Keywords
starch, sago, corn, hydroxypatite, bone replica, 3d, biomaterials, design, manufacturing, artificial, porosity, density, phase
One of the bone implant candidate materials that have similarities to the original bone is porous Hydroxyapatite (HA) based 3D bone replica. Most previous studies primarily focused on the manufacture of porous ceramics in a form that is still far from the original appearance of bone. Manufacturing the femur bone with a shape that resembles the original is expected to be closer to the application. The characteristic of the replica produced have a shrinkage range of 34,28–47.46 %, a porosity range of 18,89-37.75 %, a density range of 1.97-2,56 gr/ cm3, a bending strength range of 0.33-6,49 MPa, and pores sizes of 80-230 µm. Based on the findings, HA scaffolds for bone replicas were successfully created and incorporated in physical and chemical features.
starch, sago, corn, hydroxypatite, bone replica, 3d, biomaterials, design, manufacturing, artificial, porosity, density, phase
Porous ceramic morphology can be formed through several methods, using air or direct foaming,1,2 protein and starch in protein foaming-consolidation,3 templates impregnated by suspension of biomaterials,4 or starch consolidation. The use of starch has several advantages, that is, it is easily combustible, has a low price, is environmentally friendly, and can produce ceramics with evenly distributed pore distribution.5
Porous ceramic processing approaches have focused on the formation of porous materials, such as fiber bonds, which utilized gambas fiber as a template,6 banana stems,7 as well as protein foaming-consolidation.8 The prototype produced from previous studies has limitations for mass production because the shape and size do not have a similar structure to the original bone. Therefore, efforts are needed to produce a prototype that is similar to the original bone structure using 3-Dimensional (3D) mold technology.
Bone is a tissue that serves as a skeleton, supports and protects organs, and connects muscles to allow for movement.9 Damages or flaws in the bones cause these functions to be disrupted, necessitating healing. The use of biomaterials as bone implants has become a popular alternative in recent years as they aid in repairing and rebuilding living tissue that has been injured.10 Ca10(PO4)6(OH)2 is the chemical formula for hydroxyapatite (HA), an apatite compound and the main inorganic component of biological hard tissues such as bones and teeth.11 HA is biocompatible, osteoconductive, as well as chemically and biologically compatible with bone tissue.12 These characteristics make it appropriate for usage as a component of bone and tooth implants.
Dense HA is more resorbable and osteoconductive than the porous type or scaffold which has a substantial surface area. The attachment of living tissue cells and the formation of new bone phases are both aided by these pores.13‐16 Pores on the HA scaffold are essential for the creation of bone tissue, as well as osteoblast migration, proliferation, and vascularization. Furthermore, the porous surface can improve the mechanical bond between the implant and the bone, thereby increasing mechanical stability.16
Drying and sintering are two crucial processes in the production of ceramics to eliminate water from the slurry. Water molecules spread to the surface, where the evaporation process takes place. Meanwhile, sintering is a high-temperature heating technique that increases the material's mechanical strength. This method is commonly used in the manufacturing of a material with a regulated microstructure and porosity. Sintering can be divided into two types namely the solid and liquid phase depending on the material type at the sintering temperature. The material's particle structure will increase (coarsening) and combine to form a unified mass (densification) during sintering.17
A new application of stereolithography (SLA) has been used to develop molds with interconnected porosity. The molds produced were combined with gel-casting ceramics to make calcium phosphate bone replacement materials.18 Gyger et al.,19 used a computer numerical control (CNC) milling machine to produce molds which were then combined with alumina-based gel-casting ceramics using activated carbon as a pore forming agent.
This study aims to create a porous ceramic with similar structure to the original bone. Additionally, the effect of adding sago and corn starch on the physycal properties was evaluated, while the 3D structure of replica bone was fabricated using CNC technology.
The materials used in this study included hydroxyapatite (Lianyungan Kede Chemical Industry co. Ltd., China), sago starch (PT Indofood Sukses Makmur Tbk, Indonesia), corn starch (PT Exspot Chemical, Medan) and palm oil (PT Multimas Nabati Asahan, Indonesia).
This study was divided into several stages, namely making bone replica molds with CNC technology and porous HA structure replica slurry, as well as characterizing the duck bone replicas. The fabrication of 3D-printed duck bone replicas was carried out using CNC technology, while those used as a model was obtained from a local market in Pekanbaru, Riau. Figure 1 shows the process of using a 3D print to create a bone replica beginning with making mold design drawings using CAD (computer-assisted design), followed by G-Code stage performed with master CAM (computer assisted manufacturing). G-code is a language which instructs computerized machine tools how to make something by telling the device’s motors where to move, how fast to move, and what path to follow, and final mold printing using CNC machine with aluminum material.
The preparation of the slurry was initiated by mixing 20 grams of HA powder with 25 mL of distilled water; then starch was added. The specific type of starch used in this study was sago and corn, which were added in increments of 8, 10, 12, and 14 grams. At 400 rpm, the slurry was stirred with the mixture being shaped into a mold that has been lubricated with palm oil as a lubricant and then heated for 30 minutes at 100°C. Afterward, the green bodies were removed from the mold and dried in an oven at 80°C for 24 hours and 110°C for 8 hours. The sample was then dried and placed in the furnace. Combustion took place at a temperature of 600°C, followed by 1 hour of sintering and then the characterization of sintered bodies such as Scanning Electrom Microscopy or SEM, density, porosity, bending test, shrinkage, and XRay Difractometry or XRD.
The shrinkage of a wt% HA scaffold made with a 3D mold was measured during the drying and sintering processes. The addition of 8 grams of sago starch culminated in a larger shrinkage than the additions of 10, 12, and 14 grams in the drying process, while 14 grams caused the most shrinkage in the sintering process. The viscosity of the slurry composition also affected shrinkage. As the starch content increases, the viscosity rises, causing the slurry composition to thicken.
