Keywords
Anticancer; Bioactive compounds; GCMS; MTT assay
This article is included in the Oncology gateway.
Sea cucumbers can be explored as alternative raw materials by the pharmaceutical and fishery industries as anticancer agents because they contain potential bioactive compounds.
This study aimed to determine the anticancer activity of Paracaudina australis extract against breast cancer cells (T47D) using an MTT assay. The secondary metabolites found in P.australis are steroids, terpenoids, saponins, and phenolics. The Thin-Layer Chromatography test results are indicated by Rf values, and steroid compounds in the ethyl acetate fraction are included in the standard Rf values. The isolates obtained were identified by Gas Chromatography-Mass Spectrometry, Fourier-Transform Infrared Spectroscopy, High-performance liquid chromatography, and UV-Visual spectrophotometry.
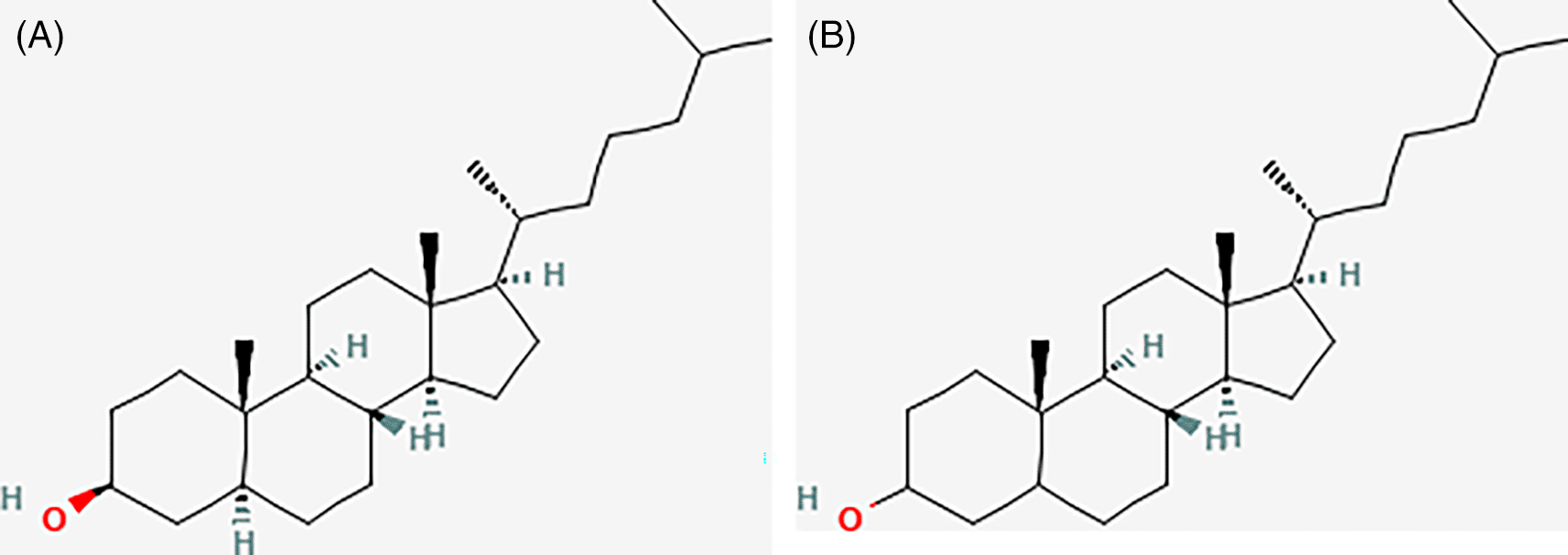
The isolated compounds were Dihydrocholesterol and Cholestan-3-ol, with a molecular formula (C27H48O). The ion weight and molecular mass of the compound were m/z 388,7.
These compounds may be responsible for the anticancer activity of P.australis. The IC50 of Isolate F4 was 25,3 μg/ml, and IC50 of Isolate F7,8 was 13,76 μg/ml.
