Keywords
Gallbladder Neoplasms, Bile Duct Neoplasms, Biological Therapy, Molecular Targeted Therapy, Immunotherapy, Palliative Care, Review Literature as Topic
Biliary tract cancers (BTCs) have low survival rates in advanced stages. Anticancer drugs (ACDs) are usually recommended, but may be associated with important toxicity and lower quality of life (QoL). Best supportive care (BSC) could represent a valid alternative of treatment. We aim to synthesise evidence regarding the effects of ACDs versus BSC in patients with advanced BTCs.
We conducted a systematic review including randomised controlled trials (RCTs) comparing any type of ACD versus BSC, placebo or no active treatment. We searched in five databases. Two reviewers performed selection, risk of bias and data extraction processes. We conducted random-effects meta-analyses and assessed certainty of evidence using GRADE.
We included eight RCTs. Biological/targeted therapies may result in little to no difference in overall survival (OS) (Mean difference (MD): 1.66 months higher; 95%CI, -0.65 to 3.96; low certainty) and toxicity (Relative risk (RR): 1.38; 95%CI, 0.99 to 1.93; low certainty), with uncertain effects on QoL. Evidence is very uncertain about the effects of chemotherapy on OS (MD: 3.28 months higher; 95%CI, 0.16 to 6.39; very low certainty), and may increase toxicity (RR: 1.33; 95%CI, 1.03 to 1.72; low certainty). We identified insufficient evidence for other prespecified outcomes.
Compared to BSC, ACDs have poor OS benefit and higher toxicity. Due to overall very low certainty of evidence, the effects of ACDs on critical outcomes are still unclear. Our findings should be used to better inform decision-making processes and future research.
Gallbladder Neoplasms, Bile Duct Neoplasms, Biological Therapy, Molecular Targeted Therapy, Immunotherapy, Palliative Care, Review Literature as Topic
Biliary tract cancers (BTCs) are an heterogeneous group of tumours that include cholangiocarcinoma (CCA) —both intrahepatic (iCCA) and extrahepatic (eCCA)—, gallbladder cancer (GBC), and ampullary tumours. Despite not being among the most frequent neoplasms (less than 5% of the incident cases of overall cancer), BTCs are important due to its poor prognosis, since they are aggressive and often diagnosed in an advanced stage.1–3 GBCs have an overall 5-year survival of 20.4%, but 44.0% of these patients are diagnosed in a distant stage, with a decreased 5-year survival of 3.2%, with other BTCs presenting similar trends.1
Anticancer drugs (ACDs) (such as chemotherapy, immunotherapy and biological/targeted therapies) constitute a commonly used therapeutic option for patients with advanced cancer. For patients with advanced BTCs, cisplatin–gemcitabine has been widely recommended for patients with a performance status (PS) of 0-1 and gemcitabine as monotherapy for PS 2 in the first-line setting, although recent evidence suggests that cisplatin-gemcitabine-durvalumab may be a new therapeutic alternative.4 On the other hand, FOLFOX is used as second-line therapy, although approximately 40% of patients with BTC harbour targetable genetic alterations, with IDH1, FGFR2, BRAF and HER2 being promising targets for molecular therapy.5
Therapy recommendations are mainly based on survival-related outcomes.6,7 Nevertheless, if we consider thresholds from other neoplasms, it is possible that some patients with advanced BTCs find the magnitude of improvements in survival to be non-significant for them.8 Besides, other patient-important outcomes (such as quality of life, toxicity and quality of end-of-life care) should be also explicitly considered for optimal shared decision-making, in an effort to balance all the possible benefits and harms of a certain treatment option.9–14
Considering the above mentioned limitations of current treatments, best supportive care (BSC) could be considered a valid therapeutic approach for these patients. BSC can be broadly considered as all the efforts and integration of treatments and techniques that aim to improve quality of life and relieve symptoms.15–17 Specifically, in patients with advanced BTCs, BSC also includes active identification and management of biliary obstruction.5 Since BSC alone is a less aggressive treatment than the addition of ACDs to a supportive care strategy, it is possible that it may represent the preferred therapeutic option for patients in an end-of-life context, who may opt to prioritise other outcomes important for them beyond survival.18
To our knowledge, no systematic review (SR) has addressed the comparative effects of adding ACDs to a BSC approach for patients with advanced BTCs, considering all the relevant body of evidence and important outcomes.19 Therefore, we aim to conduct a SR of randomised controlled trials (RCTs) to assess the effects of ACDs versus BSC alone in patients with advanced BTCs, assessing patient-important outcomes beyond survival.
