH11 (Figure 1a). Therefore, crystallization condition (i) was considered to be the optimal condition with the least influence from the crystallization solution constituents. The growth rate of TsaGH11 crystals under condition (i) was 2–4-fold longer than the crystal growth time at pH 4.6. The TsaGH11pH8.5 crystal diffracted up to 1.95 Å (Figure 1b), and although crystals were obtained under conditions (ii), (iii), and (iv) (Figure 1a), they did not exhibit sufficient diffraction intensity to determine the crystal structure.
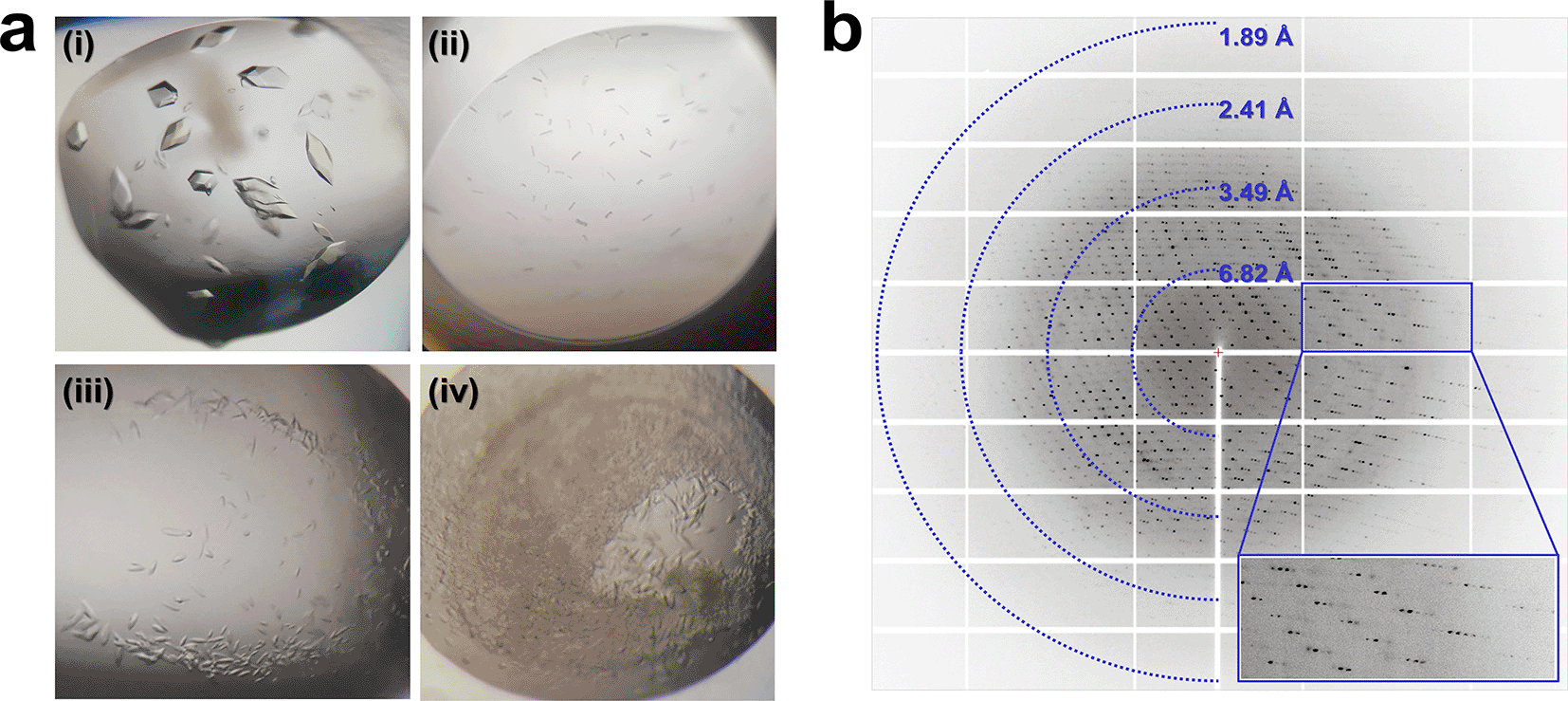
(a) Crystallization screen results for TsaGH11. TsaGH11 crystals were grown under (i) 0.1 M sodium acetate, pH 8.5, and 4.0 M ammonium acetate, (ii) 0.2 M magnesium acetate, 0.1 M sodium cacodylate, pH 6.5, and 30% (v/v) 2-methyl-2,4-pentanediol, (iii) 0.1 M HEPES, pH 7.5, and 3.0 M sodium chloride, and (iv) 0.1 M Tris-HCl, pH 8.5, and 3.0 M sodium chloride. (b) Diffraction pattern of TsaGH11 crystals grown with 0.1 M sodium acetate, pH 8.5, and 4.0 M ammonium acetate.
