Keywords
Degenerative cervical myelopathy, neurosurgery, surgery, outcomes
Degenerative cervical myelopathy (DCM) poses a significant challenge globally, often requiring surgical intervention for moderate to severe cases. Choosing between anterior and posterior surgical approaches remains controversial, highlighting the need to consider various factors such as sagittal balance and compression severity.
This retrospective cohort study described patients with DCM who underwent surgery at Carlos Van Buren Hospital between 2014 and 2021. Inclusion criteria involved clinical evidence of myelopathy and cervical spinal cord compression at two or more levels, with data collected preoperatively and postoperatively at 12 months.
Of the 66 patients analyzed, both anterior and posterior approaches demonstrated substantial clinical improvements postoperatively, with 83% of patients showing overall improvement and 59.1% achieving the minimal clinically important difference (MCID) in mJOA scores. Notably, anterior surgeries showed slightly higher rates of MCID achievement and fewer instances of disease progression postoperatively compared to posterior surgeries.
Our descriptive findings underscore the benefits of both anterior and posterior surgical approaches for DCM, with slight outcome variations. Individualized treatment, considering factors such as clinical symptoms, compression type, and cervical alignment, is crucial. Future research should prioritize comprehensive outcome measures to inform treatment strategies.
Degenerative cervical myelopathy, neurosurgery, surgery, outcomes
Degenerative cervical myelopathy (DCM) is the leading cause of spinal cord dysfunction in adults worldwide.1 The clinical complexity of DCM and its symptomatic variability requires careful treatment selection, where surgery is the treatment of choice in patients with moderate to severe disease.2 The surgical management aims to decompress the dural sac and contents, preserve spinal balance and stability, and improve neurological function. A prospective study concludes that surgery improves clinical and patient-centered outcomes such as functionality, disability, and quality of life in moderate or severe DCM.1
Both anterior and posterior surgical approaches are used to treat DCM.2 Anterior approaches include anterior cervical discectomy and fusion (ACDF) and anterior cervical corpectomy and fusion (ACCF). Posterior techniques include posterior decompression (PD), with or without instrumented fusion, and laminoplasty.
Each surgical approach offers its own set of advantages and disadvantages. However, the available evidence regarding the optimal alternative for each patient remains controversial.1,3 To decide appropriately, clinicians should balance factors such as sagittal balance, the number of levels affected, and the severity of the anterior or posterior compression.
We aim to present our local experience at the Carlos Van Buren Hospital (Valparaíso, Chile) in the treatment of patients with DCM through different anterior and posterior techniques and their clinical results after 1-year follow-up.
This study is a descriptive retrospective cohort.4 Data were collected from clinical records of patients with DCM who had undergone surgical treatment between 2014 and 2021 in the Neurosurgery Service of the Carlos Van Buren Hospital (Valparaíso, Chile). The protocol was approved by the Ethical-Scientific Committee of the Valparaíso-San Antonio Health Service (N° 001689/2023).
Patients included in the analysis met the following inclusion criteria: the presence of clinical evidence of myelopathy, defined as the presence of motor, sensory, or autonomic symptoms attributable to spinal cord compression; the presence of spinal cord compression at two or more levels of the cervical spine; and to have clinical registries at baseline (preoperative) and 12 months after either anterior or posterior surgery. The surgery choice for each patient was made by the surgeon based on the specific clinical symptoms, whether the compression was predominantly anterior or posterior, the number of levels involved, the cervical alignment, and comorbidities. The surgeon also decided on the use of intraoperative monitoring.
The exclusion criteria were: Asymptomatic patients; no evidence of myelopathy; myelopathy secondary to spinal cord compression in other segments of the spine (craniocervical junction, upper cervical, thoracic, lumbar); myelopathy of different etiologies (traumatic, tumor, infectious, etc.); previous cervical spine surgery; cervical spine deformity attributable to congenital causes, or genetic/hereditary syndromes (e.g. Klippel-Feil); previous functional disability attributable to other causes.