Anticancer; Bioactive compounds; GCMS; MTT assay
The revised version of the manuscript incorporates the corrections suggested by the reviewers. The Introduction was expanded, incorporating current bibliographic references. The Discussion was synthesized, and current quotes that better explain the results were incorporated. In the new version of the article, an extensive grammar check is done, improving the clarity and coherence of the manuscript. And we changed the conclusions because there was mistyped.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Berunok sea cucumber has not been optimally utilized, especially in the Karimun Regency, Riau Islands (Ocsandy et al., 2019). Sea cucumbers have many health benefits for humans because they contain 17 types of essential and non-essential amino acids, of which 9 are essential amino acids and 8 are non-essential amino acids (Putri et al., 2020). According to (Janakiram et al., 2015) sea cucumbers contain metabolic compounds that are potential anticancer agents. Cancer is one of the leading causes of morbidity (conditions that change quality of life and health) and mortality (death).
According to the World Health Organization (WHO, 2018), there were an estimated 18.1 million new cancer cases in 2018, and around 9.6 million people among them were declared dead due to cancer. Breast cancer is a disease in which women are at risk of suffering. Breast cancer is the leading cause of death annually. Breast cancer is a malignant tumor that occurs in breast cells and can develop and invade the surrounding tissues or metastasize deep into other parts of the body. It is common in women; however, men can also be affected (ASC, 2015).
Cancer treatments that have been carried out are surgery, radiotherapy, chemotherapy and immunotherapy (Debela et al., 2021). The cost of chemotherapy and cancer treatment is high, but the success rate of therapy is not optimal; therefore, it is necessary to find alternatives to new drugs that are more effective and selective. One of the sources that became the target of this study was sea cucumbers. The saponins contained in sea cucumbers have antifungal, antibacterial, anticancer, and antioxidant (Khotimchenko, 2018). According to Putram et al. (2017), saponins are triterpene complex glycosides containing carbohydrates that have many biological activities, such as antifungal, antibacterial, and anticancer activities. According to Sharma et al. (2013), saponins, based on their apoptotic actions on cancer cells, have been shown to have anticancer effects. The mattress sea cucumber contains bioactive compounds, such as saponins, steroids, and terpenoids (Sukmiwati et al., 2020). The ethanol extract of the spotted kridou sea cucumber has potential cytotoxic activity and can be used as a medicinal material (Kilungga et al., 2019).
The WHO data in 2018 showed that the most common cancer cases in Indonesia were breast cancer cases, with 58,256 cases out of a total of 348,809 cancer cases. Therefore, researchers are interested in testing berunok extract against breast cancer (T47D) because previous studies examined MCF-7 cells (Sukmiwati et al., 2020).
Based on this, the utilization of sea cucumbers through extraction by maceration from sea cucumbers followed by fractionation to obtain bioactive compounds can be used to prevent cancer because saponins and steroids are compounds that can inhibit abnormal cell division. T47D breast cancer cells are ductal carcinomas that have a morphology similar to epithelial cells isolated from the breast tissue of a 54-year-old woman who is included in luminal A and expresses mutated p53 protein that causes disruption in growth control and induction of apoptosis of damaged body cells (Pratiwi et al., 2020).
The ethanol extract of sea cucumber Holothuria atra Jaeger has cytotoxic activity against several cancer cells, including T47D, with the greatest inhibition occurring in T47D cells, with the mechanism of cell inhibition through induction of apoptosis. Sea cucumber extract H. atra has the ability to inhibit cancer cell growth, especially in T47D cancer cells. The inhibitory ability is mediated through the mechanism of apoptosis, which has been proven through flow cytometry testing and double staining analysis (Halimatushadyah et al., 2018). The results showed that some natural and semi-synthetic triterpenoids have tumor inhibitory properties and even cytotoxicity against breast cancer cells, including T47D (Okuda, 2016).
Cytotoxicity refers to the death of cells caused by chemical components or cell mediators. Cytotoxicity is commonly used as a guideline in the laboratory to detect cell death, regardless of the underlying mechanism. Cytotoxicity tests are the ability of cells to survive in the presence of toxic compounds. The ability of cells to survive can be interpreted as the presence of metabolites or proliferation that can be measured by increasing the number of cells and the amount of protein or DNA synthesized. A cytotoxic test was used to determine the IC50 (Inhibitory Concentration) value. The anticancer activity of the plant extract and fraction was determined from the IC50 value. The IC50 value indicates the concentration of an extract or treatment required to inhibit cell viability by 50% of the total number of treated cells. If the IC50 value is low, the ability of the compound as an anticancer substance is high.