We designed a protocol for multiple parallel SRs in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P).20 This SR adheres to PRISMA reporting guidelines.21 This is the last part of a three-stage broad evidence synthesis project (Appropriateness of Systemic Treatments for Advanced Cancer - ASTAC project).22 Briefly, we first conducted three overviews of SRs assessing the effectiveness of ACDs versus BSC for advanced non-intestinal digestive cancers (including hepatobiliary, gastroesophageal and pancreatic cancer).19,23,24 The second stage comprehended broad evidence mappings of primary and secondary evidence.25–27 This SR is part of the third stage, which aims to develop multiple SRs of RCTs aiming to update current evidence syntheses, avoiding waste research by assessing clinical questions not well responded yet.28 The protocol for this project was registered in Open Science Framework.22 We based our research question, eligibility criteria and search strategy in the ‘PICO’ framework.29 We formulated three main research questions, regarding the effects of: i) chemotherapy; ii) targeted/biological therapies; and iii) immunotherapy; versus BSC for patients with advanced BTCs.
Types of studies
We included only RCTs, without language or date restriction, published in any database, indexed or not. We excluded quasi-experimental studies, observational studies, and reviews.
Types of participants
We considered studies including adults with primary or recurrent, advanced (stages IIIb or IV) or metastatic gallbladder or bile tract cancer. We excluded neuroendocrine, stromal or lymphatic neoplasms.
Types of interventions
We considered eligible interventions using any ACD, including chemotherapy (either monotherapy or in combination), biological/targeted therapy or immunotherapy, whether individual or combined, with or without supportive care. We excluded trials considering only surgery or radiotherapy as intervention.
We considered BSC as the comparator, broadly defined as any supportive treatment administered with the purpose of symptomatic or palliative control.15,16 We considered trials that used BSC, supportive care, placebo, or that did not explicitly use ACDs as a comparator. We excluded comparisons that consisted of other treatments with non-palliative intention (e.g. radiotherapy or surgery with curative intention).
Types of outcomes
We considered overall survival (OS) (dichotomous, continuous or time-to-event outcome); quality of life (measured with validated scales); progression-free survival (PFS) (as a dichotomous and a time-to-event outcome); functional status (measured with validated scales); and toxicity (measured as moderate or severe adverse events, according to standardised classification) as critical outcomes. We also considered the following as important outcomes: Symptoms related to the disease (measured with validated scales); admissions to hospital or long-term centres, or number of emergency consultations; and quality of end-of-life care, defined as a composite outcome including any admission to hospital at the end of life (i.e. the last 30 days of life), palliative care provided during the last year, and place of death.
We conducted a sensitive search strategy for the first two stages of the overall evidence synthesis project22 in MEDLINE (access via PubMed), EMBASE (access via OVID), the Cochrane Database of Systematic Reviews, CENTRAL, and Epistemonikos until December 2019. Table S1 (Additional file 1) provides the search strategy for MEDLINE/PubMed. Using the same search strings, but only focusing on advanced BTCs, we ran an update in MEDLINE/PubMed and CENTRAL. Table S2 (Additional file 1) details this update for MEDLINE/PubMed. We conducted complementary searches in clinicaltrials.gov, and also asked experts in the field for relevant studies. Using a subset of identified RCTs, we conducted a backward and forward citation search using citationchaser.30–32
Two reviewers performed an independent title and abstract screening of the results obtained from the search. A third reviewer solved any disagreement. Afterwards, two reviewers conducted the full-text screening, also with a third author solving any disagreement. We used the Covidence software for the selection process33 (although other open access alternative softwares are available, see “Availability of data and materials” section).
Two reviewers extracted data independently from the included studies, using a previously piloted data extraction sheet. We solved disagreements through discussion. We extracted the following data for each included RCT: general characteristics of the study (country, specific study design, setting, objective, funding, conflict of interests, total patients included and randomised); type of experimental intervention (chemotherapy, targeted/biological therapy and/or immunotherapy); type of control intervention (BSC, placebo, other or non-specified); outcomes assessed; and results for each outcome. Two reviewers independently assessed all included studies using the Cochrane ‘risk of bias’ tool.34 Disagreements were resolved by consensus or by a third reviewer.
For dichotomous outcomes, we estimated treatment effect with risk ratio (RR) and its respective 95% confidence interval. For continuous outcomes, we calculated mean difference (MD) if the outcomes were reported with the same scale across the included studies, or standardised mean difference (SMD) if different scales were used. For time-to-event outcomes, we used hazard ratios (HR). Whenever a study included multiple arms, we only considered comparisons relevant for our reviews. We assessed heterogeneity both visually and using I2 through Software Review Manager 5.4.1,35 and considered it for purposes of estimation of certainty of evidence.