The following data were obtained from the clinical record of each patient at baseline and after 12 months: Age, gender, type of surgery performed, number of treated levels, use of intraoperative neurophysiologic monitoring, neurological status at baseline (preoperative) and after 12 months (postoperative), days of postoperative hospitalization, the requirement for re-surgery, occurrence and type of complications, and operative time.
We retrieved data on neurological function and degree of disability, routinely evaluated with the modified Japanese Orthopaedic Association score (mJOA). This disease-specific measure assesses various domains, such as motor function of the upper and lower extremities, sensory function of upper extremities and sphincter function.5 The total score ranges from 0 to 18 points, with lower scores reflecting greater disability. Depending on their mJOA score, mild myelopathy can be defined as 15-17 points, moderate as 12-14 points, and severe as 0-11 points.6 According to their variation in the mJOA score, we defined whether patients had achieved the minimal clinically important difference (MCID).7 The MCID on the mJOA score varies based on the severity of preoperative myelopathy: 1 point for mild preoperative myelopathy, 2 points for moderate myelopathy, and 3 points for severe preoperative myelopathy. We also classified patients according to the Nurick score, which ranges from 0 (no symptoms) to 5 (severe disability).8
We used descriptive statistics such as means (and standard deviations) and proportions. Data were processed using R.9 No statistical comparisons were made.
We followed the STROBE statement for an adequate report of our research.10
We described a total of 66 patients who met the inclusion criteria. The sample consisted of 40 men (60.6%) and 26 women (39.4%), with a mean age of 63.3±11.2 years, ranging from 31 to 87 years. Seventeen patients underwent anterior surgery, and 49 were treated with posterior surgery. Table 1 summarizes the baseline characteristics of both groups.
Intraoperative neurophysiological monitoring was not performed in any patients of the anterior surgery group, whereas 40 patients (81.6%) were monitored during the posterior surgery. Only 18 patients showed intraoperative changes in the monitoring: 14 had an improvement in evoked potentials, and four presented worsening in evoked potentials. All of these results were consistent with the postoperative clinical changes.
Seventeen patients underwent anterior surgery, of which 13 underwent ACDF without a plate, three underwent ACDF with placement of an anterior plate, and one underwent ACCF. Twenty-nine patients underwent bilateral decompressive laminectomy without fusion, seven of them were performed through a unilateral approach, and 22 were performed through a bilateral approach. Twenty patients underwent decompression surgery with instrumented fusion. Table 2 summarizes the follow-up results classified by surgical approach.
Overall, 83% (55/66) of patients showed clinical improvement after at least one year of follow-up after surgery, represented as an improvement of at least 1 point in the mJOA score. According to the severity of their myelopathy,7 39 patients (59.1%) achieved the minimal clinically important difference (MCID): 12 of the 17 patients of the anterior surgery group (70.6%) and 27 of the 49 patients of the posterior surgery group (51.1%).
At 1-year follow-up, only one patient had disease progression in the anterior surgery group (a decrease of 1 point in the mJOA score), and one patient showed no differences after surgery. The remaining 15 patients (88.2%) in the anterior surgery group showed an improvement of at least 1 point. In the posterior surgery group, four patients (8.2%) showed disease progression (from 1 to 5 points), and five patients (10.2%) had the same score as before the surgery. The remaining 40 patients (81.6%) presented an improvement of at least 1 point in the mJOA score. Table 3, Figure 1, and Figure 2 show the results of the patients at baseline and after 12 months of follow-up according to the severity of myelopathy measured by the mJOA score.