The TsaGH11pH8.5 crystal belongs to the tetragonal space group P43212 with unit cell parameters (a = b = 72.79 Å and c = 165.63 Å) and contains two molecules in an asymmetric unit (Table 1). The space group and unit cell parameters of the TsaGH11pH8.5 crystal are nearly identical to those of the TsaGH11pH4.6 crystal (P43212, a = b = 73.11 Å, and c = 165.42 Å). The crystal structure of TsaGH11pH8.5 was solved up to 1.95 Å, with Rwork and Rfree values of 0.171 and 0.209, respectively. The electron density map of the TsaGH11 structure was clearly observed for all amino acids (Thr29–Trp211). TsaGH11pH8.5 exhibits a β-jelly roll fold, resembling that of the right hand. The catalytic residues and substrate-binding cleft are located between the finger and thumb domains (Figure 2a). In the asymmetric unit of TsaGH11pH8.5, molecule A has a rigid conformation due to crystal packing, whereas molecule B has relatively high flexibility of the thumb domain, exposed to the solvent region in crystal packing (Figure 2b). The average temperature factors of the A and B molecules of TsaGH11pH8.5 were 28.17 and 37.16 Å2, respectively. The superimposition of the A and B molecules of TsaGH11pH8.5 showed similarity with a root-mean-square (r.m.s.) deviation of 0.222 Å. The palm and finger domains of molecules A and B display high similarity, but the positions of their thumb domains differ significantly (Figure 2c and 2d). At the thumb domain, Cα atoms from Arg139, Pro143, Asp146, and Thr148 between molecules A and B exhibited subtle movements of 1.09, 1.86, 1.26, and 1.60 Å, respectively (Figure 2d). The surface structure of molecule A of TsaGH11pH8.5 displays an open conformation for the substrate-binding cleft located between the thumb and finger domains (Figure 2e). At subsites +1 