The MTT assay method was chosen because it has several advantages, including ease and efficiency in terms of time because the process is relatively fast and is considered more sensitive than other cytotoxic tests such as the LDH and protein methods. The MTT method can detect cell activity (proliferation) based on the ability to convert the yellow MTT substrate into purple formazan crystals by the enzyme succinate dehydrogenase contained in living cells. The intensity of formazan formation was proportional to the number of living cells. Therefore, the stronger the cytotoxic activity of a compound, the smaller the formazan crystals formed, followed by a low absorbance value, which indicates that the cells are alive in small numbers (Safitri, 2020).
Doxorubicin was used as a positive control, so a higher concentration would have a significant effect on the decrease in % cell viability. Some chemotherapeutic agents can increase Reactive Oxygen Species to cytotoxic levels, such as cisplatin, gefitinib, and doxorubicin on various types of cancer cells (Okon et al., 2015). Doxorubicin is an anthracycline chemotherapy that is quite potent and still used in the treatment of breast cancer (Frengki et al., 2023).
The main ingredients were berunok sea cucumber (Paracaudina australis) weighing 600-700 grams/head obtained from Tanjung Balai Karimun, Riau Islands. Breast cancer cells (T47D), trypsin-EDTA (Sigma, 3417012020-RID-186367117), culture media, RNase (Merck, 3544006000-BKR-035343301), RPMI media (Sigma, R8758), phosphate buffered saline (PBS) (Sigma, P5119), 20% MTT solution 3-(4,5-dimethylhiazolyl-2,5-diphenyltetrazolium bromide (Merck, M2003), 10 % Sodium Dodecyl Sulfate (SDS) (Sigma, L5750), DMSO 100% (Merck, 20-139), ethanol (Merck 02851):, n-hexane (Merck 1.04367.2500), methanol (Merck, 1.06009.2500), and ethyl acetate (Merck, 1.09623.2500), respectively.
The equipment used were 96 hole microplate, biosafety cabinet (Faster), inverted/phase contrast microscope (Olympus), culture flask, CO2 incubator, spectrophotometric microplate reader (Thermo), liquid nitrogen tank, water bath, ELISA reader, hemocytometer, sterile conical tube, scraper, ampoule, Laminar Air Flow, 6 well plates (TCD), micropipettes (10, 20, 200, 1000 μl), 1.5 ml centrifuge tube, centrifugator, water bath, FACS-Calibur, and hemacytometer.
Maceration was carried out by placing sea cucumbers (500 g) in a dark bottle, adding 1000 ml methanol, left for 3 days, and shaking occasionally. After three days, the macerate was filtered and repeated up to three times. Methanol macerate was concentrated using a rotary evaporator until it was thick and weighed.
The thick methanol (Merck, 1.06009.2500) around 200 mL extract was fractionated using a separatory funnel using solvents of different polarity each 4 × 200 ml. The nonpolar solvent n-hexane was used to obtain the n-hexane and water fractions. The n-hexane fraction was evaporated to a thick n-hexane fraction using a rotary evaporator. The water fraction was fractionated with acetone to obtain the ethyl acetate and water fractions. The acetone fraction was evaporated into a thick ethyl acetate (Merck, 02851) around 200 mL fraction using a rotary evaporator.
The water fraction was further fractionated with methanol to obtain the methanol and remaining fractions. The ethanol fraction was evaporated in a thick fraction of methanol using a rotary evaporator. The residual fraction was taken as 5% to determine its dry weight of the residual fraction. Each viscous extract of the fraction was tested for cytotoxic activity.
The TLC test using silica gel stationary phase and 10 mL n-hexane (Merck, 1.04367.2500) and 10 mL ethyl acetate (Merck, 02851) (1:1) mobile phase was performed using sulfuric acid anisaldehyde stain. Sapogenin is indicated by purple-red staining. Two milligrams of sea cucumber extract was dissolved in methanol (0.5 mL), and the extract solution was photographed on a silica plate with a length of 5 cm and a width of 4 cm. The solvent combination of n-hexane:ethyl acetate (1:1) was used as the eluent to fractionate the extract. Bottling was performed at a distance of ±1 cm from the bottom of the KLT plate, using a capillary tube.
