Keywords
Head and neck cancer in children, Radiation therapy, Primary teeth, Silver diamine fluoride, Microhardness of primary teeth
This article is included in the Datta Meghe Institute of Higher Education and Research collection.
Head and neck cancer in children, Radiation therapy, Primary teeth, Silver diamine fluoride, Microhardness of primary teeth
A large number of children around the world suffer greatly from cancer, and it is considered one of the most fatal diseases known to mankind (more information here).
Although head and neck tumours in children are very uncommon, they have been on the rise in the recent years and becoming more and more common.1 Head and neck cancers (HNC) make for 5% of all pediatric cancers.1 Radiation therapy (RT) is a crucial component of the multimodality approach to treating HNC in children.1 Complex considerations in treatment planning may be necessary depending on a number of variables, such as the location, tumour size, and molecular features.1 Treatment results in direct and indirect harmful effects on the oral cavity, one of the most severe being radiation induced caries.2
Post-radiation caries advance quickly and are quite damaging to the dental tissue.3 All the common side effects can manifest within the first three months of RT.3 Xerostomia induced due to radiation of the head and neck is one of the most common adverse effects seen which causes alteration in the saliva production which in turn affects the oral flora causing increase in the incidence of caries.3
The increased amount of caries incidence could also be due to the direct effect on the mechanical and physical properties of the teeth.2 Surface hardness, mineral and organic contents of enamel and dentine respectively with carbonate, phosphate, hydrocarbons and amide are all shown to be reduced after RT.2 The rods in primary teeth are thinner and denser, there are many micro porosities, some of the rods are exposed, and there is a significant amount of carbonate addition. Due to all of these circumstances, primary teeth are extremely vulnerable to radiation caries and develop severe carious lesions.2 Therefore, all efforts to manage such type of patients with severe caries should be redirected towards prevention. Effective preoperative dental care, routine dental examination, and regular topical fluoride administration can help in solving this problem.4
SDF is an effective agent in remineralising initial carious lesions and arresting advanced caries.5 Streptococcus mutans and lactobacilli respond favourably to its antibacterial activity, demonstrating its anticariogenic effects.5 Its mechanism of action includes piercing the bacterial cell wall causing the impairment of deoxyribonucleic acid (DNA) replication and cell wall damage by the silver nitrate nanoparticles. Hence it prevents further demineralization of the already carious lesion.5 Staining caused by SDF on the tooth and adjacent structures is a significant drawback.6 However, SDF has been proved to increase the microhardness of the tooth in various in vivo and in vitro studies.5,7
Children with HNC have to undergo frequent radiation exposure compromising the oral health and causing extensive carious lesions.2 For such patients comprehensive dental treatment may be required. Due to such issues, primary teeth enamel of such children can be protected by pre-treating with SDF application prior to radiation exposure. Considering the beneficial effects of SDF the current study was undertaken to evaluate and compare the effect of RT on microhardness and surface morphology of pre-treated primary teeth with SDF and without any pre-treatment.
The sample size was calculated using 95% probability, showing a statistically significant difference using the alpha level and power 80% by using nMaster software (Version 2.0), Rstudio (RRID:SCR_000432) may be able to perform similar tasks and is available for free. It was calculated using the formula for Qualitative data with the mean and standard deviation obtained from previous research. The formula utilised where Zα is the level of significance at 5% level of significance i.e. 95% confidence interval, Zβ is the power of test, the is the standard deviation of control group,is the standard deviation of SDF group and being the difference between the mean of the two groups. According to this formula n was determined as 10.35 per group. So, the minimum sample size calculated was 11 for each group and total 22 samples were taken.
Inclusion criteria was caries free primary teeth and primary teeth nearing exfoliation. Exclusion criteria was carious primary teeth and primary teeth with developmental defects and cracks. Tooth samples were collected from the patients who visited the department of Pediatric and Preventive Dentistry and had to undergo extraction. Teeth were not specifically extracted for this study but which were indicated for extraction. Written informed consent was taken from the parents of the patients before extraction. Out of these, samples which met the inclusion criteria were included in the study.
A total of 22 freshly extracted or exfoliated primary teeth were taken from children ranging in the age of 6-12 yrs. 5.25% sodium hypochlorite (Prime dental) was used to clean the primary teeth by soaking for one minute and then ultrasonic scaling (Woodpecker UDS-P ultrasonic scaler) was performed to remove any debris on the surface of the teeth. The samples were kept immersed in distilled water for no more than three months before the study was carried out.