Green bodies experienced shrinkage in the range of 31.65—41.20% due to the drying process as shown in Figure 2. The addition of 8 grams of sago starch produced the highest shrinkage of 41.20%, while 14 grams had the lowest value of 31.65%. The shrinkage of sintered samples ranged from 34.24—48.26%. The highest value of 48.26% was found in samples added with 14 grams of sago starch, while the lowest of 34.24% was produced by 8 grams. Visually, differences were observed in the body of the HA scaffold bone replica produced. The sample added with 10 grams and 14 grams of sago had a wt% shrinkage value of 38.94% and 48.26%, respectively.
Furthermore, there was a decrease in the length of the HA scaffold bone replica that was created, in addition to the difference in the value of w % shrinkage in the sample as shown in Figure 3. A scaffold with a length of 7.4 cm was obtained with the addition of 10 grams sago starch, while 7.2 cm was obtained with 14 grams. Both samples had an initial length of 10 cm before sintering, hence, the length shrinkage for each sample was 26% and 28%, respectively.
Based on the results, corn starch had a shrinkage value similar to sago when used as a pore forming agent. Green bodies' shrinkage ranged from 31.55 to 40.62% due to the drying process as shown in Figure 4, while sintered bodies shrank by 35.40—47.46%. The addition of 14 grams of sago starch produced the maximum shrinkage value of 47,46%, while 8 grams of corn starch yielded the lowest value of 8.46% after sintering.
Shrinkage can also be induced by the loss of starch or the migration of gases trapped in the pore area out to the surface which causes the ceramic to solidify, thereby increasing its mechanical strength. Consequently, sintered bodies containing the most starch will shrink the most, causing the ceramic particles to become more compact and closely joined.17 After the sintering process, the bone reproductions shrank in both size and weight. Green bodies with an average length of 10.2 cm and a tip diameter of 1 cm were fused into sintered bodies with values of 8 cm and 0.7 cm, respectively.
Figure 5 shows that shrinkage of the HA scaffold bone replicas occurred at a wt%. The sintered bodies also shrank in the range of 34.28—39.46%, according to the graph in Figure 5. Bone replicas sintered at 1300°C shrank the most, by 39.47%, 39.02%, and 36.26%, respectively, at sintering rates of 2°C, 3°C, and 4°C/minute. The shrinkage becomes significantly higher as the sintering temperature increases. A previous study20 produced suitable Tri Calcium Phosphate TCPs, with shrinkage of the sample body increasing from 34.91—51.35% when the sintering temperature was increased from 1000—1100°C.
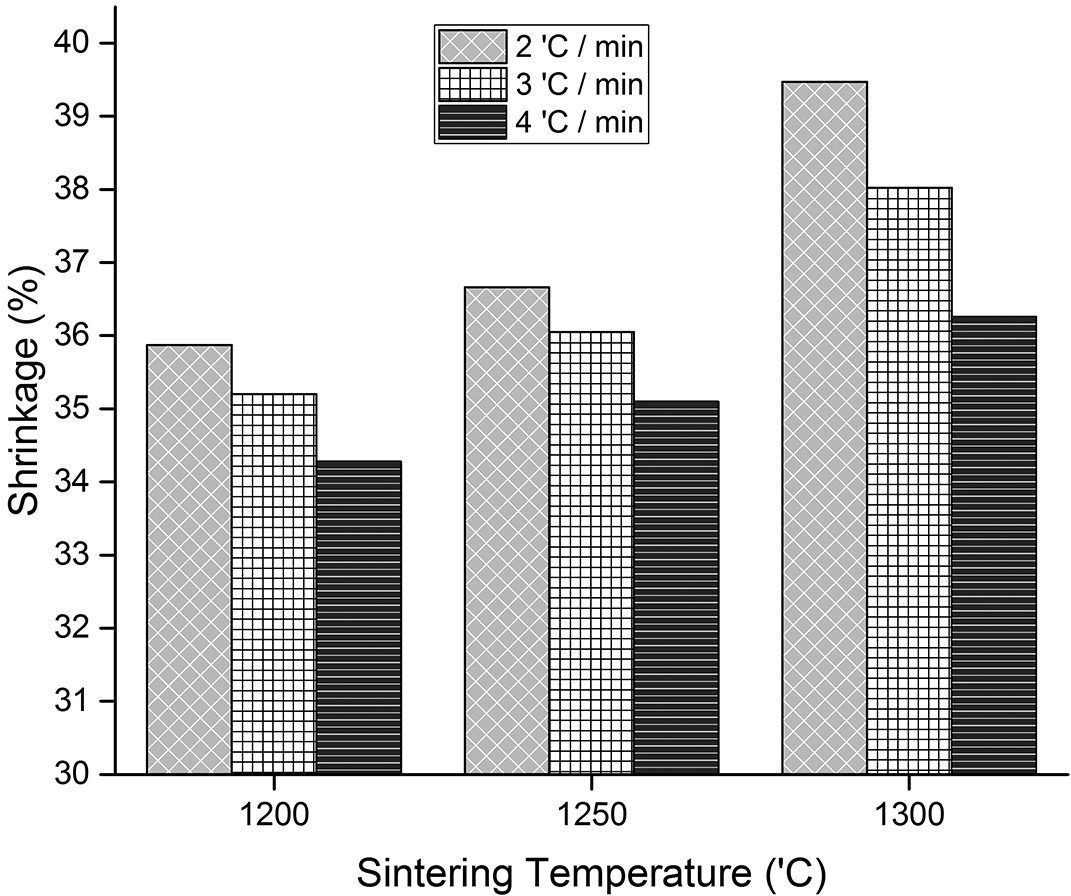
The smallest shrinkage percentage, which is 34.28%, was obtained in the sample sintered at a temperature of 1200°C and the sintering rate of 4°C/minute. The effect is inversely proportional to the sintering temperature, wherein the sample becomes smaller as the temperature increases. This is because the sintering time will be shorter when the rate is increased, thereby making the densification process in the implant smaller.21
The MDS-300 Densimeter was used to determine the density value and Figure 6(a) shows the density of HA scaffold generated using a 3D mold. The density values for the scaffold with sago starch addition ranged from 1.89—2.34 gr/cm3 and 1.97—2.46 grams/cm3 for the corn starch. Figure 6(a) shows that the corn starch has a higher density with 8 grams of HA producing the greatest density value of 2.46 grams/cm3.