and +2, the distance between Pro40 (atom: CB) and Trp40 (CH2) was 5.84 Å (Figure 2e). At subsites –1 and –2, the distances between Asn62 (OD1) and Arg139 (NH2) and between Arg139 (NH1) and Tyr200 (OH1) were 7.02 and 7.93 Å, respectively. The distance between the catalytic Glu105 (OE2) and Glu198 (OE1) residues was 5.17 Å. Conversely, the surface structure of molecule B of TsaGH11pH8.5 displayed a closed conformation between the thumb and finger domains (Figure 2f). At subsites +1 and +2, the distance between Pro40 (CB) and Trp40 (CZ2) was 3.71 Å (Figure 2f). At subsites −1 and −2, the distances between Asn62 (OD1) and Arg139 (NH2) and between Arg139 (NH1) and Tyr200 (OH1) were 5.71 and 8.38 Å, respectively. The distance between the catalytic Glu105 (OE2) and Glu198 (OE1) residues was 5.13 Å (Figure 2f). Consequently, molecules A and B in the asymmetric units of TsaGH11 represent the open and closed conformations of the substrate-binding cleft, respectively, due to the crystal packing effect. These differing conformations of TsaGH11pH8.5, influenced by crystal packing, are similar to those observed in the previously reported TsaGH11pH4.6 crystal because of identical protein crystal packing within the same space group.6
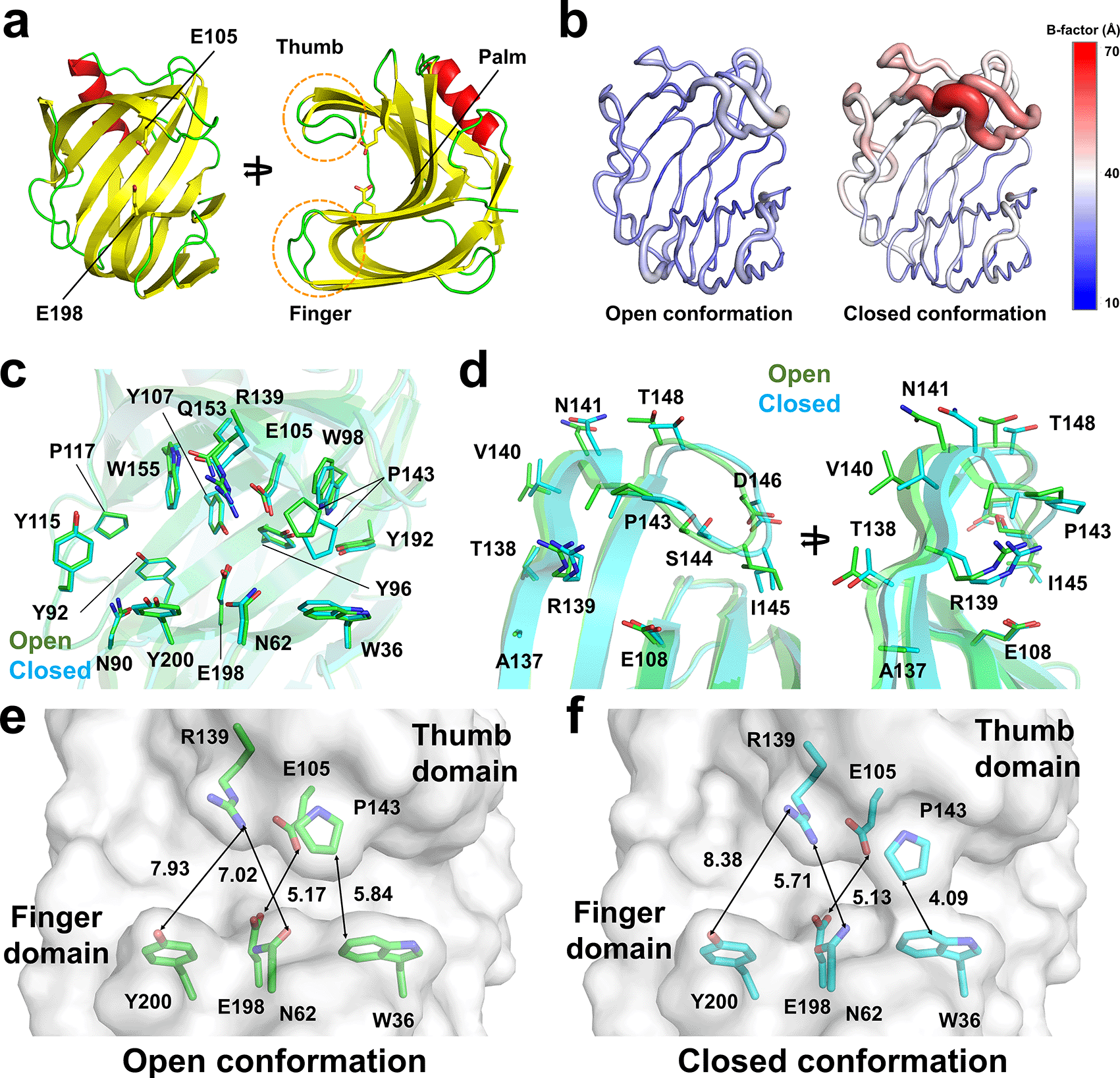
(a) Cartoon representation of TsaGH11, resembling the right-hand structure with palm, finger, and thumb domains. (b) B-factor putty representation of TsaGH11pH8.5 for molecules in the asymmetric unit. (c) Superimposition of two TsaGH11pH8.5 molecules in the asymmetric unit. (d) Close-up view of the superimposition of the thumb domain of the open (green) and closed (cyan) conformations of TsaGH11pH8.5. Surface structure of the (e) open and (f) closed conformations of the substrate-binding cleft in TsaGH11pH8.5.