If there were less than two studies reporting for a specific outcome, or in case they were considerably heterogeneous, we did not perform meta-analysis and only reported results descriptively.36 If studies were reasonably homogeneous (both clinically and methodologically), we performed meta-analysis, using a random effects model. For each type of intervention in the experimental arm, we grouped all the interventions in the control group (i.e., placebo, supportive care, best supportive care, usual treatments and others) as one unique comparator. We planned to assess publication bias by visually analysing funnel plots only if 10 or more studies provided data for a specific outcome. We conducted subgroup analysis according to line37: specific types of chemotherapy, specific types of immunotherapy, specific types of biological/targeted therapy.
We assessed the certainty of the evidence for each primary outcome according to GRADE guidance.38 We created Summary of Findings (SoF) tables, classifying the certainty of evidence for each outcome as high, moderate, low, or very low. We also report the main findings of the SoF table in plain language, according to their specific assessment of the certainty of evidence.39
We screened a total of 70,076 references. After title and abstract screening, 2,841 references were assessed by full text, of which we excluded 2,656. Finally, eight RCTs (within nine publications) met our eligibility criteria.6,7,40–46 Figure 1 provides the PRISMA flow diagram of the selection process.
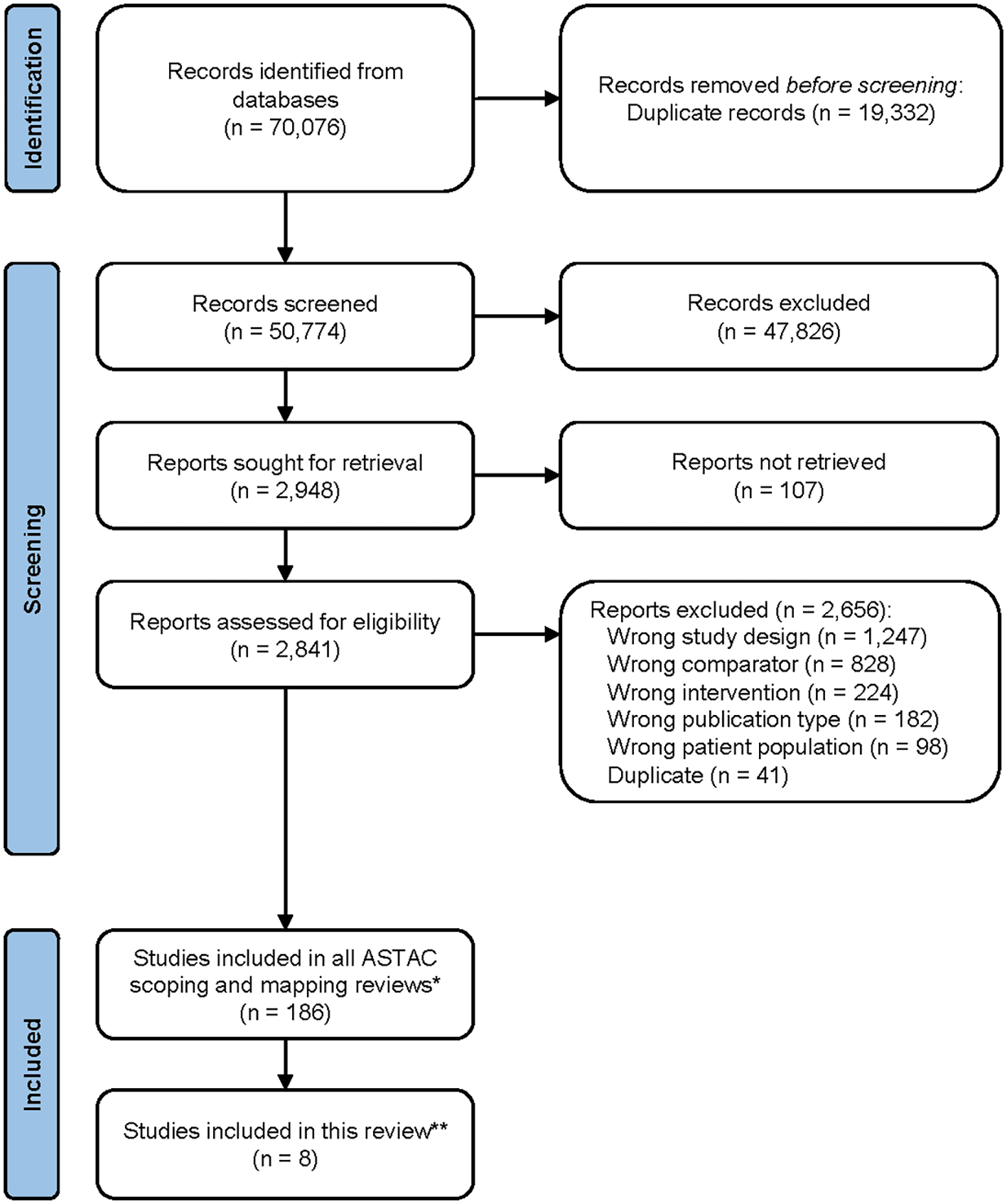
* Considers all the reports included for the ASTAC project, including gastroesophageal, pancreatic, hepatic and biliary tract localisations, as well as observational studies, quasi-experimental and experimental primary studies, and systematic reviews
** Considers only randomised controlled trials assessing the effects of anticancer drugs versus best supportive care in advanced biliary tract cancer patients. We included two relevant publications for the ClarIDHy trial.