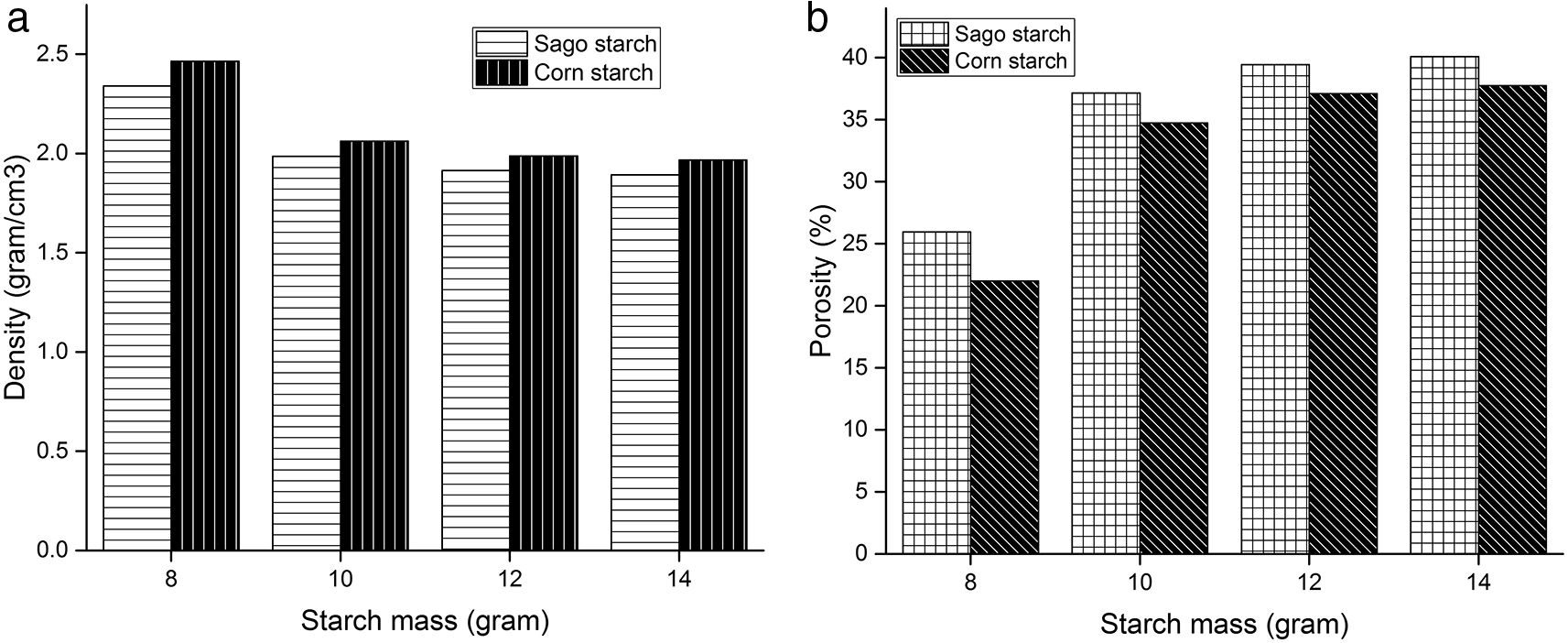
The density of the sample increased as the amount of additional starch was reduced, and this is because the lesser the amount of starch used, the fewer the number of pores generated, causing the mass of the bodies to be heavier. The particles became more compact and hardened (densify) at high temperatures, causing an increase in density. Furthermore, the material's particle structure will expand (coarsen) and coalesce to form a cohesive mass during the sintering process (densification). When the temperature rises, the pressure increases, the particle size decreases, and the sintering time lengthens with the rate of densification.9
Figure 6(b) shows the relationship between the porosity of the HA scaffold and the addition of starch using a 3D mold, while 6(a) demonstrates that the link between porosity and starch addition improved. The porosity of the scaffold ranged from 25.85—40.1% when sago starch was added and 22—37.75 % with corn. The addition of 8 grams corn starch produced the lowest porosity of 22%. Due to the increased swelling process of starch particles in the slurry composition, the porosity of the scaffold increases with the addition of starch.22
The porosity produced with the addition of sago starch was greater than that of corn and this is caused by the larger particle size. The particle size of sago starch is 30 μm while that of corn is 14 μm.23,24 Interparticle densification improved when smaller starch was added to HA.16 The quantity of porosity in the porous HA sample will be reduced throughout the densification or compaction process.25
Figure 7(a) shows that the sample density values ranged from 2.31—2.56 gr/cm3 with the largest namely 2.56 grams/cm3 occurring at a temperature of 1300°C with an increase in the sintering temperature of 2°C/minute. The higher the density, the smaller the pores, culminating in a denser particle structure and lower porosity. A previous study26 demonstrated this by fabricating TCP at 950, 1050, and 1150 °C with densities of 2.27, 2.84, and 3.22 grams/cm3 as well as porosities of 24, 6, and 0% respectively. The porosity of the scaffold improved as shown in Figure 7(b), ranging between 18.89 and 26.93%. The highest porosity was achieved at a sintering temperature of 1200°C and a heating rate of 4°C/minute. This demonstrates that the higher the sintering heating rate, the higher the porosity values, while the lower the temperature, the higher the porosity percentage.