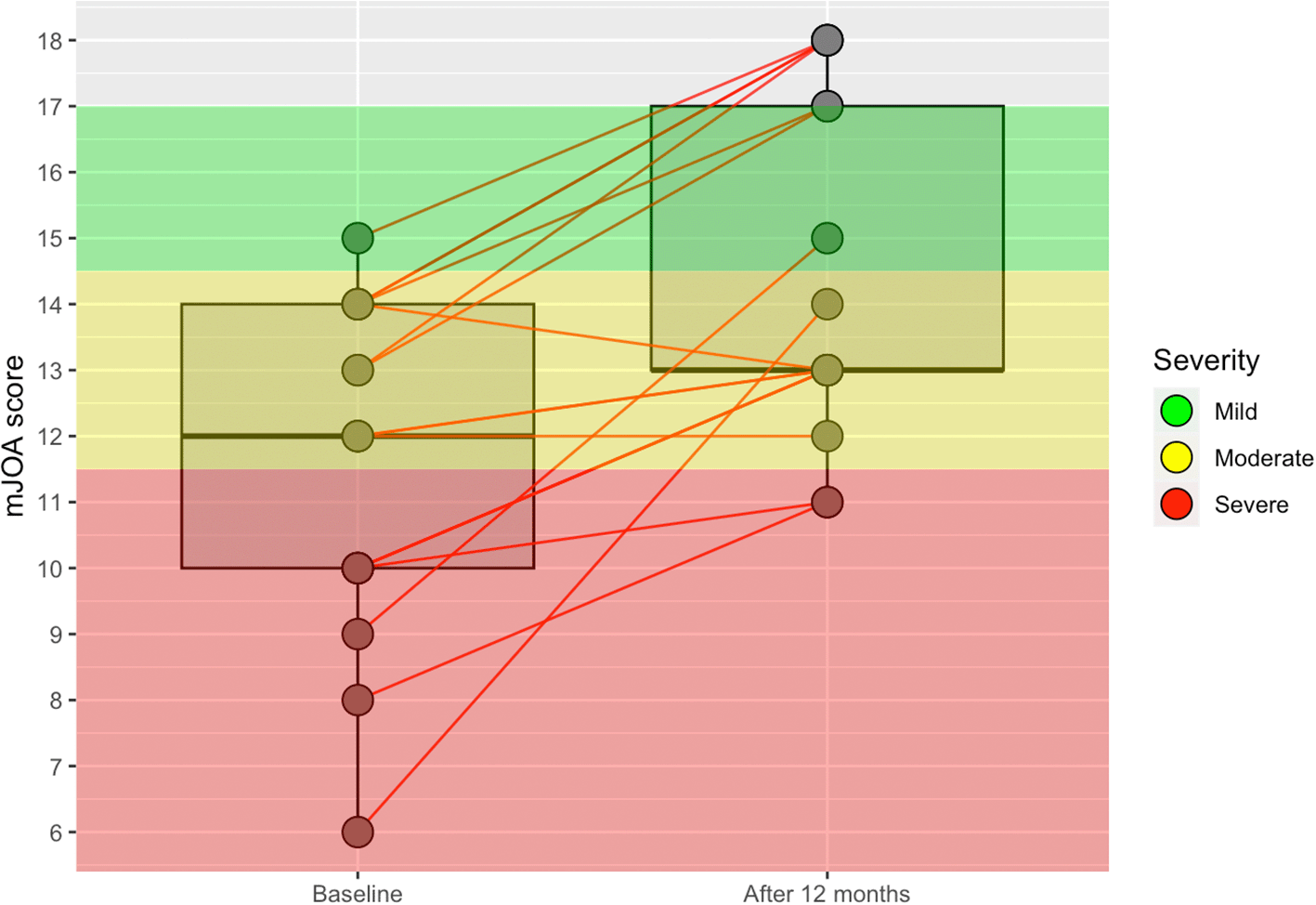
mJOA: Modified Japanese Orthopaedic Association.