The tooth samples were divided randomly into two groups of 11 samples each. Group 1: the SDF group and group 2: the control group. The primary teeth samples were cut 2mm below the cemento-enamel junction (CEJ) with diamond disks (Brasseler USA, Savannah, GA) and high speed handpiece (Air Rotor) (NSK, Japan). They were then mounted in self-cure acrylic resin using circular metallic moulds of 1.5-centimeter (cm) diameter and 1.5 cm height (Circle Frame Die Cut, Topiky), and the buccal surfaces of the teeth were exposed. An area of 5 × 5 mm was exposed on the buccal surface of all the samples. Polishing of the mounted teeth was carried out using silicon carbide paper of 200, 600, 800, and 1200-grit sequentially for one minute each to obtain a smooth surface on all sides except on the exposed buccal surface of the primary teeth. They were then stored in artificial saliva of pH7.0 containing 0.9 mM PO4, 1.5 mM Ca and 150 mM KCl in 20 mM Tris buffer for 37 c. To achieve this ratio 122.48 milligram (mg) of monopotassium phosphate (KH2Po4),220.53 mg of calcium chloride (CaCl2), 11.175 gram (gm) of KCl and 2.422 g of Tris buffer was used in 100 ml of deionized water. All the quantities were measured using balanced weighing machine (ADVENTURER ANALYTICAL, AX124/E, OHAUS). The pH of the artificial saliva was adjusted to 7 using digital pH meter. Standardized instructions were followed from the manufacturer for SDF (Kids-e-Dental) application before radiation therapy.
All the 22 samples were exposed to a daily fraction of 2 Gy each day for five days, followed by a two-day interval for six weeks resulting in a total dose of 60 Gy. A total of 30 daily fractions were completed. This protocol was developed in the “American College of Radiology (ACR)” and is traditionally used for patients suffering from Hodgkin’s lymphoma.8
The irradiation process was carried out in a cancer hospital with the facility of RT. The samples were irradiated on a Vital Beam (Life saving radiotherapy linear accelerator) (Varian Medical System international, India) with photon energy of 6 megavolt (MV) at a distance of 100 cm from the surface of the samples with 15X15 cm2 field size. The monitor units were calculated for 2 Gy per fraction (Figure 1 and 2). A uniform irradiation was received by all the samples of both the groups. After every fraction of 2 Gy of radiation, the samples were placed in new artificial saliva which was renewed daily. All the samples were then transferred in an incubator (Sesw 28 L bacteriological incubator) at 37o C to stimulate the normal body temperature of an individual.
Surface microhardness (SMH) of the samples was tested by a Vickers microhardness testing machine (MITUTOYA, Japan) under a load of 50 grams for 10 seconds. The microhardness for each sample was assessed at five different sites within the sample, 300 μm apart from each other and their mean was considered as the final microhardness for that particular sample. Two representative samples from each group were prepared to be evaluated using a SEM (JEOL JSM-6380A). The samples were scanned at power of 100X, 500X and 1000X magnification and photomicrographs were taken to observe the structural changes in the enamel.
Descriptive and analytical statistics were done. The data was represented in mean, median and standard deviation. The normality of continuous data was analyzed by Shapiro-Wilk test. A nonparametric test called the Mann-Whitney U test was used which enables two groups to be compared without assuming that the data are regularly distributed. SPSS software (Statistical Package for Social Sciences) Version 24.0 (IBM Corporation, Chicago, USA) (RRID:SCR_002865) was used. The p value <0.05 was considered to be statistically significant.
In Figure 3 the mean microhardness values after RT of the control and SDF group is compared27,28. It was found that there was statistically significant difference (p<0.001) in mean microhardness values between the two groups. The mean microhardness of the SDF group (254.07 ± 35.84 Vickers pyramid number) was significantly higher that the control group (88.18 ± 6.79 Vickers pyramid number).
SEM images29 of SDF samples at 100X (Figure 4) revealed a surface that was smooth and had less dentine collagen fiber exposed. At 500 X (Figure 5), some amount of surface roughness was seen. At 1000X (Figure 6) showed dense granular structures of spherical grains. Such appearance is visible only in sample viewed under high power magnification. No evident discontinuity was observed on the enamel surface.
