Keywords
CVD, plaque progression, blood velocity, atherosclerosis, arterial resistance
Atherosclerosis is a build-up of low-density lipoproteins (LDL) in the channels of blood vessels. This occludes the vessels and, occurring in the carotid arteries, portends conditions that favour stroke. This work is an attempt to mathematically represent the physiological process of atherosclerosis caused by plaques on the walls of the human arteries.
Provide insight into the effect of blood flow velocity on wall shear stress and its implications on atherosclerosis progression in a human carotid artery via computational simulation.
The effect of blood velocity on plaque growth and progression is simulated using COMSOL multi-physics. The human carotid was modeled in 2-D with Stokes law for model flow. The simulation began with a plaque-free vessel with velocities of 30 m/s – 125 m/s.
Results showed that the rate of plaque initiation dropped as the blood velocity increased from 30 m/s to 125 m/s; higher inlet velocities gave lower plaque growth; the highest degree of 30% stenosis was recorded at a blood velocity of 30 m/s. Plaque height significantly affects the Plaque wall Stress, PWS, and its distribution around the plaque and arterial wall; higher plaque heights experience higher velocity distribution around the plaque, causing a higher force associated with blood flow around the plaque, resulting in higher compression stress. More compressional stresses are localized around the root, which would encourage growth as well as possible rupture at higher velocities. These ruptured plaques potentially narrow or block the arteries and prevent blood flow. This is atherosclerosis and can lead to a heart attack.
Results from this study can find significant use in the understanding, management, and treatment of atherosclerosis since the regulation of blood velocity and pressure plays a major role in the progress of atherosclerosis in the carotid artery which raises the risk of stroke.
CVD, plaque progression, blood velocity, atherosclerosis, arterial resistance
Strong et al. (2007)1 highlighted that stroke was the second leading cause of death globally and the number one reason for neurological dysfunction in adults. In 2010, the Global Burden of Disease estimated that approximately 16.9 million individuals experienced a stroke each year 2 Following Alzheimer’s disease, stroke stands as the second leading cause of dementia, and it is associated with depression and fatigue.3 It has also been observed that there is now an increased incidence of ischemic stroke in the younger population, aged 55 and below, which has surpassed the incidence of myocardial infarction.4 Cardiovascular Diseases (CVDs) are also on the rise globally, which may be connected to vessel changes with age.5 Furthermore, as the world population is projected to be an aging population, the number of stroke cases is expected to rise, and it has been projected that by 2030, today’s numbers will rise by about 50%.6
Atherosclerosis is a disease that propagates in large and intermediate blood vessels, usually arteries where fatty lesions are initiated and developed in the inner surface of the arterial wall (Tunica Intima). It occurs as a result of the hemodynamic processes in the blood and its interaction with the arterial wall. Atherosclerosis is usually initiated when dysfunctional damage to the arterial endothelial cells.7 Atherosclerosis is caused by the blockage or stiffening of arteries induced by plaque growth within the tunica intima of the arterial wall. This is a frequent cause of CVD (cerebrovascular diseases) – a major cause of morbidity, worldwide.7,8
During flow, blood exerts a tangential force, “shear stress” on the endothelial surface of the blood vessel. In laminar flow, shear stress is predominantly high and helps with alignment in the flow direction and also, facilitates endothelial cell survival. On the contrary, turbulent flow gives low-shear stress that enhances endothelial proliferation and platelet aggregation.9 The pathogenesis of atherosclerosis is connected to deviations from normal shear stress, and it is imperative to fully understand the impact of this abnormal shear stress.
The pathobiology of atherosclerosis is quite complex, being a function of multifactorial processes, and shear stress associated with blood flow has been an essential factor in the initiation and overall development of the disease.8 The control of physiological laminar shear stress is essential for normal vascular function, influencing vascular diameter regulation, inhibiting proliferation, reducing arterial wall inflammation, and preventing thrombosis.10,11 The wall shear stress on the vessel is paramount in regulating the athero-protective, normal physiology, and pathobiology, through complex molecular mechanisms that influence the initiation and progression of the diseases.11 Gibson et al. (1993)12 in their study, were able to show that shear stress is an independent local hemodynamic factor that can act independently of other known risk factors and direct correlations can be found between the disease progression and the wall shear stress, which is related to the velocity of the blood.