The value of the bending stress contained in the sample decreased with the addition of sago starch as a pore-forming agent. The bending stress in the sample added with sago starch ranged from 0.33 to 1.33 MPa. The sample with the addition of 8 grams sago starch had the highest stress value of 1.33 MPa, and the maximum allowable loading value is 0.4 N. Meanwhile, the 14 grams of sago starch caused the lowest stress value of 0.33 MPa, with the maximum allowable loading value being 0.1 N.
The value of the bending stress in the sample made with corn starch as a pore-forming agent ranged from 0.67 MPa–2.33 MPa. The highest stress value of 2.33 MPa was found in the sample prepared with 8 grams of corn starch and the maximum acceptable loading value was 0.7 N. The lowest stress value of 0.67 MPa was produced by 12 grams of sago starch with the maximum loading value being 0.2 N. Furthermore, the value of the bending stress in the scaffold with the addition of corn starch varied. The addition of 8 grams to 12 grams culminated in a decrease in the value of bending stress, while 14 grams caused an increase.
Based on the results, as the rate of the sintering temperature increased, the maximum bending stress allowable by the sample decreased. This is also due to the decreasing density value at the same variation, causing the densification process to decrease and the bending strength to increase. The sintering temperature is directly related to the maximum bending stress that the sample can withstand, while the rate of increase is inversely proportional to the maximum bending stress. According to a study,27 when the porosity of porous ceramics decreases, the compressive strength increases. Various samples fall into the cancellous bone compressive strength range, which is between 1—100 MPa in general.28
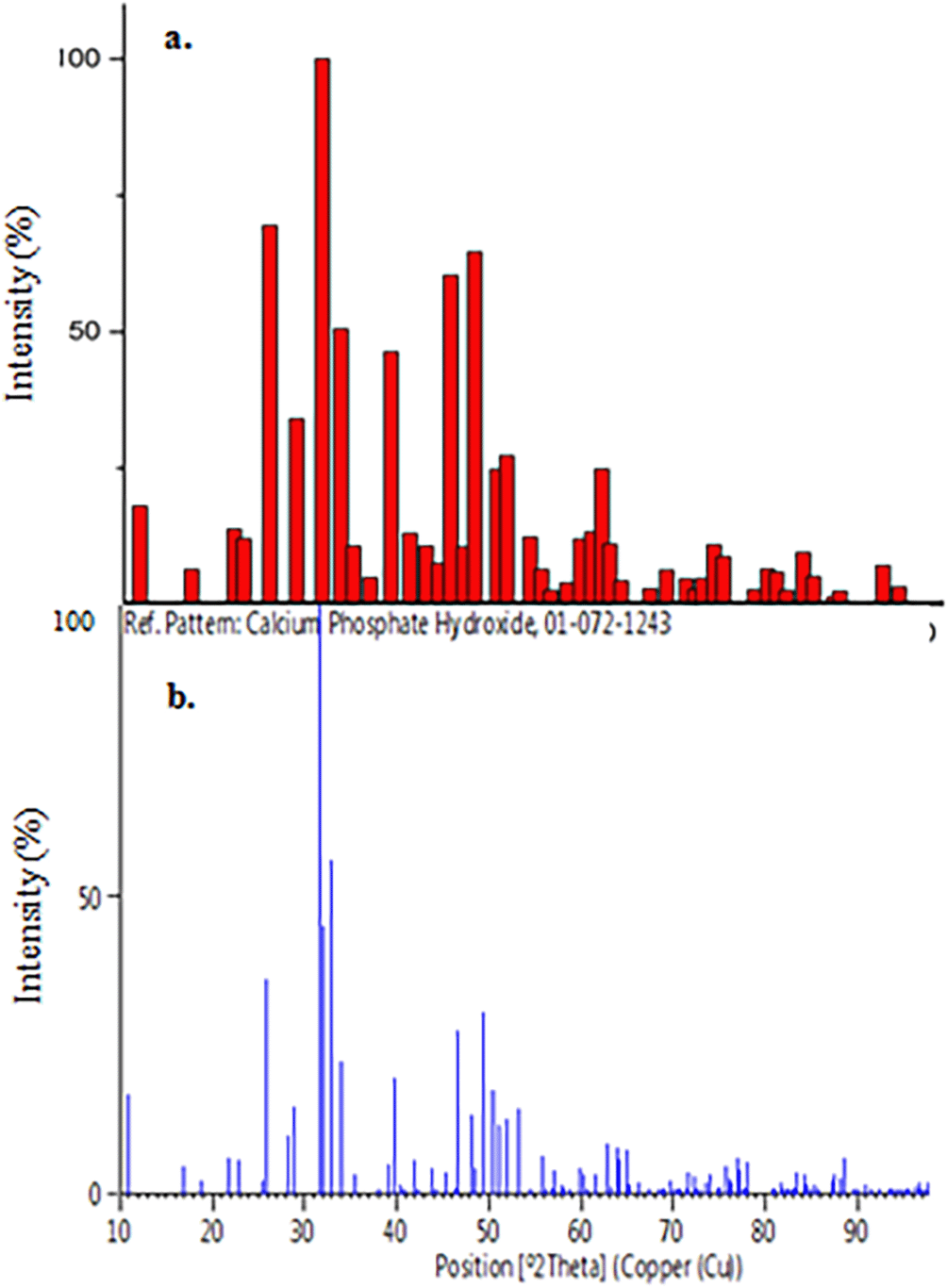
The number of crystals and the size of the chemicals contained in the sample were indicated by diffraction peaks obtained from XRD data analysis. The greater the number of compound crystals in a sample, the bigger the diffraction peak produced and vice versa.29 The results of the XRD examination for HA after processing and raw materials are shown in Figure 8. The porous HA diffractogram of the sample added with 8 grams of sago starch is shown in Figure 8(a) with the peaks/intensity at two theta of 25.87oand 32.19°, while Figure 8(b)'s sample had peaks at two theta angles of 25.85° and 31.72°, respectively. According to the results of the diffractogram in Figure 8(a), the constituent compounds' composition is HA with a percentage of 100%.