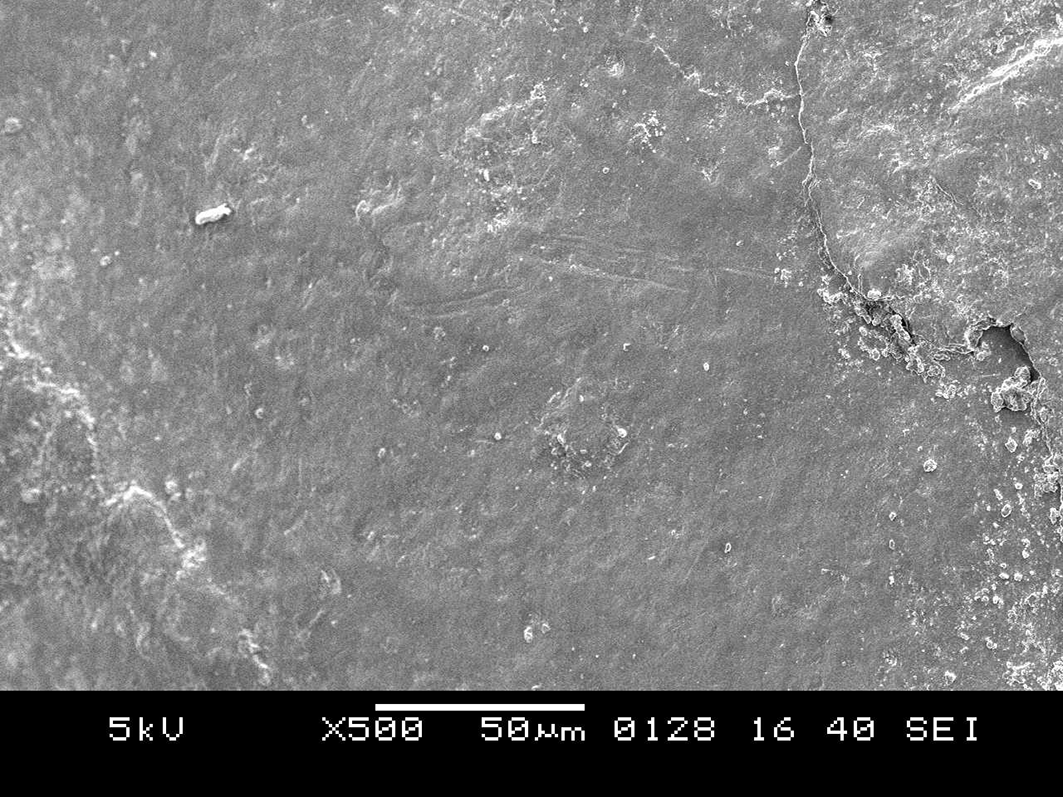

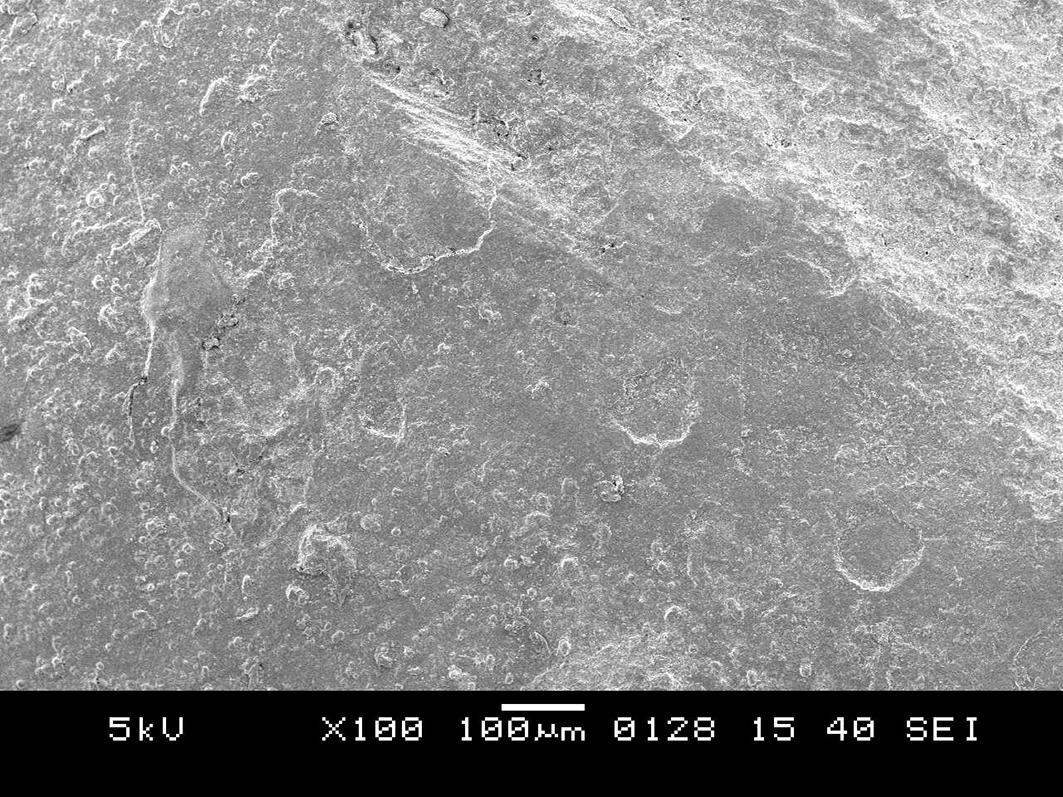
SEM images of the control group samples at 100X (Figure 7) showed discontinuity of the buccal contour due to exposure from radiation. At 500X (Figure 8) showed progressive morphological alterations on the enamel surface. The enamel surface showed surface irregularities and cracks. SEM images of the control group samples at 1000X (Figure 9) indicated that the peritubular structure was gradually destroyed, which caused the tubules to gradually get obliterated. It also showed amorphous structures. After comparing Figure 6 and Figure 9, it was found that the SEM image of the enamel of the control teeth displayed loss of smooth homogeneous surface which was very evident in comparison to the SDF group. More amorphous structures were seen in the SEM images of the control group than the SDF group.
Topical fluorides to prevent radiation caries in patients HNC has been suggested by various authors.9,10 Kielbassa AM et al (1997)11 and Fränzel W et al (2006)12 recommended the biannual or quarterly use of 1.25% and 5.6% of NaF varnish respectively. Use of 1% neutral NaF gel has been suggested by Dreizen S et al (1977)9. Together, these studies showed how fluoride therapy can be used to prevent radiation caries, and dentists should always recommend its use while dealing with patients of HNC. The positive results obtained from the present study that is the mean microhardness of the SDF group being significantly higher from the control group suggest fluoride application in the form of SDF in preventing radiation caries in children.
Both an antibacterial and remineralising agent, SDF is a solution consisting of silver nitrate and fluoride. By lowering biofilm counts and preventing collagen deterioration, the product encourages the remineralization of enamel and dentin, halting the spread of caries which in turn arrests the carious lesion and its progression.13 In cases where other options aren't accessible, SDF can be used to control caries. To combat fear and anxiety in very young children to extensive dental treatment, SDF which is a low-cost simple method, can be used as a conservative treatment. It has therefore grown its acceptance in caries management.13
Chu CH et al (2008)7 in his ex vivo study found that SDF aided in prevention of progression of arrested coronal caries in primary teeth of preschool children and it also showed potential to promote enamel SMH, decrease enamel surface mineral loss, and inhibit cariogenic growth.7 Their results are in accordance to the current study in which the enamel SMH of the SDF group were greater than the control group. Findings from the study of Prakash M et al (2021)14 suggested that single application of SDF treated tooth is beneficial in increasing the microhardness of dentin surfaces as compared to non-SDF treated surfaces. But in their study SDF in combination with Potassium iodide (KI) was used which could improve carious dentin microhardness closer to that of a sound dentin surface in comparison to 38% SDF.14 However, in the present study a single application of commercially available SDF solution was used without the combination of KI.