Wada and Karino (2000)13 theoretically investigated the effect of flow patterns and wall shear stress induced by the flow on LDL concentrations at endothelial boundaries which begin in childhood and advance in size and numbers over time.14 Results identified that the shear stress, among other factors, was responsible for a pile-up of LDL occurring at the vessel wall. The LDLs are laden with cholesterol that becomes fixed on arterial walls, forming plaques. The presence of plaques on the arterial wall perturbs blood flow which eventually leads to occlusion.15,16 Their work also revealed low LDL concentrations in regions of low shear stress.13,17 Plaque stenosis has been reported to present higher anterograde motion in patients with stenosed carotid arteries. This anterograde motion was found to be directly proportional to plaque severity, and blood flow patterns in stenosed carotid arteries notably gave better estimates of motion compared to non-stenosed arteries.18 Variations of blood flow within a blood vessel can lead to vessel occlusion in the presence of a thrombus. Our research attempts to give a clearer understanding of the effect of blood flow velocity on wall shear stress and its implications.
Two domains (the arterial wall and lumen domains) were considered in the carotid artery 2-D straight model. It was assumed that the arterial wall was homogeneous, isotropic, and linear-elastic.19 The boundary conditions taken for the flow are described in Figure 1 and Table 1. Modified vessel dimensions on the carotid artery from previous work were adapted8 as indicated in Figure 1. The vessel and carotid model characteristics are presented in Table 1.

The diameter, length and thickness of the artery were taken as 6, 10 and 0.5 mm, respectively.
| S/N | Parameter | Value | Source |
|---|---|---|---|
| 1 | Youngs Modulus | 0.9 MPa | 19 |
| 2 | Poisson Ratio | 0.45 | 19 |
| 3 | Vessel length | 10 mm | 20 |
| 4 | Vessel Radius | 6 mm | 20 |
| 5 | Vessel Thickness | 0.5 mm | 19,21 |
| 6 | Arterial wall density | 1050 kg/m3 | 19,22 |
The assumption is that blood is Newtonian, incompressible, and viscous in carrying out Wall Shear Stress, and WSS (frictional forces exerted by the moving blood in the artery) studies in nature under the arterial conditions under investigation.23,24 Laminar flow was also assumed for the blood rheology for the conditions under investigation. That is, Reynolds number values are less than 2300. This completely rules out the turbulent considerations. In the absence of underlying risks, constant blood parameters are presumed throughout the flow process. The rheological properties and density of blood are assumed to be 0.0035 Pa.s and 1050 kg/m3 respectively.22
Since arteries undergo constant deformation, a linearly elastic arterial wall was assumed during the cardiac cycle of blood flow.8 The linear elastic model has been observed to yield results that closely align with experimental data.25 The area under examination was the common carotid artery, with edge constraints implemented to ensure model stability during blood flow.26 For fluid-structure interaction between the domains, the remaining nodes were designated as free.19,27,28 The inlet velocity and traction boundary pressure were fully developed. Boundary conditions were applied at the artery’s inlet and outlet, respectively.11,20,29 A constant mean (inlet) velocity of exposure was assumed throughout time. A no-slip condition at the arterial wall was also assumed during the cardiac cycle. The distribution of velocity along the cross-section of the vessel is as defined in the work of Alagbe et al. (2022).8
A fluid-solid interaction (FSI) system was taken as the arterial flow model – with a simultaneous interaction between the solid and liquid domains. Blood flow in the artery was simulated using Navier-Stokes equation, incorporating model equations of Alagbe et al. (2022)8 were used for the simulation.
The plaque’s geometry was simplified and simulated starting at the artery’s central length (5 mm). Plaque growth, forming an asymmetric blockage, extended from the arterial wall as it progressed.
The model equations by Alagbe et al. (2022)8 and Tang et al. (2008)30 for plaque initiation and development were adopted. This model gives a linear function expressed as;
σ represents principal shear stress (Plaque Wall Stress, PWS) in KPa, and τ denotes flow shear stress (WSS) in Dyne/cm2. WTI measures the increase in plaque wall thickness in cm, reflecting the difference from the initial height. This model predicts the achievable plaque height relative to the initial height every 304 days, corresponding to one cycle of geometry update.
From the modified work of Liu and Tang (2010),31 the two major stages of initiation and progression were adopted.