As shown in Figure 8(b), the raw material evaluation indicated 84.3 % HA and 15.7 % calcite. According to the two data, the HA composition accessible prior to use was 84.30 %, but it increased to 100% after usage. This is because the substance includes calcite (CaCO3) molecules, which causes a reaction.
Using the Origin Pro 2016 (https://www.originlab.com/2016) application, the percentage of sample crystallinity was determined from the XRay Difractogram data, and the crystallinity of the two variations was 61.7% for the HA raw material and 88.3 % for the scaffold sample, respectively. Therefore, it can be deduced that when the powder sample is synthesized into porous HA, the height of the crystal peak will increase, along with the crystallinity level. The proportion of crystallinity in raw materials and after processing was still below the ISO 13175 requirement of 95% HA, which was established in 2015.
The crystallinity degree of the scaffold has a relationship with its mechanical strength, hence, the higher the crystallinity of the material, the better the mechanical strength or close to that of human bone.30,31 The percentage of crystallinity in the sample was close to a good value with strong resorbable qualities in the range of 60—70 %.
The porous HA diffractogram, as shown in Figure 9, contains peaks that are comparable to the typical standard pattern found in (International Center of Diffraction Data) ICDD data. The red peaks of the sample follow the peaks of the blue lines, as observed in the graph of HA standard. In other words, the sample's level of crystallinity is the same as that of the HA standard in the ICDD data. Figure 9 shows peaks that are similar to the standard HA characterization pattern from ICDD data which has peaks at two theta angles of 25.87° and 32.19°, while the peaks found in the sample were at 25.84° and 31.74°, respectively.
The chemical structure of the sample is unaffected by the variation in the rate of increase in the sintering temperature, as shown in Figure 10. This is because the chemical structure is not affected during the sintering process when the sample is sintered at different increasing rate but at the same temperature.
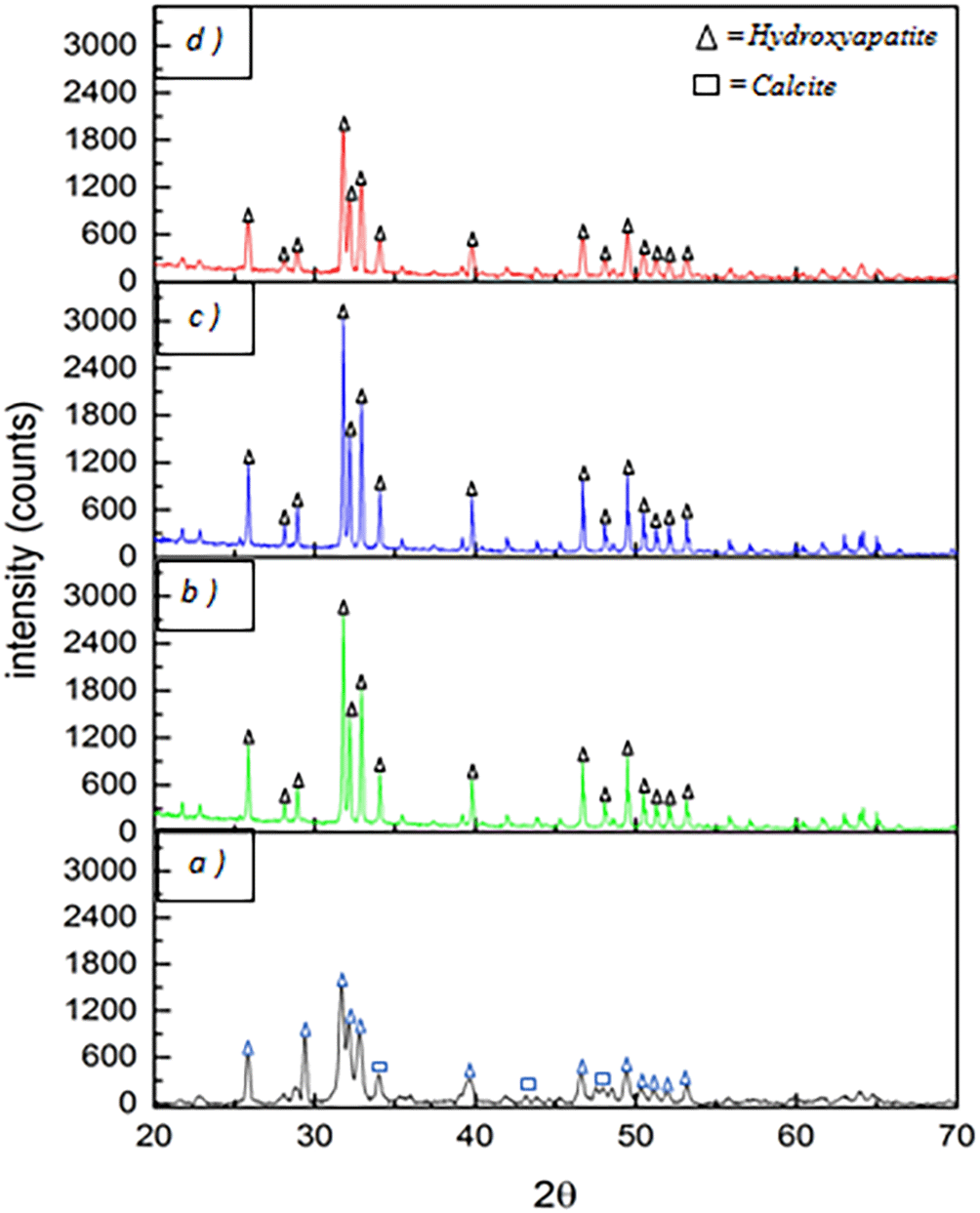
Figure 11 is a diffractogram graph of the raw material which was analyzed and yielded 84.3% HA and 15.7% calcite. The graph of XRD analysis shows that the intensity of the diffractogram also increased with rising sintering temperature. The peak intensities at sintering temperatures of 1200°C, 1250°C, and 1300°C were 1630.06 cts, 2782.76 cts, and 2807.28 cts respectively. Based on the calculation performed using the Origin-Pro 2016 software, the crystallinity value of the HA powder sample was 61.70%, which then increased with the temperature starting from 1200°C, 1250°C, and 1300°C with values of 84.61%, 88.30%, and 90.31%. The graph peaks in red, which represent the sample with a sintering temperature of 1250°C, follow the peaks of the blue lines for HA standard (ICDD 01-072-1243) as shown in Figure 12. In other words, the sample's crystallinity is the same as that of the HA standard in the ICDD data.