The included studies were conducted in Sweden,7 Japan,40 India,6,42 Korea,41 Belgium,43 and UK.45 One trial was conducted in various countries, including France, Italy, Spain, UK, US and South Korea.44 RCTs included patients with advanced cholangiocarcinoma,7,41,44 gallbladder cancer,6,42 or both.40,43,45 One study included exclusively patients with IDH-1 mutation.44 Two trials also included patients with pancreatic adenocarcinoma.7,40 Four RCTs provided the intervention as first line therapy,7,40–42 while three studies specified that patients were previously treated.43–45 One RCT admitted patients with previous treatment if it was completed at least six months before enrolment.6 None of the included studies assessed immunotherapy. Table 1 provides the general characteristics of the included studies
| Study ID | Country | Cancer localisation | Performance status | Total patients included | % Female | Previously treated | Intervention | Comparator | Outcomesa |
|---|---|---|---|---|---|---|---|---|---|
| Biological/targeted therapies versus BSC | |||||||||
| ClarIDHy44,46 | France, Italy, South Korea, Spain, UK, and US | CCA (IDH-1 mutant) | ECOG 0-1 | 187 | 63.6% | Yes | IVO | PLB | OS, PFS, Toxicity, Symptoms |
| Kataria 201942 | India | GC | ECOG 3 | 51 | NR | No | ERL | BSC | OS |
| REACHIN43 | Belgium | CCA, GC | ECOG 0-1 | 66 | 39.4% | Yes | REG | PLB | OS, PFS, Toxicity |
| Chemotherapy versus BSC | |||||||||
| ABC-0645 | UK | CCA, GC and ampullary carcinoma | ECOG 0-1 | 162 | 50.6% | Yes | ASC + FOLFOX | BSC | OS, PFS, Toxicity |
| Glimelius 19967 | Sweden | Biliary or pancreatic adenocarcinoma | KPS<50 | 90 | 65.6% | No | Sequential 5-FU/LV + ETO or 5-FU/LV aloneb | BSC | OS, QoL Symptoms |
| Kataria 201942 | India | GC | ECOG 3 | 51 | NR | No | CAP | BSC | OS |
| Park 201441 | Korea | Hilar CCA | KPS≥30% | 43 | 51.2% | No | PDT + S-1 | PDT (no further details) | OS, PFS, QoL |
| Sharma 20106 | India | GC | ECOG ≤2 | 81 | 80.2% | Yes and No | FU or GEM + OXA | BSC | OS, PFS |
| Takada 199840 | Japan | Pancreatic, GC and biliary tract | PSc 0 to 3 | 83 | 39.8% | No | 5-FU + DOX + MIT | BSC | OS |
One study had an overall low risk of bias.43 Most of the included studies had an unclear risk of selection bias due to unclear reports about the random sequence generation6,7,40,44 or allocation concealment.6,7,40,41 Several RCTs had high risk of performance bias,6,7,40–42,45 and high40,42,45 or unclear6,7 risk of detection bias. None of the studies was considered to be at high risk of attrition bias, with only two being unclear in this domain.40,42 Reporting bias was considered at high risk for three studies,40,41,44 and unclear for two.7,45 Table 2 provides the summary of the risk of bias assessment of the included studies.