Initiation Stage: A plaque-free artery is assumed before plaque formation. The blood flow in the plaque-free vessel is taken as the initial condition. Values of WSS and PWS are taken at plaque initiation. These values are used to obtain the WTI value. With this WTI, the plaque is now freshly initiated in a new domain.
Progression Stage: Plaque growth is considered in relation to the initial plaque condition’s memory. The values for WSS and PWS are obtained from the fluid-solid interaction (FSI) model. By adding the WTI, the next plaque height was obtained. The steps are repeated and stopped at the expected time of exposure.
The stenosis severity is expected to increase with every geometry update, leading to an increase in plaque height. Stenosis severity quantifies the degree of arterial lumen blockage by the plaque, expressed in terms of luminal diameter. This obstruction limits blood flow in the axial direction.
Navier-Stokes equation was solved with finite elements, quadratic velocity, linear pressure, and GMRES iteration, using COMSOL Multiphysics. The meshing used for the simulations were fine meshes as shown in Figure 2 because it guarantees an optimum between computational cost and result accuracy. For the developed carotid vessel geometry before the plaque is initiated, after implementing boundary conditions and selection of meshing protocol, the finalized geometry had 3 domains, 10 boundaries, and 8 vertices. The complete mesh consisted of 3078and 246 domain elements and 246 boundary elements respectively.
The meshing was done such that, they are more intricate around the boundaries and the wall/blood interface to ensure that the interactions between both domains are accurately computed.
From Figure 3, it can be observed that there is a general decline in the rate of initiation of the plaque as the velocity of blood in the artery is increased from a minimum of 40 cm/s to 125 cm/s from its initial dimensions where there is no plaque (Figure 8). The variation of plaque initiation height with velocity shows a very strong linear relationship. On comparison of the change in plaque initiation height with velocity change, a 40 cm/s to 80 cm/s change in velocity (100% change) shows an approximate difference of -8.8% in plaque height, although this may seem like a small change, accumulated changes over the years could be significant in the determination of the period in time when the patient is at a high risk of stroke. Minimum and maximum stenosis within the velocity range occurred at 125 cm/s and 40 cm/s, respectively, with plaque heights 1.02 and 0.83 mm, indicating degrees of stenosis of 17 % and 13.8 %, respectively, indicating that persons with lower blood velocities would experience stroke earlier in life. The reason for this could be a result of the higher residence time usually associated with low velocities in plug flow systems; this leads to higher recirculation of blood within the region as well as more significant leaching of LDL into the permeable arterial wall within the region, at the end of this processes, the result is more stagnation and accumulation of biochemical cells.32 This buttresses the results of Liu & Tang (2010),33 where a higher flow rate was associated with lower plaque heights.
Figure 4 shows that as time progressed, the differential increase in plaque height reduced. The work of Liu & Tang (2010)33 buttresses this observation when a similar model was implemented in the coronary artery. It is also generally observed that lower blood velocity causes a higher differential increase in plaque deposition in the vessel over the years; the highest blood velocity of 45 cm/s studied produced the least amount of plaque deposition over the period. Figure 4 buttresses the initial study of Figure 3 that higher inlet velocities correspond to lower plaque growth, so also the plaque is observed to grow at a decreasing rate over the years; this is in line with the study of Stone et al. (1993)34 which showed that higher degrees of stenosis retards the rate of growth. The velocity at the neck of the stenosis is much higher compared to other parts, as indicated in the velocity band plots. This observation aligns with the earlier statement that higher velocities around the plaque do not favor plaque progression.33,35 A maximum differential plaque increase of 0.17 mm was recorded for an inlet velocity of 30 cm/s after the time-2 (604 days), and a minimum differential plaque height increase of 0.1 mm was observed for a velocity of 45 cm/s after the second year (608 days).

The plaque height is steadily higher for lower blood flow velocity over the period investigated.