The sample's pore size was estimated using a digital imaging analysis of SEM with ImageJ 1.51j8 software (http://wsr.imagej.net/distros/). The average pore size was obtained after processing the data with varying area values to produce the pore diameter. During the sintering process, the pores that are the source of voids will travel from the center to the outside of the surface, and the particles will move into the ceramic body simultaneously. The rise in sintering temperature will cause volume shrinkage to increase as the particles move more intensively thereby reducing porosity and the pore size in the ceramic body.3
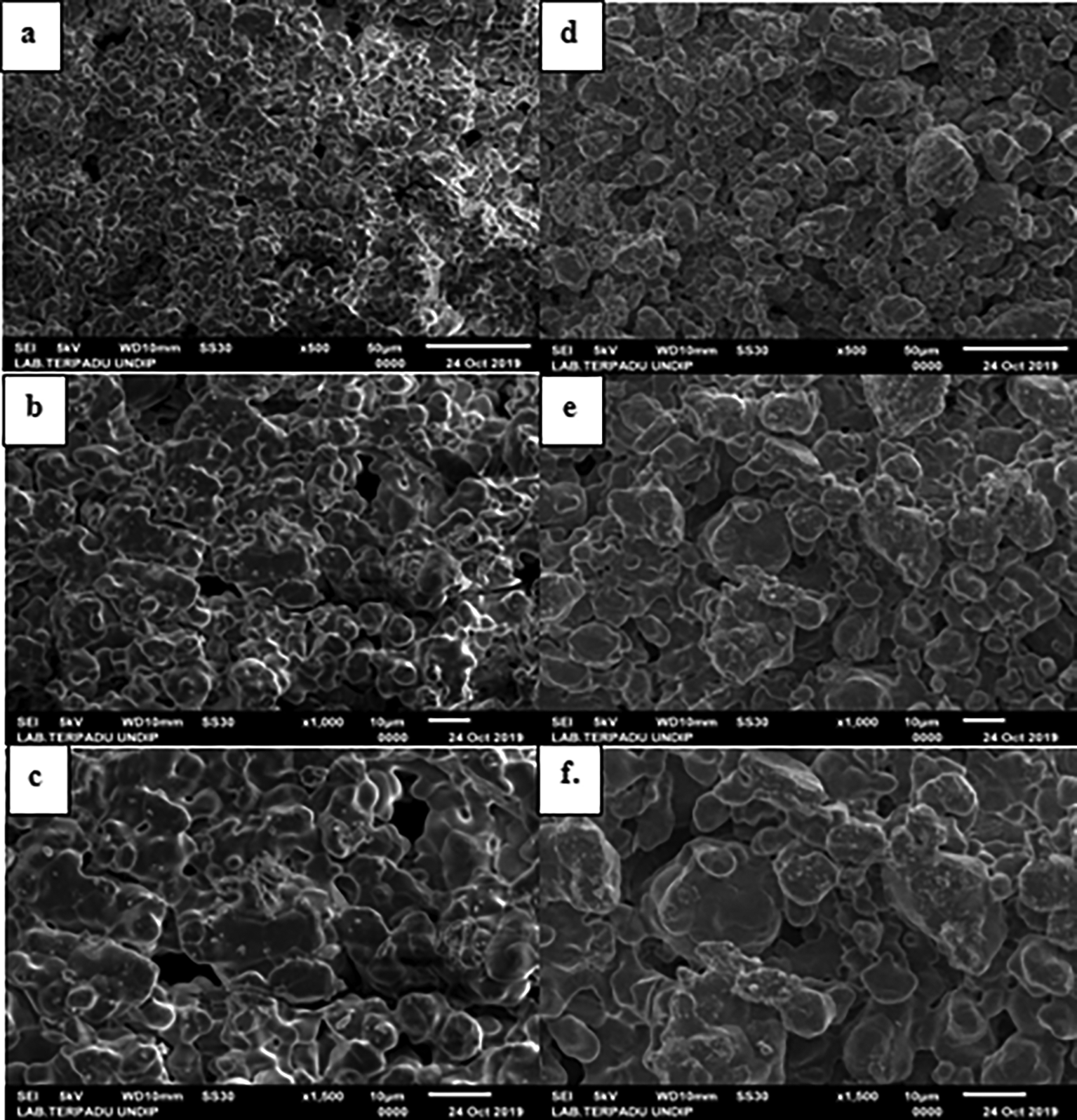
Figure 14 presenting the morphological characterization results shows that the porous ceramic produced had an average pore diameter ranging from 100—200 μm. This was formed with the use of 8 grams sago starch as a pore forming agent. Meanwhile, the average pore diameter produced by using corn starch with the addition of 8 grams ranged from 80—150 μm. This implies that the pore size formed varied with the difference in the use of starch. The pore diameter produced by the use of sago starch is larger than that of corn starch.