| Study ID | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting |
|---|---|---|---|---|---|---|
| REACHIN43 | Low | Low | Low | Low | Low | Low |
| ClarIDHy44 | Unclear | Low | Low | Low | Low | High |
| Glimelius 19967 | Unclear | Unclear | High | Unclear | Low | Unclear |
| Park 201441 | Low | Unclear | High | Low | Low | High |
| Sharma 20106 | Unclear | Unclear | High | Unclear | Low | Low |
| Takada 199840 | Unclear | Unclear | High | High | Unclear | High |
| Kataria 201942 | Low | Low | High | High | Unclear | Unclear |
| ABC-0645 | Low | Low | High | High | Low | Unclear |
Three RCTs provided data for this comparison.42–44 One RCT compared erlotinib as first line therapy versus BSC in patients with advanced gallbladder cancer.42 The other two used biological/targeted therapies in previously treated patients, comparing its effects to placebo. One used regorafenib in patients with advanced cholangiocarcinoma or gallbladder cancer,43 and the other assessed the effects of ivosidenib in patients with IDH-1 mutant cholangiocarcinoma.44
Our analysis showed that the use of biological/targeted therapies in patients with advanced BTCs may result in little to no difference in OS (MD: 1.66 months higher; 95%CI, 0.65 months lower to 3.96 months higher; low certainty of evidence). This outcome was informed by the three RCTs,42–44 with no significant heterogeneity observed between the studies. The two RCTs assessing previously treated patients43,44 also showed that biological/targeted therapies probably result in little to no difference in PFS (MD: 1.44 months higher; 95%CI, 0.83 months higher to 2.05 months higher; moderate certainty of evidence), and suggest that they increase toxicity (RR: 1.38; 95%CI, 0.99 to 1.93; low certainty of evidence). The evidence is very uncertain about the effects of biological/targeted therapies on quality of life. There were no studies reporting data for the other prespecified outcomes. Table 3 shows the SoF and figure S1 (Additional file 2) provides the meta-analyses for the comparison of biological/targeted therapies versus BSC.
| Patient or population: Advanced biliary tract cancers Intervention: Biological/targeted therapies Comparison: No anticancer drugs | ||||||
|---|---|---|---|---|---|---|
| Outcome N° of participants (studies) | Relative effect (95%CI) | Anticipated absolute effects (95%CI) | Certainty | What happens | ||
| Without biological/targeted therapies | With biological/targeted therapies | Difference | ||||
| Overall survival (OS) assessed with: months № of participants: 281 (3 RCTs) | - | The mean overall survival was 5.8 months | - | MD 1.66 months higher (0.63 lower to 3.96 higher) | ⨁⨁◯◯ Lowa,b | Biological/targeted therapies may result in little to no difference in overall survival. |
| Overall survival (OS) № of participants: 253 (2 RCTs) | HR 0.78 (0.59 to 1.05) [Overall survival] | 15.0% | 11.9% (9.1 to 15.7) | 3.1% fewer (5.9 fewer to 0.7 more) | ⨁⨁◯◯ Lowa,b | Biological/targeted therapies may result in little to no difference in overall survival. |
| Progression-free survival (PFS) assessed with: months № of participants: 251 (2 RCTs) | - | The mean progression-free survival was 1.45 months | - | MD 1.44 months higher (0.83 higher to 2.05 higher) | ⨁⨁⨁◯ Moderatea,c | Biological/targeted therapies probably result in little to no difference in progression-free survival. |
| Progression-free survival (PFS) № of participants: 251 (2 RCTs) | HR 0.41 (0.30 to 0.56) [Progression-free survival] | 10.0% | 4.2% (3.1 to 5.7) | 5.8% fewer (6.9 fewer to 4.3 fewer) | ⨁⨁⨁◯ Moderatea,c | Biological/targeted therapies probably result in little to no difference in progression-free survival. |
| Toxicity assessed with: Patients with grade 3 or more adverse events № of participants: 248 (2 RCTs) | RR 1.38 (0.99 to 1.93) | 32.6% | 45.0% (32.3 to 62.9) | 12.4% more (0.3 fewer to 30.3 more) | ⨁⨁◯◯ Lowa,d | The evidence suggests biological/targeted therapies increase toxicity. |
| Quality of life (1 RCT) | One study assessed quality of life (QoL) using the EORTC QLQ-C30 scale. For Global health status/QoL assessed at cycle 2, the least square mean difference of ivosidenib versus placebo showed a non-significant difference favouring ivosidenib (p=0.12), with a statistically significant effect for the domains physical functioning, cognitive functioning and emotional functioning, and a non-statistically significant effect for social functioning and role functioning. The same measure at cycle 3 showed a non-significant difference favouring placebo (p=0.54), with only physical functioning and emotional functioning showing a significant effect favouring ivosidenib. | ⨁◯◯◯ Very lowe,f | The evidence is very uncertain about the effect of biological/targeted therapies on quality of life. | |||
| Functional status - not measured | None of the included studies for this comparison reported changes from baseline in ECOG or Karnofsky scales. | - | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). To calculate absolute differences for time-to-event measures of OS and PFS, authors assumed a basal risk of 15.0% and 10.0% without the intervention, respectively. CI: Confidence interval; HR: Hazard ratio; MD: mean difference; RCT: Randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Most patients for this comparison correspond to cholangiocarcinoma, and one study included only patients with IDH-1 mutation. Therefore, for specific populations (other cancer locations or cholangiocarcinoma patients without IDH-1 mutation), the certainty of evidence may be downgraded due to indirectness.