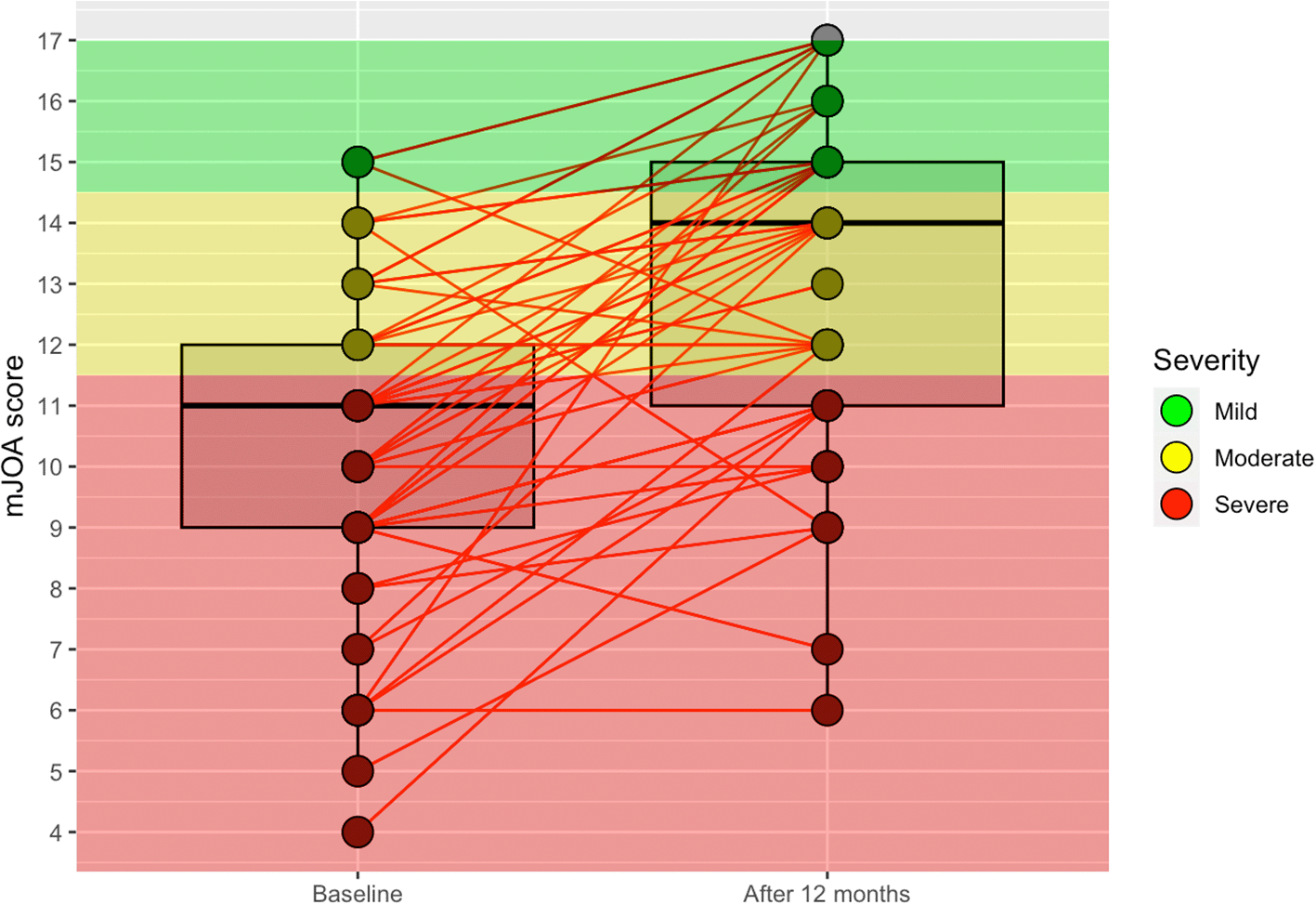
mJOA: Modified Japanese Orthopaedic Association.
According to the Nurick score at follow-up, one patient from the anterior surgery group worsened, and two patients showed no changes, while the remaining 14 patients (82.4%) had an improvement of at least 1 point. In the posterior surgery group, three patients had a lower score on the Nurick score at follow-up, and 17 patients had no changes. The remaining 29 patients (59.2%) had an improvement of at least 1 point.
Table 4 summarizes the results of the secondary objectives. Six patients (35.3%) in the anterior surgery group presented treatment-related complications (three cases of permanent, moderate to severe dysphonia; two cases of neurological deterioration with paraplegia and sensory level with caudal anesthesia, and one of dural tear), as well as six patients (12.2%) in the posterior surgery group (one case of surgical site infection; two cases of dural tears, two cases of neurological deterioration with increased severity of paresis; and one of severe neck pain).