A tooth's mineral composition has a direct impact on the changes seen in the microhardness.15 It was reported in the study of Li YJ et al (1984)16 that the microhardness of demineralized enamel and dentine increased after SDF treatment. When treated with SDF, silver chloride and silver phosphate were reportedly almost completely insoluble or only slightly soluble and were found on the dentine surface from various studies.16 These precipitates suggest that a layer of insoluble protection was formed to stop demineralization of enamel and dentine also further losing calcium and phosphorus. Another ex vivo study by Mei ML et al (2014)17 reported that after the application of SDF, a dense and highly remineralized surface layer was discovered that was abundant in calcium and phosphate.17 This is how SDF treatment hardened the surface of the arrested lesions.
Considering the beneficial effects of SDF towards the microhardness of the teeth. The present study had planned to pretreat the surfaces of primary teeth before exposure to RT.
Several studies have been conducted using permanent teeth to evaluate the effect of radiation exposure in the context of oral cancer.3 But in the present study primary teeth were considered as children are likely to undergo radiation exposure at 6-12 years old. For such an age group both sets of teeth are present in the oral cavity.2 The primary teeth have thin and aprismatic enamel with irregular enamel rods which further can get affected with structural disturbances while undergoing radiation exposure.2 Hence only primary teeth were selected for the study.
Lu H et al (2019)18 and Gonçalves LMN et al (2014)19 observed a reduction in the enamel microhardness close to the dentinoenamel junction region, while de Siqueira Mellara T et al (2014)20 observed, first, a decrease and then an increase in microhardness. No changes have been reported by some studies,21,22 while others recorded an increase in microhardness,19,20 and some have reported decreases in microhardness21,22 after RT. In the current study, a statistically significant difference (p<0.001) was observed in the enamel of primary teeth pretreated with SDF compared to the control group where no pretreatment with SDF was done. A 188.12 % increase in microhardness was seen with SDF group when compared to control group after RT.
After six weeks treatment of radiation exposure primary teeth pretreated with SDF samples had values of 254.07 mean Vickers pyramid number which is more when compared to a study by Mante FK et al (2022).23 Variation in results with respect to study by Mante FK et al (2022)23 is because in their study they had used permanent teeth samples. But the present study had used primary teeth samples. The difference in microhardness values can be attributed to morphological differences in primary teeth enamel as compared to permanent teeth enamel.
Contrary to other studies on permanent teeth, which either found that the microhardness of irradiated enamel is lower than that of nonirradiated enamel24 or that the microhardness is unaffected by radiation.22,10 The current study, however used primary teeth, and it is possible that they would react to RT differently. Ionizing radiation may cause mineralized tissues' crystal structures to reorganize, which would change their physical properties, including structural microhardness, according to studies using permanent teeth.20
SDF is said to form a coating with a high silver content when applied on enamel, deposits of silver were discovered on the surface penetrating into a depth of 20–40μm.25 Other researchers have shown that SDF treatment causes silver to be incorporated into hydroxyapatite, and it may help to enhance surface hardness.12,17 Surface hardness may rise as a result of rapid production of fluorohydroxyapatite due to SDF's high fluoride content (44,800 ppm).17 The ability of teeth treated with SDF to resist demineralization, stop the progression of caries, and simultaneously inhibit the development of new caries can be explained by the significant increase in enamel hardness. Because of this, SDF is more effective than other caries preventative treatments in preirradiated patients, such fluoride gels containing 5% NaF (22600 ppm) and stannous fluoride (1,100 ppm).23 However, the blackish stain formed after the application of SDF may encourage its application only on the posterior teeth.23
The orientation of the prisms that make up tooth enamel determines its anisotropic performance and impacts its mechanical properties. As previously documented for bovine teeth,22 SEM revealed morphological changes in the enamel structure, which were characterized by a more disordered prismatic structure as the cumulative dosage of radiation grew to 60 Gy. This change in the crystalline structure of the enamel is one of the possible reasons for the increased risk of dental caries following RT.20 Taking these changes in consideration, SDF application was done in the current study to prevent these ill effects from occurring which was not done in the previous studies.
The SEM images of the enamel surface of the SDF pre-treated tooth samples showed a relatively smooth surface with few dentine collagen fibres exposed. Dense granular structures of spherical grains were found at higher magnification of 1000X. The control group's contour discontinuity was more obvious than it was in the SDF group. The SDF-pretreated enamel showed no obvious discontinuity. Prisms were clearly structured and surrounded by interprismatic zones in the enamel of the SDF samples.