b Downgraded by two levels due to confidence interval including threshold for large effect in favour of ACD, and no effect. Also, optimal information size not met.
c Downgraded by one level due to optimal information size not met, but with a confidence interval not including the minimally important difference.
d Downgraded by two levels due to optimal information size not met, and confidence interval including threshold for large effect in favour of no ACD, and no effect.
Six RCTs provided data for this comparison,6,7,40–42,45 including patients with advanced gallbladder6,40,42,45 and bile duct7,40,41,45 cancer. Two studies also included patients with pancreatic cancer.7,40 Four RCTs studied ACDs as first line therapy,7,40–42 and one study exclusively enrolled patients previously treated.45 Other RCT considered ACDs as first line therapy or more.6 Most of the studies described their comparator as BSC,6,7,40,42,45 with one study describing only photodynamic therapy as a cointervention, with no further description of the control group.41
Our meta-analysis of five RCTs6,7,41,42,45 showed that the evidence is very uncertain about the absolute effects of chemotherapy on OS (MD: 3.28 months higher; 95%CI, 0.16 months higher to 6.39 months higher; very low certainty of evidence). The findings derived from a meta-analysis of two RCTs6,41 providing data about the absolute effects on PFS was also very uncertain (MD: 4.27 months higher; 95%CI, 0.25 months higher to 8.78 months higher; very low certainty of evidence). Only one study provided clear data for toxicity,45 suggesting that chemotherapy increases adverse events compared to BSC (RR: 1.33; 95%CI, 1.03 to 1.72; low certainty of evidence). Two studies assessed quality of life using validated scales. Two studies7,41 applied the EORTC QLQ-C30 global health status subscale, ranging from 0 to 100 points7 and the Digestive Disease Questionnaire-15 (DDQ-15) ranging from 1 to 5 points41 to assess QoL. Overall, the effect was a non-significant trend in favour of the intervention group (SMD: 0.08; 95%CI, -0.65 to 0.81; very low certainty). Another study also measured quality of life, but the full results have not been published yet.45 One study7 reported the change in Karnofsky scale from baseline to last follow-up, showing a decrease of 5.19 points from randomisation to last follow-up in the experimental group, and a decrease of 16.06 points in the control group (MD: 10.87). Table 4 shows the SoF and figure S2 (Additional file 2) provides the meta-analyses for the comparison of chemotherapy versus BSC
| Patient or population: Advanced biliary tract cancers Intervention: Chemotherapy Comparison: No anticancer drugs | ||||||
|---|---|---|---|---|---|---|
| Outcome N° of participants (studies) | Relative effect (95%CI) | Anticipated absolute effects (95%CI) | Certainty | What happens | ||
| Without chemotherapy | With chemotherapy | Difference | ||||
| Overall survival (OS) assessed with: months № of participants: 346 (5 RCTs) | - | The mean overall survival was 4.85 months | - | MD 3.28 months higher (0.16 higher to 6.39 higher) | ⨁◯◯◯ Very lowa,b,c | The evidence is very uncertain about the effect of chemotherapy on overall survival. |
| Overall survival (OS) № of participants: 286 (3 RCTs) | HR 0.60 (0.43 to 0.83) [Overall survival] | 15.0% | 9.3% (6.7 to 12.6) | 5.7% fewer (8.3 fewer to 2.4 fewer) | ⨁⨁⨁◯ Moderatea | Chemotherapy probably increases overall survival. |
| Progression-free survival (PFS) assessed with: months № of participants: 124 (2 RCTs) | - | The mean progression-free survival was 2.6 months | - | MD 4.27 months higher (0.25 lower to 8.78 higher) | ⨁◯◯◯ Very lowa,d,e | The evidence is very uncertain about the effect of chemotherapy on progression-free survival. |
| Progression-free survival (PFS) № of participants: 124 (2 RCTs) | HR 0.44 (0.25 to 0.77) [Progression-free survival] | 10.0% | 4.5% (2.6 to 7.8) | 5.5% fewer (7.4 fewer to 2.2 fewer) | ⨁◯◯◯ Very lowa,d | The evidence is very uncertain about the effect of chemotherapy on progression-free survival. |
| Toxicity assessed with: Patients with grade 3 or more adverse events № of participants: 162 (1 RCT) | RR 1.33 (1.03 to 1.72) | 51.9% | 69.0% (53.4 to 89.2) | 17.1% more (1.6 more to 37.3 more) | ⨁⨁◯◯ Lowf,g | Chemotherapy may increase toxicity. |
| Quality of life assessed with: EORTC QLQ-C30, global health status subscale* N° participants: 75 (2 RCT) | - | - | - | SMD 0,08 SD higher (0.65 lower to 0.81 higher) | ⨁◯◯◯ Very lowh,i,j | The evidence is very uncertain about the effect of chemotherapy on quality of life |