The xylanase activity of TsaGH11 at pH 4.6 and pH 8.5 was approximately 97% and 35%, respectively,6 and the crystal structures of TsaGH11pH4.6 and TsaGH11pH8.5 therefore represent structural conformations at relatively high and low activity ranges, respectively. Among the crystal structures of TsaGH11pH4.6 and TsaGH11pH8.5, molecules A and B have the same conformation and were consequently compared. pH induces alterations in the ionization state of amino acid side chains and can affect the secondary structure of proteins.29,30 Consequently, the secondary structures of TsaGH11pH8.5 and TsaGH11pH4.6 were analyzed using DSSP and ESSS method. In the TsaGH11 open conformation, secondary structural differences were observed in Val40, Gly89, Asn90, and Asn167 (Figure 3a). Val40, Gly89, and Asn90 were located above the substrate-binding cleft in the finger domain, and Asn167 was positioned on the surface opposite the substrate-binding site and did not directly influence substrate-binding or activity (Figure 3b). In the TsaGH11 closed conformation, secondary structural differences were present in Asn59, Cys60, Gly61, Gly89, Asn90, Ser144, Gly147, Thr166, Asn167, Qln201, and Ser202 (Figure 3a). Asn59, Cys60, Gly61, Gly89, Asn90, Qln201, and Ser202 are located in the finger domain and lie above the substrate-binding cleft, and Ser144 and Gly147 are positioned in the thumb domain and are presumed to be involved in substrate recognition. Thr166 and Asn167 are located opposite the substrate-binding cleft and do not directly affect substrate-binding (Figure 3c). Accordingly, changes in the secondary structure were commonly observed in the main chain of Gly89 and Asn90 of the finger domain, as well as of Asn167, positioned on the opposite surface of the substrate-binding cleft in both the open and closed conformations of TsaGH11. Although differences in secondary structure were noted in the main chain, no significant structural changes were observed in the conformation of the side chains.
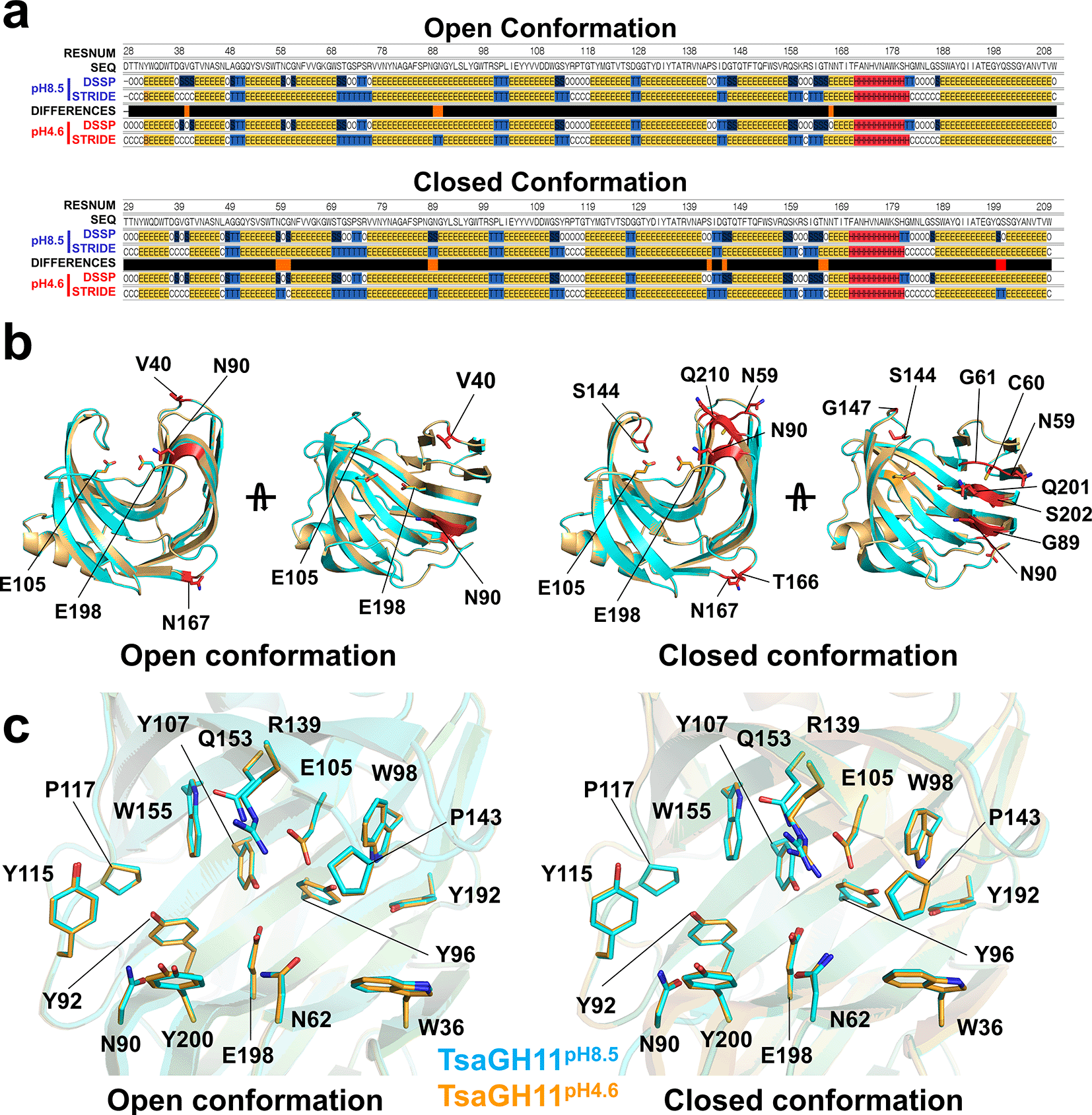
(a) Secondary structure analysis of the TsaGH11 structures using DSSP and STRIDE. Different secondary structure regions are indicated by red bars. (b) Cartoon representation of the different secondary structure regions between TsaGH11pH8.5 and TsaGH11pH4.6. (c) Superimposition of the catalytic and substrate-binding clefts of the open and closed conformations of TsaGH11pH8.5 and TsaGH11pH4.6.