The pore size is in accordance with the obtained porosity, where the values produced with 8 grams of sago and corn starch was 25.95% and 21.99%, respectively as shown in Figure 13. Therefore, it can be concluded that the greater the porosity value, the larger the pore size. The amount of sago starch used affected the pore size in general. The bigger the pore size, the higher the amount of sago starch needed. The larger the pore size, the higher the porosity value. Meanwhile, when the sample's density and compressive strength increase, the pore size will shrink thereby causing the number of grains linked to one another to become higher. The porous ceramic's compressive strength increases due to the interfacial bond between the grains.17
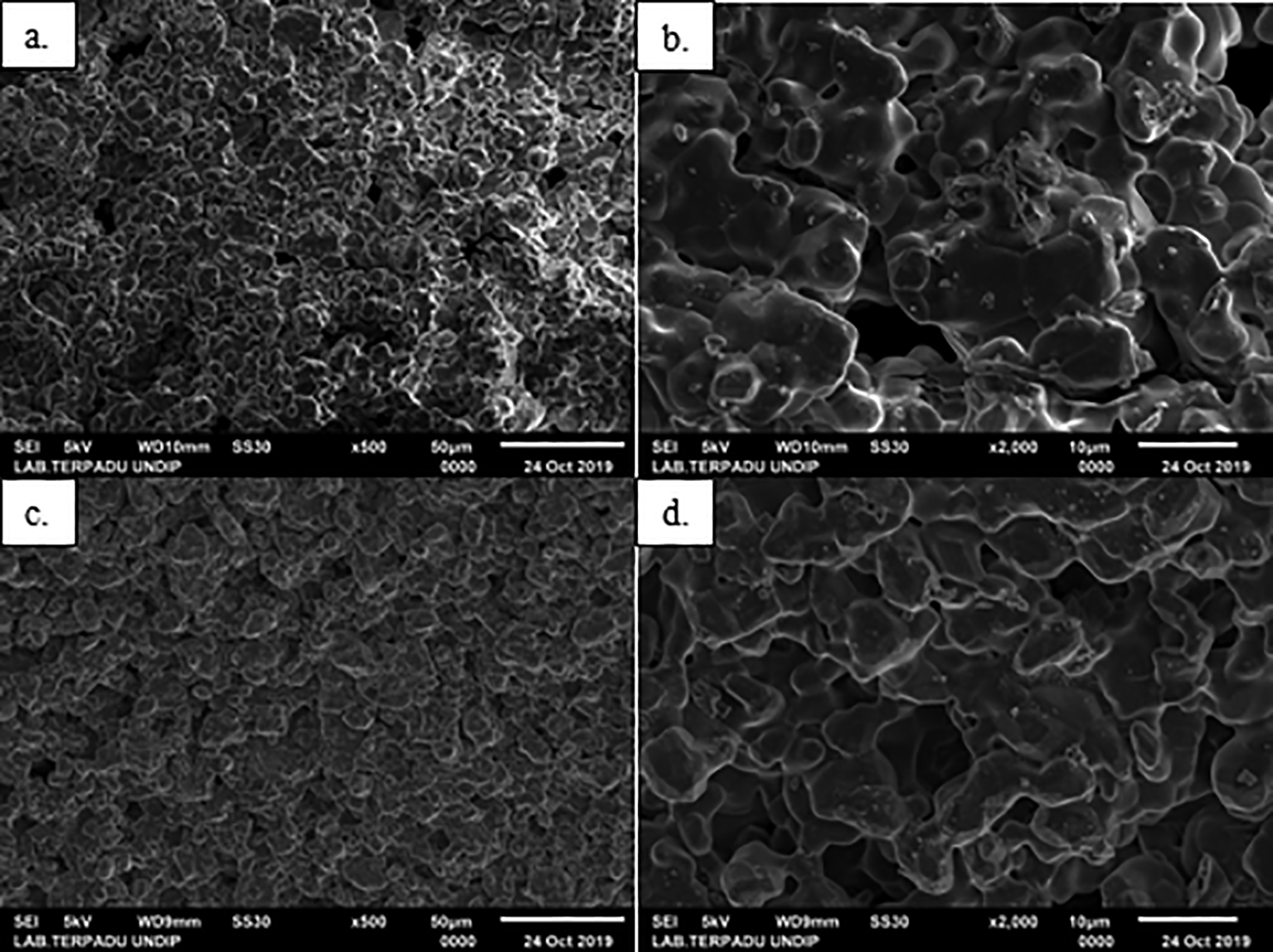
The effect of sintering temperature on the morphology of the HA scaffold bone replica is shown in Figure 15. The higher sintering temperature altered the microstructure of the sample, producing smaller pore sizes. This occurred because the grains bond to each other due to particle fusion. Based on the results, the average pore size produced at a sintering temperature of 1300°C was smaller than 1250°C and 1200°C. The pore size obtained is also in accordance with the porosity, where the values temperature of 1200—1300°C were 20.38%, 19.53% and 18.89%, respectively. Therefore, it can be concluded that the greater the porosity value, the larger the pore size.