| Functional status № of participants: 45 (1 RCT) | One study reported a decrease in Karnofsky scale of 5.19 points from randomisation to last follow-up in the treated group, and a decrease of 16.06 points in the control group (mean difference: 10.87 more). | ⨁◯◯◯ Very lowh,i | The evidence is very uncertain about the effect of chemotherapy on functional status | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). To calculate absolute differences for time-to-event measures of OS and PFS, authors assumed a basal risk of 15.0% and 10.0% without the intervention, respectively. CI: Confidence interval; HR: Hazard ratio; MD: mean difference; SMD: standardised mean difference; SD: standard deviations; RCT: Randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
* EORTC QLQ-C30, global health status subscale with a score transformation made by the authors from 0 to 100, and with Digestive Disease Questionnaire-15 (DDQ-15) ranging from 1 to 5 points. For both, scales higher scores represent higher response level.
a Downgraded one level due to risk of bias, especially unclear risk of selection bias for most of the included studies.
c Downgraded one level due to imprecision, since the confidence interval (despite being statistically significant) includes the minimally important difference, predefined as two months.
d Downgraded two levels due to inconsistency, which may be due to differences in specific chemotherapy schemes. Compared to no ACDs, a study reports no differences for FUFA and a significant difference in favour of mGEMOX, and another study reports a significant difference in favour of S-1.
e Downgraded two levels due to imprecisions. The confidence interval includes the minimally important difference, and may lead to opposite clinical decisions; and there are few patients included for this comparison.
g Downgraded two levels due to imprecision, since the confidence interval (despite being statistically significant) includes the minimally important difference, predefined as a difference of 10% of events between groups, and there are few patients and events included for this comparison.
h Downgraded two levels due to risk of bias, since both included studies have unclear risk of selection bias, and high risk of performance bias. Also, one study showed high risk of reporting bias.
Our SR identified, assessed and synthesised the evidence of eight RCTs assessing the effects of ACDs versus BSC for patients with advanced BTCs. For most of the assessed survival and toxicity outcomes, our results show low or very low certainty of evidence, with a lower magnitude of benefit for ACDs in second or further lines of therapy (figures S1-S2, additional file 2). Nevertheless, toxicity was less frequently or systematically reported than survival outcomes. Even more, most of the available RCTs have not systematically reported or even assessed quality of life, functional status, symptoms, or outcomes related to the quality of end-of-life care.
The comparison of biological/targeted therapies versus placebo needs a thoughtful interpretation. First, the certainty of evidence for OS and toxicity is low, which should be translated into an enhancement of shared decision-making processes. Since there is still important uncertainty about the true (positive and negative) effects of the intervention, patients’ perspectives and costs of the intervention should play a key role in decision-making. Second, an analysis only of the relative magnitude of effect of survival outcomes (HR reported in table 3) may lead some clinicians to overestimate the findings. When analysed as absolute difference, our analysis showed an improvement in OS of only 1.66 months. An improvement of less than 2 months, even in the context of an advanced disease, should not be considered a clinically important difference by default —even if it represents an apparently important reduction in HR. In third place, one of the three included studies for this comparison, the ClarIDHy trial, included a biological therapy (ivosidenib) focused in IDH-1 mutant patients.44 This may hinder the extrapolation of the findings to non-IDH-1 mutant patients (see table 3 footnotes), where the treatment is not considered a targeted therapy. On the other hand, since in this study, the primary objective was PFS and not OS, the patients could make a cross-over to the active treatment, which could have biased the results towards the null effect. Also, this study provided the data informing the quality of life outcome for the comparison of targeted/biological therapies versus BSC, but it showed discrepant results over time.