The superimposition of the TsaGH11pH8.5 and TsaGH11pH4.6 open conformations had an r.m.s. deviation of 0.075 Å (Figure 3c). In the open conformation, the distances between the side chains of the two catalytic residues Glu105 and Glu198 from TsaGH11pH8.5 and TsaGH11pH4.6 were 5.17 and 5.17 Å, respectively. At subsites +1 and +2, the distance between Trp36 (CZ) and Pro143 (CB), which determines the size of the opening between the thumb and finger domains, in TsaGH11pH8.5 and TsaGH11pH4.6 were 5.84 and 5.60 Å, respectively. At subsite −1, the distances between Asn62 (OD1) and Arg139 (NH2) from TsaGH11pH8.5 and TsaGH11pH4.6 were 7.02 and 6.84 Å, respectively. At subsite −2, the distances between Arg139 (NH1) and Tyr200 (OH) from TsaGH11pH8.5 and TsaGH11pH4.6 were 7.93 and 8.30 Å, respectively. Overall, the active site regions of the TsaGH11pH8.5 and TsaGH11pH4.6 open conformations are almost identical, whereas the distance between the finger and thumb domains involved in the substrate recognition site of TsaGH11pH8.5 is slightly wider than that in TsaGH11pH4.6.
The superimposition of the closed conformations of TsaGH11pH8.5 and TsaGH11pH4.6 had an r.m.s. deviation of 0.094 Å (Figure 3c). In the closed conformation, the distances between the side chains of the two catalytic residues Glu105 and Glu198 in TsaGH11pH8.5 and TsaGH11pH4.6 were 5.13 and 5.21 Å. At subsites +1 and +2, the distances between Trp36 (CZ) and Pro143 (CB) and between TsaGH11pH8.5 and TsaGH11pH4.6 were 3.71 and 3.82 Å, respectively. Notably, different side chain conformations of Arg139 involved in substrate recognition were observed between TsaGH11pH8.5 and TsaGH11pH4.6. At subsite −1, the distances between Asn62 (OD1) and Arg139 (NH2) from TsaGH11pH8.5 and TsaGH11pH4.6 were 5.71 and 4.99 Å, respectively. At subsite −2, the distances between Arg139 (NH1) and Tyr200 (OH) from TsaGH11pH8.5 and TsaGH11pH4.6 were 8.02 and 8.41 Å, respectively.
The flexibility of protein conformation can be affected by pH.31 To understand whether pH affects the flexibility of TsaGH11, the temperature factor of TsaGH11pH8.5 and TsaGH11pH4.6 was investigated. The overall B-factor values of the open/closed conformations of TsaGH11pH8.5 and TsaGH11pH4.6 were 28.17/37.16 and 18.14/21.80 Å2, respectively (Figure 4a). At both pH 8.5 and 4.6 for TsaGH11, the flexibility tendencies of the closed and open conformations differed, with the closed conformation exhibiting higher flexibility than the open conformation, which can be attributed to protein packing. In the B-factor plot, the tendencies of each open and closed conformation for TsaGH11pH8.5 and TsaGH11pH4.6 were similar. However, the B-factor values for these conformations of TsaGH11pH8.5 were 1.55 and 1.70-fold higher than those of TsaGH11pH4.6. This result indicates that the molecular flexibility of TsaGH11 is relatively high at basic pH. The crystal structures of TsaGH11pH8.5 and TsaGH11pH4.6 were obtained from different crystals, and B-factor effects may occur because of variations in the crystal conditions. Accordingly, the normalized B-factor of TsaGH11pH8.5 and TsaGH11pH4.6 was analyzed and compared (Figure 4b). In the open conformation, Asn88 in the finger domain and Arg139–Gln149 in the thumb domain in TsaGH11pH4.6 showed relatively higher flexibility than TsaGH11pH8.5. In the closed conformation, the area around Lys180 of the palm domain in TsaGH11pH4.6 has a relatively higher flexibility than that in TsaGH11pH8.5.