Figure 5 shows how the overall plaque height increases with time, causing more obstruction of the blood vessel termed “stenosis,” which is measured as the amount of the arterial lumen domain (measured as a function of diameter) the plaque has blocked. Initially, at the initiation of the plaque, there is a sharp increase in plaque height from 0 mm to around 1.0 mm (Figure 9) for all cases of inlet velocity; this is a result of the associated low WSS effect on the initiation of plaque; However, as the plaque continued to develop, as a result of diminishing differential plaque deposit (as indicated by the plaque progression curve getting dampened), the changes in height became significantly less compared to the initiation stage. After about five years, the carotid vessel that was exposed to the 30 cm/s blood velocity had the highest degree of stenosis of about 30% (Figure 10), as indicated by the overall plaque height of 1.78 mm, and the artery exposed to the 45 cm/s inlet velocity experienced the least plaque height of 1.32 mm equivalent to a stenosis degree of 22% (Figure 11). These values may not seem significant at this early stage. Still, as indicated by the curve trend, the plaque height of the 30 cm/s and 45 cm/s inlet velocities, the divergence between the two curves seems to widen as time elapsed (in this case, after each geometric update); this indicates that after a long period in time, the significance of overall plaque height would be too massive to overlook (Figures 10 and 11).
From Figure 6, it can be observed that, on a general note, the peak WSS values are localized at the peak of the plaque. This observation is in tandem with that made by Razavi et al. (2011)22 in the study of the inlet velocity cycle in the carotid artery. It can also be observed that WSS values are relatively lower and flattened before and after the region of the plaque location. As indicated in Figures 8 to 11, velocity contour plots indicate that areas of high velocity are localized just above the stenosis neck (peak), which could have been responsible for high WSS values around that region of plaque growth. This corroborates the fact that high wall stress is caused by high flow velocity. A high difference of about 500 dyne/cm2 was observed between the far-developed stenosis of 80% and initial stenosis of 17%. Since high WSS values tend to retard plaque growth, this study gives a better insight into how high WSS values can go, depending on the level of stenosis and the probability or magnitude of plaque growth associated with these values. Taking a closer look at how WSS is distributed around the plaque, it can be seen that the lower region of the plaque tends to grow at a faster rate from lower WSS regions compared to the neck of the plaque.
Figure 7 shows that the plaque height (measured in terms of the degree of stenosis) plays a significant role in PWS and its distribution around the plaque and the entire arterial wall. The higher the degree of stenosis, the more influential the PWS; 80% stenosis plaque neck experienced the maximum tensile stress of 0.3 kPa, while the initiation stage plaque neck experienced the minimum tensile stress of 0.09 kPa. Taking a holistic look at Figure 7 concerning Figures 8–11, higher plaque heights experience higher velocity distribution around the plaque, causing a higher force associated with blood flow around the plaque, resulting in higher compression stress. From the growth perspective, higher tensile stresses lead to a lower growth rate of the plaque and higher compression stresses lead to faster growth rates as indicated by the growth model in equation 1, this automatically implies that since higher plaque heights lead to higher tensile stresses around the plaque, hence, lower growths rates would be observed. However, as the plaque geometry increases in size, more compressional stresses are localized around the root, which would encourage growth as well as possible rupture at higher velocities. The PWS shock wave seen in Figure 7 at the region of arterial length 4 and 6 mm for 17.3% stenosis and 2 and 8 mm for 50% stenosis, could be a result of reflective backflow caused by the plaque obstruction.

At the plaque initiation stage, there is a sharp increase in plaque height from 0mm to 1.0 mm.

Simulation for 5 years gives a 30% stenosed carotid artery at a blood velocity of 30 m/s.
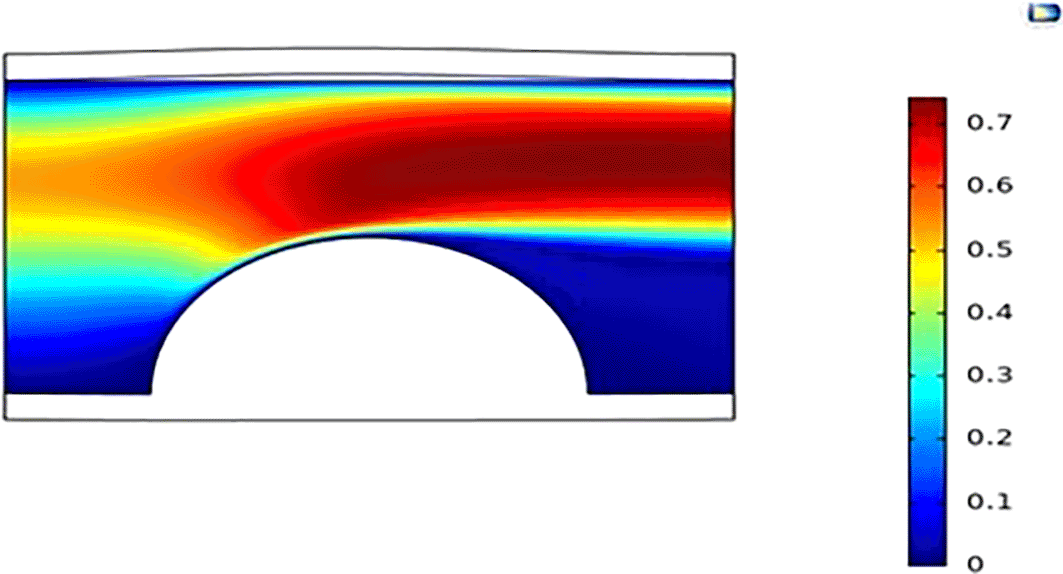
Simulation for 5 years gives a 22% stenosed carotid artery at a blood velocity of 45 m/s.