In the case of the comparison of chemotherapy versus BSC, the results should be cautiously considered in terms of lines of treatment. As shown in figure S2, additional file 2, survival outcomes tend to be more favourable to chemotherapy in the first line setting, with a pooled benefit of 5.45 months for this subgroup in terms of overall survival. Nevertheless, and despite the magnitude of effect, the certainty of evidence is very low for the absolute effects of the therapy. Also, the assumption that the therapy has better outcomes as a first line treatment cannot be currently extrapolated to other assessed outcomes, since the identified RCTs showed both non-significant and dissenting results for quality of life (as first line therapy),7,41 and only one provided comparative data regarding toxicity (as second line or more).45
We identified several outcome reporting gaps for both comparisons. Only two studies reported information about symptoms, with one reporting improvement in symptoms based on the clinicians perception,7 and the other reporting pain as a symptom in the context of a quality of life assessment.44 We acknowledge that the reporting of symptoms carries important challenges, such as differentiating it from adverse events, or avoiding redundancy with the data reported for quality of life. These issues may explain why we found no evidence regarding this specific outcome. Regarding functional status, most studies consider it as a baseline measurement, but only few assess a change in functional status as an outcome. None of the included RCTs measured patients’ admissions or emergency consultations, provision of palliative care, or place of death. All these outcomes may be important for most patients, and, therefore, should be routinely considered by clinicians for shared decision-making.
Considering the findings from our previous research within the ASTAC project,19 this evidence synthesis constitutes an appropriate research aiming to deal with a question not well responded yet by systematic reviews. We conducted an exhaustive search strategy, and performed the selection, data extraction and analysis process by at least two independent reviewers. Besides adhering to the GRADE guidance for assessing certainty of evidence and in order to ensure consistency for the dissemination of our results, we adhered to the GRADE recommendations for communicating our findings. Despite our efforts to retrieve all the relevant evidence, our findings are limited due to the scarce number of included studies and, in some cases, the heterogeneity of the included populations (e.g. including other cancer localisations) or interventions.
This SR may serve as a reliable basis for communicating the current research about the effects of using versus not using ACDs in advanced BTCs to patients, including current uncertainties in a transparent way. Future guideline panels may draw upon our findings to improve question prioritisation and evidence to recommendation processes. This review also highlights the current evidence gaps in the primary research. Future RCTs should consider the questions included in our SR, since there are still areas with low and very low certainty of evidence, and a lack of consideration of patient-important outcomes.
CR: Conceptualisation; Methodology; Investigation; Validation; Data curation; Formal analysis; Writing - original draft; Visualisation. JB: Conceptualisation; Methodology; Investigation; Validation; Data curation; Formal analysis; Writing - original draft; Visualisation. NM: Investigation; Validation; Data curation; Formal analysis; Writing - Review & Editing. PR: Validation; Formal analysis; Writing - Review & Editing. EG: Validation; Formal analysis; Writing - Review & Editing. XBC: Conceptualisation; Methodology; Writing - Review & Editing; Project administration; Supervision; Funding acquisition.
Most of the software used for this article were publicly available, expect for Covidence. An open access alternative that may replace this software is Rayyan (https://www.rayyan.ai/).
Open science framework: Anti-cancer drugs versus supportive care for advanced biliary tract cancers: a systematic review, DOI 10.17605/OSF.IO/WDRMC. 47
This project contains the following extended data:
Table S1: MEDLINE/PubMed search strategy for the ASTAC project (until December 2019)
Table S2: Updated MEDLINE/PubMed search strategy for this systematic review (until May 2022)
Figure S1: Meta-analyses for the comparison of biological/targeted therapies versus BSC
Figure S2: Meta-analyses for the comparison of chemotherapy versus BSC
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication) (http://creativecommons.org/publicdomain/zero/1.0/)
Open science framework: PRISMA checklist for “Anti-cancer drugs versus supportive care for advanced biliary tract cancers: a systematic review”, DOI 10.17605/OSF.IO/WDRMC. 47
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication) (http://creativecommons.org/publicdomain/zero/1.0/)
Carolina Requeijo is a PhD candidate at the Doctorate Program on Biomedical Research and Public Health, Universitat Autònoma de Barcelona, Barcelona, Spain.
Appropriateness of Systemic Oncological Treatments for Advanced Cancer (ASTAC) Research Group: Acosta-Dighero R, Antequera Martín A, Auladell-Rispau A, Bonfill Cosp X, Bracchiglione J, Cantero-Fortiz Y, Hernandez ED, Irassar J, Meade AG, Meinardi P, Merchán-Galvis AM, Meza N, Quintana MJ, Requeijo C, Rodríguez-Grijalva G, Salas-Gama K, Salazar J, Santero M, Savall-Esteve O, Selva A, Solà I, Urrútia G.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)