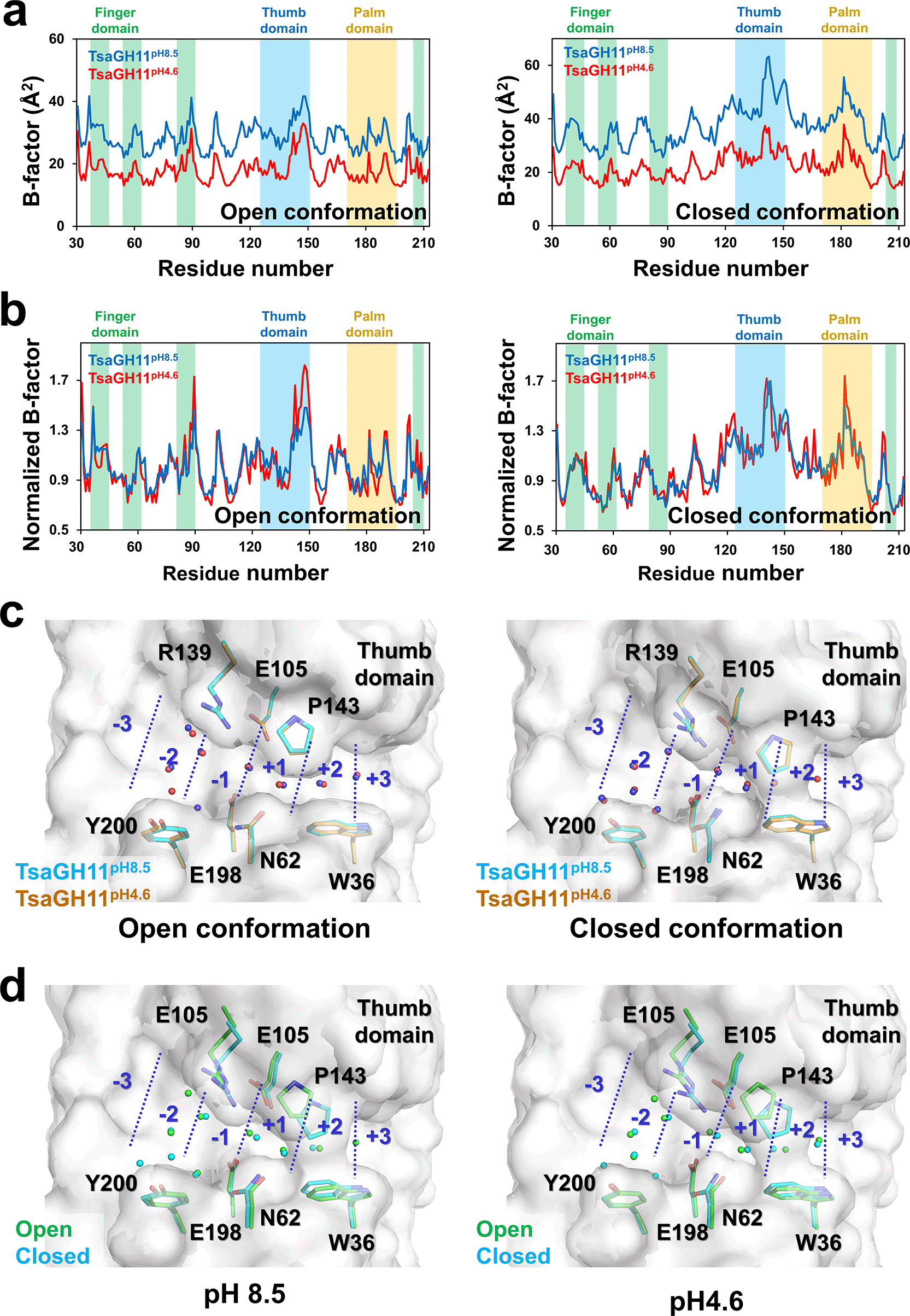
Profile of (a) B-factor and (b) normalized B-factor for the open and closed conformations of TsaGH11pH8.5 and TsaGH11pH4.6. (c) Superimposition of water molecules in the substrate-binding cleft from TsaGH11pH8.5 (blue) and TsaGH11pH4.6 (red). (d) Superimposition of water molecules in the substrate-binding cleft for the open (green) and closed (cyan) conformations of TsaGH11pH8.5 and TsaGH11pH4.6. Water molecules are indicated by spheres.