WSS is seen to increase with plaque height.
Figure 12, shows the WSS distribution in the carotid artery model at plaque heights of 4.8 mm representing 80% of stenosis in the artery. Studies have shown that at this degree of stenosis, the plaque is usually in a very vulnerable state of rupture.34 This study gives insight into the WSS and PWS distribution at the stage of plaque development, insights for further growth of plaque would also be drawn. It can be observed that WSS values are generally extremely high with 125 cm/s inlet blood velocity showing the highest WSS of about 2800 Dyne/cm2 and 30 cm/s inlet velocity showing the lowest WSS value of about 500 Dyne/cm2. The implication for these high WSS values is that; WSS-associated growth would be unlikely around this region, WSS WSS-associated plaque growth is more likely at lower shear stress values below 400 Dyne/cm2.12,30
As earlier seen in Figure 7, which shows how PWS increases as stenosis height increases, Figure 13 also exhibits these characteristics between inlet blood velocity and the PWS. It can be conveniently inferred that blood velocity constitutes more force within the plaque as the plaque tends to resist the flow. At this 80% degree stenosis, the maximum tensile stress was experienced at the neck of the plaque with the value of about 4 kPa for a velocity inlet of 125 cm/s. This plot gives a revelation that at the root of the stenosis at vessel length 1 mm to about 4 mm for 125 cm/s to 30 cm/s respectively, relatively high compressive force is observed for 125 cm/s inlet velocity with the value of -10 kPa. This gives an insight as to how this compressive force could cause a possible rupture of the plaque at the root and also how blood flow with higher velocities in fully-developed plaques has a higher probability of rupture, hence leading to stroke. The maximum compressive PWS was higher than the maximum tensile PWS.
The results have shown that blood velocity is inversely related to plaque initiation rate and progression rate, although the variation of plaque growth with blood velocity in the artery is not overly significant, however, long-term exposure effects are significant, as this would determine the overall plaque height after a couple of years. It can also be concluded that WSS and PWS increased with blood velocity, at regions around the stenosis neck. Similarly, the WSS and PWS increased with the degree of stenosis. Regions farther from the point of initiation of the stenosis experience lesser WSS and PWS, therefore, as the plaque tends towards being fully developed, regions on the plaque, that are away from the neck of the plaque, that is, around the root, would tend to grow at a faster rate compared to the neck of the plaque, which could further affect the structural stability of the plaque.
Results from this study can find significant use in the understanding, management, and treatment of atherosclerosis since the regulation of blood velocity and pressure significantly contributes to the onset and progression of atherosclerosis in the carotid artery, elevating the risk of stroke.
Mendeley Data: Dataset on blood flow velocity’s impact on atherosclerosis progression, https://doi.org/10.17632/cdx38fx5vp.1. 36
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The Team is appreciative of the publication fee support for this manuscript by the management of Covenant University and the collaborative support of the Brody School of Medicine at East Carolina University, Greenville NC.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Theorist, Biofluid Mechanics, Computational Mechanics
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Ahmed H, Podder C: Hemodynamical behavior analysis of anemic, diabetic, and healthy blood flow in the carotid artery.Heliyon. 2024; 10 (4): e26622 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: I am passionate about studying the movement of fluids, and I employ computational methods to gain a deeper understanding of these flows. This includes a particular focus on both atmospheric dispersed flow and the intricate dynamics of blood flow.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: fluid mechanics, computational fluid dynamics, biofluid mechanics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 30 Apr 24 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)