The 5 cm long plate was eluted by placing it vertically in a glass cup. The elution process was stopped when the eluent reached ¾ of the KLT plate. The separation stains were observed by spraying reagents on a 2-hole drop plate, and 10 mL concentrated sulfuric acid (Supelco, 100731) was added to another hole to show terpenoid/steroid compounds in the fraction. The KLT parameter is the retention factor (Rf ), which is the ratio of the distance traveled by the solute to that traveled by the mobile phase.
Preparation of culture media
Cell culture medium was prepared by mixing 10 ml of 10% PBS (Sigma, P5119) with 0.5 ml of 0.5% fungizone (Merck, 71375) and 2 ml of 2% penstrep (Merck, P4458) added to 100 ml DMEM solution. The culture medium was then stored at 4°C.
Cell preparation
Inactive cells in cryotubes were collected from a liquid nitrogen tank at -85°C, immediately thawed at 37°C, and then sprayed with 70% ethanol (Merck, 02851). The cryotube was opened and the cells were transferred into a sterile conical tube containing 10 ml DMEM (Sigma-Aldrich, F0415).
The cell suspension was centrifuged at 750 rpm for 5 min and the supernatant was discarded. The pellet was added to 5 ml DMEM growth medium, resuspended until homogeneous, and the cells were grown in small tissue culture flasks (2-3 pieces). The cells were then incubated in a 5% CO2 incubator at 37 oC. After 24 h, the medium was replaced, and cells were grown again until confluence and sufficient for the study.
Cell harvesting
After the number of cells was sufficient, the medium was discarded, and the cells were washed by adding PBS solution and gently resuspended; the solution was discarded, and the cells were added to 700 μl of 0.05% trypsin (Sigma-Aldrich, 650345-1MG).
solution and placed in a CO2 incubator for 5-10 minutes so that the trypsin worked properly. Cells were observed under an inverted microscope to ensure that they had been released from the tissue culture flask. After the cells were released, 5 ml of the culture medium was added to stop the 0.05% trypsin reaction. The cells were transferred into a sterile conical tube, and 10 ml PBS was added. Furthermore, 10 μl was taken, and the number of cells was counted using a hemocytometer. A certain amount of culture medium was added to the cell suspension to obtain the required cell concentration (1.5 × 104 cells per 100 μl) and was ready for use in the cytotoxic test.
Preparation of test solution
The sample stock solution was prepared by dissolving 20 mg of the sample in 100 μl of DMSO and adding 900 μl of the culture medium. Thus, a concentration of 20,000 μg/ml was obtained. The test concentrations were 120; 60; 30; 15; 7.5; 3.750; 1.875; 0.9375 μg/ml. Concentration series were prepared in small tubes and transferred to 96 microplate. All of these processes were carried out in a laminar airflow cabinet.
Cytotoxic test
Cell suspension in culture medium as much as 100 μl (density of 1.5 × 104 cells/well) was placed into a 96-well plate and the plate was incubated for 24 h in a 5% CO2 incubator. Then, 100 μl of the sample was added to the medium in each well so that the final levels of the sample were obtained at various levels (120, 60, 30, 15, 7.5, 3.750, 1.875, and 0.9375 μg/m). Next, the plate was incubated in a 5% CO2 incubator for 24 h, the medium was discarded, and 110 μl of a mixture of culture media and MTT (Merck, M2003) 5 mg/ml was added (100 μl culture medium + 10 μl MTT 5 mg/ml). The cells were then incubated in a CO2 incubator. After incubation for 4 h, 100 μl/well of 10% SDS was added, and the mixture was shaken for 5 min. The plate was then incubated for 24 h at room temperature to dissolve formazan, which is the result of the reaction between the mitochondrial enzymes of living cells and MTT. The absorbance of each well was measured using a spectrophotometric microplate reader at 570 nm.
Bioactive compounds were identified to qualitatively determine the content of secondary metabolite compounds in the extract of Paracaudina australis. The test results were in the form of colors that were adjusted to the color standards of each reagent. The test results of the secondary metabolite compounds in the n-hexane, ethyl acetate, and ethanol extracts are presented in Table 1.
The results of testing the secondary metabolic compounds in each extract revealed the presence of flavonoids, steroids, terpenoids, and saponins. Compounds that are thought to have potential as anticancer agents in sea cucumber extracts include steroids, terpenoids, and saponins. Doughari (2012) stated that steroids and triterpenoids in sea cucumbers are anti-inflammatory, anticancer, tranquilizers, and insecticides. These compounds were detected in the n-hexane and ethyl acetate extracts, which were then tested for their TLC profiles.