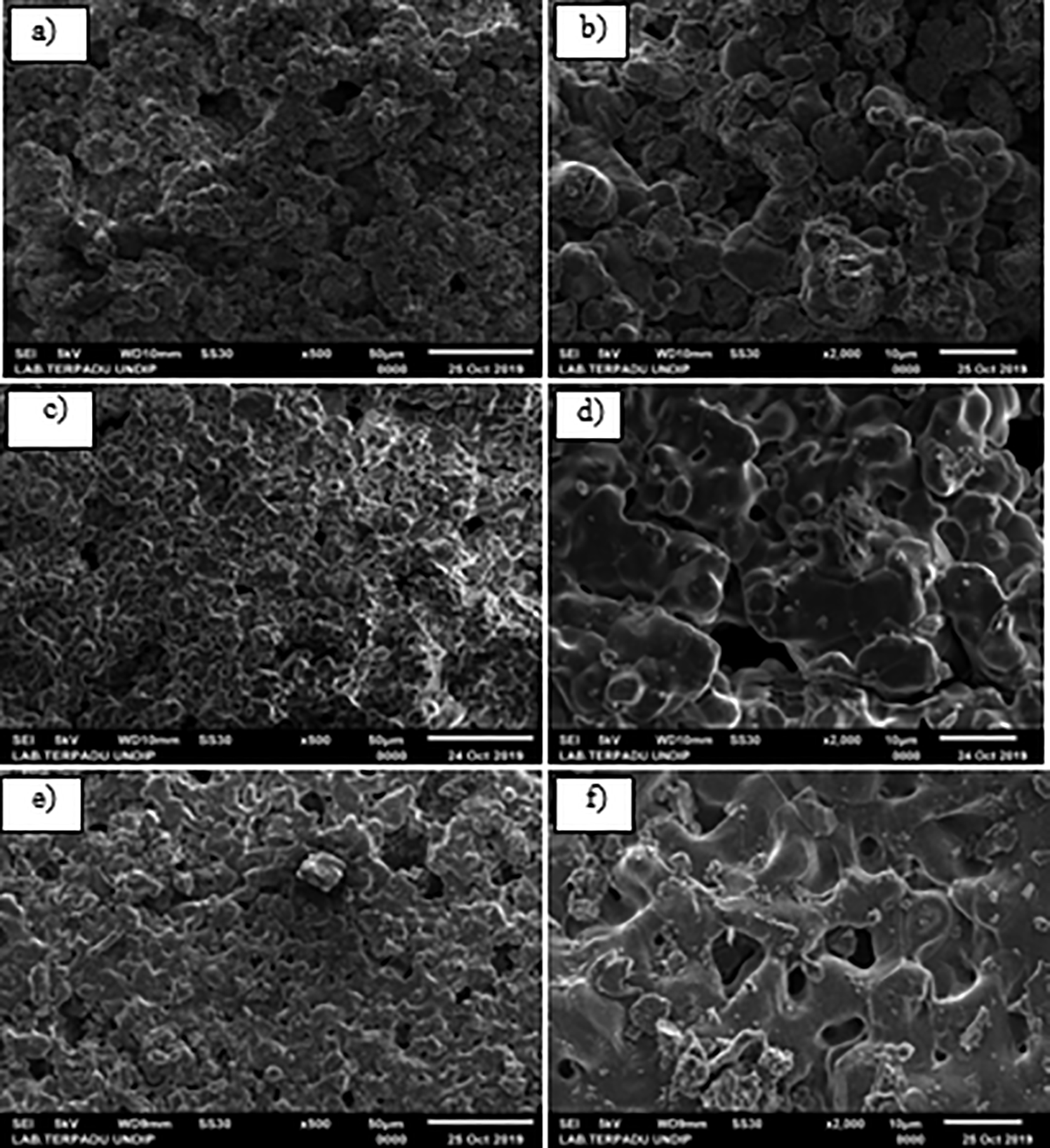
Differences in the sintering heating rate also generated changes in the microstructure and pore size of the HA scaffold samples, as shown in Figure 16. The morphology of the bone replica samples revealed that as the rate of sintering increased, the pore size decreased. Figure 16 shows that the distance between the particles became more tenuous as the heating rate of sintering decreased, culminating in small pore size. The range of pore sizes generated in the characterization of HA scaffold samples at a sintering rate of 2°C/minute and 3°C/minute was 100—200 μm, and 100—210 μm, while at a rate of 4°C/minute, it was 110—230 μm. Meanwhile, the pore size of HA is an essential concept in bone development, with pores ranging from 1—20 μm playing a role in cell formation and growth,32 while biomaterials with pores smaller than 1 μm can only interact with proteins. Pore sizes of 100—150 μm are required for bone development and blood circulation, while osteoid development occurs in pores with a diameter of 50 μm.4

A porous bone replica with HA raw material was successfully made using the starch consolidation method. Its physical, chemical, and mechanical properties were influenced by the addition of sago and corn starch. The greater the amount of sago starch added, the lower the bending stress with higher porosity and smaller density. The density of the HA scaffold using sago starch was in the range of 1.89—2.34 gr/cm3 and 1.97—2.46 gr/cm3. Moreover, the porosity at 25.95—40.09% was greater than that of corn, which ranged from 21.99—37.75% with bending stress values of 0.33—1.33 MPa and 0.67 —2.33 MPa respectively. The higher temperature and lower rate of sintering culminated in greater sample shrinkage of 34.28-39.46%, and smaller porosity of 18.89-26.93%. This was also accompanied by a higher density of 2.31—2.56 grams/cm3, bending stress of 2.55—6.49 MPa, and crystallinity value of 84.61-90.3%.
Figshare: Underlying data for “Utilization of Various Starches for 3D Design of Femur Bone Replica” https://doi.org/10.6084/m9.figshare.22107194. 33
Data are available under the terms of CC BY Attribution International License (CC BY 4.0)
The authors are grateful to The Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia for the financial support towards this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)