Changes in pH can affect the charge state of amino acids and subsequently influence the coordination or position of water molecules around protein amino acids.32,33 Water molecules share characteristics with the hydroxyl group in the xylan substrate, and their positions around the active substrate can subsequently offer insights into the substrate-binding moiety. Therefore, water molecules in the substrate-binding cleft and the active site of TsaGH11 were investigated. In the open conformation of TsaGH11, the subtle movement of water molecules in TsaGH11pH8.5 and TsaGH11pH4.6 at subsites −2, –1, +1, and +2 were approximately 0.22–0.77, 0.26, 0.48, and 0.34 Å, respectively, and between the catalytic residues in TsaGH11pH8.5 and TsaGH11pH4.6 was approximately 0.19 Å (Figure 4c). In the closed conformation of TsaGH11, the subtle movement of water molecules from TsaGH11pH8.5 and TsaGH11pH4.6 at subsites –2, −1, +1, and +2 were approximately 0.16–0.48, 0.09–0.22, 0.42, and 0.26 Å, respectively, and between the catalytic residues from TsaGH11pH8.5 and TsaGH11pH4.6 was approximately 0.17 Å (Figure 4c). Consequently, the average change in the position of these water molecules at different pH levels was <0.5 Å, and no specific directionality was observed in these positions.
Unlike the difference in the position of the water molecules caused by pH, the overall change in the position of water molecules in the open and closed structures in TsaGH11pH8.5 and TsaGH11pH4.6 was substantial. In the superimposition of the open and closed conformations of TsaGH11pH8.5, the subtle movements of water molecules at subsites −2, –1, +1, and +2 were approximately 0.23–0.38, 0.22, 0.26, and 0.71 Å, respectively, and between the catalytic residues in TsaGH11pH8.5 was approximately 0.61 Å (Figure 4d). In the superimposition of the open and closed conformations of TsaGH11pH4.6, the subtle movements of water molecules at subsites −2, −1, +1, and +2 were approximately 0.12–0.41, 0.37, 0.72, and 0.92 Å, respectively, and between the catalytic residues in TsaGH11pH4.6 was approximately 0.64 Å (Figure 4d). Overall, a minor structural change occurred in the position of water molecules in the substrate-binding cleft of TsaGH11 because of the pH. However, the change in the position of water molecules resulting from the open and closed conformational changes in the substrate-binding cleft of TsaGH11 was more significant than the pH effect.
Xylanase GH11 is of significant industrial importance. The high enzyme activity of TsaGH11 exhibits pH-dependent hydrolase activity, with increased activity at acidic pH and reduced activity at basic pH. To understand the correlation between pH-dependent function and structure, a crystallography study on TsaGH11 was conducted, producing four new crystallization conditions. The crystal structure of TsaGH11 under condition (i) was determined at 1.95 Å. However, diffraction data for TsaGH11 crystals under conditions (ii), (iii), and (iv) could not be collected. Although crystal optimization and diffraction data collection for conditions (ii–iv) were not obtained in this study, this crystallization information can be used for future crystallographic studies for elucidation at basic or other pH values (see below).
To understand the changes in TsaGH11 activity caused by different pH values from a structural perspective, the crystal structure of TsaGH11pH8.5 determined in this study was compared with that of the previously reported TsaGH11pH4.6. Upon comparing the open and closed conformations of TsaGH11 at the two pH conditions, subtle structural differences in the side chain shift, flexibility, and water molecule position were observed. Comparing the TsaGH11pH8.5 and TsaGH11pH4.6 structures, alterations in the secondary structure were commonly noted at the Gly89, Asn90, and Asn167 regions in both open and closed conformations. Amino acids in the Gly89 and Asn90 regions, which are located near the substrate access region in the substrate-binding cleft, may be implicated in substrate recognition. However, Asn167, situated opposite the substrate-binding site, may not be involved in direct substrate recognition or activation. Conversely, changes in the secondary structure of more amino acids in the finger domain were observed between the closed and open conformations. Given the crucial role of the finger domain, alongside the thumb domain, in substrate recognition, alterations in the main chain of related amino acids might directly or indirectly affect enzyme activity. The variations in the secondary structure between the open and closed conformations indicate that the structural effect of pH on the main chain varies depending on the TsaGH11 conformation.