The n-hexane and ethyl acetate extracts of sea cucumber berunok that were tested by the TLC method were fractionated by the Vacuum Liquid Chromatography (VLC) method ( Table 2) by as much as 10 g. The main purpose of fractionation is to simplify the composition and homogeneity of substance properties so that they can be easily isolated into single compounds or pure substances (Djamal, 2011).
VLC is a method in which the stationary phase used is silica gel 60 (230-400 mesh) with a mobile phase in the form of an eluent with a gradient system whose polarity is increased starting from the eluent of n-hexane, ethyl acetate, and methanol. The VLC technique is carried out with a system that works under continuous vacuum conditions, such that the maximum packing density is obtained, or by using low pressure to increase the flow rate of the mobile phase. The sequence of eluents used in vacuum chromatography begins with eluents that have a low level of polarity, and then the polarity is slowly increased (Hostettmann et al., 1995).
Furthermore, thin-layer chromatography (TLC) was carried out to determine the components of chemical compounds present in the sea cucumber berunok isolates, using ethyl acetate:methanol (1:1) and n-hexane:ethyl acetate (1:1) eluents. Observations were made under a UV lamp λ254 nm and λ366 nm (Gandjar and Rohman, 2007). A good standard Rf value is 0.2-0.8 (Rohman, 2009) Several strains can be observed in the following Figure 1.
The GC-MS test results for isolate F7.8 showed the highest peak at a retention time of 31.740. Fragmentation results with an area of 33.89 showed that there were several compounds contained in isolate F7.8 including Dihydrocholesterol and Cholestan-3-ol (C27H48O) with a molecular weight of 388.7. Next, an HPLC test was performed, and the results showed that there was only one peak in the HPLC graph.
The IR (KBr) spectrum presented in Figure 4 shows twin and weak absorption bands at wave numbers 3440.86 cm-1 and 3369.38 cm-1 for the primary amine group. The absorption bands at 2953.39 cm-1, 2926 cm-1 and 2864.76 cm-1 indicate aliphatic C-H absorption. The absorption band at 1629 cm-1 shows C=C sp2. The absorption band at 1443 cm-1 shows the sp2 aryl group. The presence of a carbonyl ester group is indicated by sharp absorption bands at 1746 and 1629 cm-1. The vibration of the ether bond was indicated by a strong absorption band at 1036 cm-1. Based on the results of FT-IR identification, the combined fraction (7.8) is thought to contain steroid compounds which can be seen in the absorption wave number 3440.86-33369.38 cm-1 which is thought to produce free OH functional groups which are supported by the absorption of secondary OH functional groups. The presence of C-H absorption at wave number 2953.39 is supported by symmetric -CH2 absorption at wave number 2854.76. There is moderate-weak intensity at wave number 1746.88 indicating the presence of C=O bonds. The typical absorption band for the steroid group also appears at strong intensity at a frequency of 1443.82 cm-1 indicating the presence of a C-H group and at a frequency of 1370.91 with a functional group -C (CH3)2 or commonly known as dimethyl gem (Astuti et al., 2014). Steroid compounds themselves are non-polar because they are composed of isoprene, but steroids also have a more polar -OH group which is also called sterol.
The cytotoxicity test of sea cucumber fraction on T47D cancer cells showed that the ethyl acetate fraction using doxorubicin as a comparison, where the IC50 value of the ethyl acetate fraction was 193.184 μg/mL, control cells was 34.4807 μg/mL and doxorubicin was 0.5479 μg/mL.
Based on the IC50 results of isolates F4 and F7,8, which showed the results of anticancer testing, the isolates were classified as active. Inayah et al. (2012) reported that the active compounds of sea cucumber H. scabra can inhibit MCF-7 breast cancer cells and HeLa uterine cancer cells with IC50 28.03 and 18.09 μg/mL. Nursid et al. (2019) reported that B. marmorata and Holothuria atra had the highest cytotoxicity against T47D cells, with IC50 values of 28.1 and 23.0 μg/mL. Putram et al. (2017) examined the IC50 of n-hexane, ethyl acetate, and methanol-water fractions of H. atra sea cucumbers against HeLa and MCF-7 cells using a cytotoxic test. They found that the methanol-water fraction was more toxic to HeLa and MCF-7 cells than the n-hexane and ethyl acetate fractions. Nimah (2012) reported that the cytotoxicity test for H. scabra on the n-hexane fraction had an IC50 value of 62.86 μg/mL, an ethyl acetate fraction of 43.56 μg/mL and a methanol-water fraction of 18.85 μg/mL.