The buccal contour was disrupted in the enamel without any previous treatment. Images obtained through SEM examination revealed both enamel and dentin to have undergone gradual morphological changes. After 60 Gy, superficial fractures on the enamel surface were visible. The tubules were gradually destroyed as a result of the peritubular structure degrading over time. Loss of smooth homogeneous surface is very evident in comparison to the SDF group. It also showed amorphous structures.
The interprismatic area of enamel, is where the initial radiation damage exposes itself, despite the fact that the majority of the enamel's composition is inorganic. Hydrogen peroxide and hydrogen free radicals are created during the oxidation of water molecules, and they denature the organic components.26 As a result, the integrity and mechanical characteristics of the enamel are impacted. The prismatic structure of the enamel was shown to have been affected by irradiation in the current investigation, suggesting that changes to both the organic and inorganic chemicals in the enamel are what cause the clinically recognizable radiation effects. Nevertheless, the measured abnormalities in the tissue's mechanical and physical properties in the current study imply that the disturbance of the enamel and its SMH is a contributing component in the clinically reported alterations.
Several limitations exist in this study as evaluation of the SMH would have hampered the SDF application on the samples for example: SMH before RT and after application of SDF was not evaluated, change in SMH after each interval of weekly radiation exposure within six weeks schedule was not evaluated, change in the ionic concentration such calcium, phosphate and bicarbonate ions after RT and SDF application was not evaluated.
Based on the observations and results of the current study it can be drawn that the SMH of primary teeth pretreated with SDF after RT was significantly higher than normal primary teeth without any pretreatment. SEM analysis of primary teeth pretreated with SDF after RT revealed smooth homogeneous surface and well-formed enamel prism. Within the limitation of the study, it can be concluded that SDF significantly improved the SMH and morphology of the primary teeth. Therefore, it is recommended that more research be done in order to completely understand how fluoride treatments work to support the restoration of the physical properties of teeth following RT for oral cancer.
In the current in-vitro study institutional ethical committee clearance was obtained from “Datta Meghe Institute of Higher Education and Research (Deemed to be University), Sawangi (Meghe), Wardha” with reference number DMIMS (DU)/IEC/2020-21/9413. Written informed consent for study and data publication was taken from the parents of the patients whose tooth samples were used for the study.
Zenodo: Microhardness for SDF group after Radiation Therapy. https://doi.org/10.5281/zenodo.7744176. 27
This project contains the following underlying data:
Zenodo: Microhardness of control group teeth after Radiation Therapy. https://doi.org/10.5281/zenodo.7744252. 28
This project contains the following underlying data:
Zenodo: SEM image of control group and SDF treated teeth after radiation therapy
https://doi.org/10.5281/zenodo.8072843. 29
This project contains the following underlying data:
- 1 CONTROL 1000X.jpg
- 10 CONTROL 100X.jpg
- 11 CONTROL 300X.jpg
- 12 CONTROL 500X.jpg
- 13 CONTROL 1000X.jpg
- 14 CONTROL 5000X.jpg
- 15 CONTROL 100X.jpg
- 16 CONTROL 500X.jpg
- 17 CONTROL 1000X.jpg
- 2 CONTROL 500X.jpg
- 3 CONTROL 1000X.jpg
- 4 CONTROL 500X.jpg
- 5 CONTROL 100X.jpg
- 6 CONTROL 500X.jpg
- 7 CONTROL 1000X.jpg
- 8 CONTROL 5000X.jpg
- 9 CONTROL 27X.jpg
- SDF 1000X 16.jpg
- SDF 1000X 4.jpg
- SDF 1000X 6.jpg
- SDF 100X 1.jpg
- SDF 100X 9.jpg
- SDF 300X 2.jpg
- SDF 5000X 5.jpg
- SDF 500X 7.jpg
- SEM 1000X 11.jpg
- SEM 100X 10.jpg
- SEM 100X 13.jpg
- SEM 300X 14.jpg
- SEM 500X 12.jpg
- SEM 500X 15.jpg
Zenodo: STROBE Checklist. https://doi.org/10.5281/zenodo.7766253. 30
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
This research was supported by Datta Meghe Institute of Higher Education and research, Sawangi (Meghe), Wardha. Various facilities like RT and central laboratory containing incubator were utilised in the institution.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Restorative dentist
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Operative dentistry, Dental materials, Caries, Esthetic dentistry
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 23 Apr 24 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)