In the superimposition of the crystal structures of TsaGH11pH8.5 and TsaGH11pH4.6, a slight positional shift of approximately <0.5 Å is observed for the side chains of amino acids involved in the substrate-binding cleft and the active site of TsaGH11. However, these small changes in the positions of these amino acids are limited in explaining the structure and activity variations observed because of the influence of pH. Conversely, a substantial conformational change was noted in the side chain of Arg139 in the closed conformation of TsaGH11 in response to pH alterations. This residue is involved in substrate recognition on the thumb domain, and this pH-induced conformational change in Arg139 may therefore be noteworthy. Nevertheless, considering that the pKa of Arg is 12.48, its charge remains constant at pH 4.6–8.5. Therefore, the preferred conformation of the Arg139 side chain results from complex factors such as the arrangement and mobility of water molecules around Arg139 that are influenced by changes in pH.
B-factor analysis revealed that the flexibility of amino acids in TsaGH11pH8.5 was similar to that in TsaGH11pH4.6 in both open and closed conformations. However, the B-factor value of the TsaGH11pH8.5 structure was >1.5-fold that of the B-factor value of TsaGH11pH4.6. The higher flexibility of TsaGH11 at this basic pH may provide insight into future understanding of the function of GH11 depending on pH. However, even if the crystallization conditions are similar and the data collection conditions are the same, an absolute comparison cannot be made because the two crystals are not identical.
Charge state of amino acids can be influenced by pH, potentially affecting the arrangement of water molecules around the substrate-binding site. Our analysis of water molecules in the TsaGH11 structures revealed a difference in the number of water molecules in TsaGH11 between basic and acidic pH. This disparity may result from crystal structure resolution rather than a direct effect of pH, making direct comparison challenging. In addition, a small movement in the position of water molecules in the substrate-binding cleft was observed depending on pH, but no specific trends or significant changes were notable. To quantitatively analyze water molecules according to pH, they need to be investigated according to changes at various pHs, which requires crystal structures formed at these different pHs. The change in the position of the water molecules in the open and closed conformation structures at TsaGH11pH8.5 and TsaGH11pH4.6 was greater than the change in the position of the water molecules caused by changes in pH. Consequently, the location of the water molecules indicates the influence of pH on protein conformation. Accordingly, to accurately compare water molecules in the active site depending on pH, proteins of the same conformation must be compared.
In summary, to understand the relationship between the structure and function of TsaGH11 at different pH values, the crystal structure of TsaGH11pH8.5 was determined and compared with that of TsaGH11pH4.6 to explain the structural differences between the different pH values. Structural comparison of TsaGH11pH8.5 and TsaGH11pH4.6 identified differences in secondary structure, conformational changes of amino acids in the substrate-binding cleft, and flexibility depending on pH. Although these results could provide useful insight into understanding the pH-dependent function of GH11, no significant changes in the crystal structure were observed to be the cause of the decreased activity of TsaGH11 at basic pH. Accordingly, in future research, crystal structures at basic pH (>pH 9.0) must be determined or at various other pHs and trends in molecular movement investigated to observe structural changes induced by changes in pH. Nevertheless, this result offers valuable information, suggesting that structural changes in TsaGH11 may occur due to pH variations. When combined with crystal structures obtained at other pH values in the future, these findings will contribute useful insights for explaining the pH-dependent structure and function in future studies.
The atomic coordinates and structure factor for the TsaGH11 at pH 8.5 has been deposited in the Protein Data Bank (https://www.rcsb.org/), accession code 8X1D: https://www.rcsb.org/structure/8X1D.
I thank the staff at the 11C beamline at Pohang Accelerator Laboratory for their assistance with data collection. The editing and publication were supported by Kookmin University.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular biology, Enzymology, Bioprocess, enzyme kinetics, protein characterization
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biomass conversion, fungal strain improvement, cellulases, hemicellulases, and auxiliary enzymes, genomics and proteomics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 02 Apr 24 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)