The Anticancer activity of MCF-7 cells in Holothuria nobilis Selenka showed an IC50 value of 0.82 μg/mL, compared to doxorubicin with an IC50 value of 1.32 μg/mL (Zhang & Zhu, 2017). The IC50 value was calculated by interpolation using the aforementioned equation to produce IC50 value of 17.25 μg/ml, 15.11 μg/ml, 13.32 μg/ml. The IC50 value was calculated by interpolation to obtain an IC50 value of 2.55 μg/ml. Based on the research of Dhinakaran and Aaron (2014), the hexane extract from sea cucumber meat and innards had an IC50 value of and 42.5 g/mL for HeLa cells, whereas the extract with ethyl acetate from the body had an IC50 value of 98.3 μg/mL.
Based on the National Cancer Institute, an extract is declared active and has anticancer activity if it has an IC50 value < 30 μg/ml and moderately active if it has a value of 30 < IC50 100 μg/ml (Wijaya, 2015). The results showed that isolates F4 and F7.8 from the berunok sea cucumber fraction were active against T47D cells with IC50 values of 25.3 μg/ml and 13.76 μg/ml. Cytotoxicity tests showed that several sea cucumbers had good cytotoxicity against T47D cells, where Holothuria atra and Bohadschia marmorata showed strong cytotoxicity with IC50 values of 23.0 and 28.1 ug/mL (Nursid et al., 2019).
Doxorubicin has a stronger IC50 value than T47D cells because doxorubicin is a chemotherapeutic agent in the anthracycline class, which has broad-spectrum anti-cancer activity and has been used in various types of cancer. The use of doxorubicin as a chemotherapeutic agent is limited by its toxic effects on normal tissues, especially the heart, and suppression of the immune system. Reducing the dose of doxorubicin can reduce side effects (Wattanapitayakul et al., 2005). Anticancer drugs and healthy commercial ingredients derived from marine invertebrates provide diversity for pharmaceutical research on marine biodiversity (Senthilkumar et al., 2013).
These compounds may be responsible for the anticancer activity of P.australis. The IC50 of Isolate F4 was 25,3 μg/ml, and IC50 of Isolate F7,8 was 13,76 μg/ml.
Mery Sukmiwati as Writing – original draft, Writing – review & editing
Susilawati as Formal Analysis
Noveri Rahmawati as Methodology
Deri Islami as Visualization
Zenodo: Identification and Testing of Cytotoxic Activity of Bioactive Compounds of Berunok Sea Cucumber (Paracaudina australis) Against Breast Cancer Cells (T47D), [Dataset], https://doi.org/10.5281/zenodo.10690480 (Sukmiwati et al., 2024a).
Zenodo: Figure 1. Results of Thin-Layer Chromatography (TLC) testing of Isolate (a) F4, (b) F7,8 at UV lamp λ254 nm [Images], https://doi.org/10.5281/zenodo.10780589 (Sukmiwati et al., 2024c).
This project contains the following underlying data:
• Images from Figure 1. Results of Thin-Layer Chromatography (TLC) testing of Isolate (a) F4, (b) F7,8 at UV lamp λ254 nm
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Zenodo: The ARRIVE guidelines 2.0: author checklist Animal Research: Reporting in vitro Experiments, https://doi.org/10.5281/zenodo.10602918 (Sukmiwati et al., 2024b).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors thank the Director of the Research Institute and the Extension University of Riau Indonesia for their financial support.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Prabhakar, P., Mukherjee, S., Kumar, A., Rout, R. K., Kumar, S., Verma, D. K., ... & Banerjee, M. (2024). In Silico, In Vitro and Ex Vivo Evaluation of the Antihyperglycaemic, Antioxidant and Cytotoxic Properties of Coccinia grandis L. Leaf Extract. Food Technology and Biotechnology, 62(2), 188-204.Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Phytomedicine, Food Science and Technology, Phytochemical Extraction, Purification, and Identification
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 2 (revision) 08 Dec 25 |
read |
|
Version 1 23 Apr 24 |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)