This study describes the outcome of a cohort involving 66 patients with DCM undergoing surgical treatment. Surgery was indicated according to current clinical practice guidelines.11 Overall, patients from both anterior and posterior surgery groups showed clinical improvement after surgery, with slightly better results in neurological recovery in patients who underwent an anterior approach. Although some complications were recorded, re-surgery was scarcely required.
A prospective multicenter study by Fehlings et al. involving 278 patients with DCM that compared anterior and posterior approaches reported favorable results in both groups with no differences in neurological recovery, functionality, and quality of life.1 A recent systematic review with meta-analysis concluded that both ACDF and PD are similar regarding functional outcomes, with ACDF being beneficial in terms of less bleeding, shorter length of stay, and lower odds of complications.1,3 Other findings inform the role of posterior surgery in improving functional outcomes, quality of life, and neck pain.12
The study from Fehlings et al. also showed that surgeons selected patients with different baseline characteristics for anterior or posterior surgery: patients from the anterior surgery group were younger, had more focal cervical pathology, and had less neurological impairment. Although our study did not explore statistical associations, patients who underwent anterior surgery were also younger and had less baseline neurological impairment, those being aspects that predict a better neurological prognosis after surgery.7,13
A retrospective study from Northern Ireland of 102 patients (32% treated with a posterior approach and 68% with an anterior technique) reported a longer hospital stay in patients who underwent a posterior surgery,14 as well as patients with lower baseline mJOA scores or presented treatment-related complications, which is concordant with our findings.
Seven patients from our study underwent bilateral laminectomy through a unilateral approach, a technique scarcely described in the literature. A recent study suggested that both unilateral laminotomy and hemilaminectomy (both followed by an over-the-top undercutting procedure for decompression) may improve functional outcomes in patients with DCM.15 Another study highlighted shorter surgical times, less blood loss, and a reduced incidence of postoperative axial pain for hemilaminectomy, compared to laminoplasty.16
The choice of the surgical approach should be individualized for each specific patient, considering aspects such as age, comorbidities, and clinical presentation, as well as whether the compression is predominantly anterior or posterior, the number of levels involved, the cervical alignment, the presence of ossification of the posterior longitudinal ligament, and the joint decision between the surgeon and the patient.17
One of the limitations of our study is the lack of assessment of neck pain and its impact on the functional status and quality of life of our patients. Surgery plays a role in the neurological improvement of patients with DCM and determines significant improvements in neck pain and functional status scores.17 Other aspects, such as the presence and severity of postoperative deformity, were also impossible to evaluate in our center due to the lack of serial radiological evaluations and the absence of baseline sagittal balance assessments.
Our results elucidate comparable improvement in patients with DCM undergoing anterior and posterior surgery, as measured by specific instruments such as mJOA and Nurick scores. However, novel instruments encompassing the different aspects of DCM by pooling neurological impairment, neck pain, and quality of life are required to facilitate routine clinical practice.
This study describes our local experience in the surgical treatment of patients with DCM, which is consistent with the findings described throughout the literature. Our results show that both anterior and posterior procedures benefit patients with DCM. Although our results favor neurological recovery in patients treated with an anterior approach, we believe the treatment choice should be individualized and embedded in a shared decision-making process.
New explorations are required to assess both core clinically relevant and patient-centered outcomes, such as postoperative sagittal balance or impact of cervical pain, among other non-surrogative results.
Before initiating, our protocol was approved by the Ethical-Scientific Committee of the Valparaíso-San Antonio Health Service (N° 001689/2023, minute N°50/2023) in accordance with the Declaration of Helsinki, on September 13th, 2023. After all the ethical requirements were accomplished, the Committee granted a waiver of informed consent, allowing us to analyze a protected and anonymized database of the clinical registries, due to the retrospective nature of this study.
PA: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Project Administration, Writing – Original Draft Preparation, Writing – Review & Editing; PM: Resources, Validation, Writing – Review & Editing, Supervision; PY: Resources, Validation, Writing – Review & Editing, Supervision.
OSF: DCM Data Frame Neurosurgery HCVB. https://doi.org/10.17605/OSF.IO/NF87T. 18
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Spine surgery; Neurosurgery
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Spine Surgery
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 26 Apr 24 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)