Keywords
Rorschach; Comprehensive System; Neuroimaging; fMRI; resting-state networks.
The Rorschach test is one of the most popular tests used in clinical settings for psychopathology and personality assessment; however, there is still little evidence regarding the functional correlates of test responses. Functional magnetic resonance imaging (fMRI) allows for the investigation of biological correlates associated with different psychological functions. Among other applications, fMRI has been used to identify different networks that reflect brain activity in the resting state (rs-fMRI), that is, when an individual is not engaged in any specific task. Among these resting-state networks (RSN), the best-known and most studied are the Default Mode Network (DMN), along with other networks such as salience, frontoparietal, sensorimotor, temporo-parietal, visual, and cerebellar networks.
We used this type of analysis to obtain new evidence regarding Rorschach. This study aimed to analyze the functional brain architecture underlying Rorschach’s personality variables, administered according to Exner’s Comprehensive System (CS). In particular, the aims are: 1) to ascertain the existence of correlations between CS variables and activity of the RSN, and 2) to use these profiles of activity to develop a new data-driven clustering of the CS variables. Archival data from twenty-four non-clinical subjects were analyzed. Independent Component Analysis (ICA) and partial least squares regression (PLS) were used to analyze the fMRI data.
The results showed specific associations with the given Rorschach variables (several of which could be grouped into higher-order latent factors) and activity of the main RSN. Moreover, the cluster analysis outlined important groupings of Rorschach variables, particularly regarding their clinical implications.
Our study could be comprised in the existing literature providing strong evidence about the neurobiological validity of the Rorschach test.
Rorschach; Comprehensive System; Neuroimaging; fMRI; resting-state networks.
The Rorschach Comprehensive System (CS) is a standardized performance-based test that shows in vivo how subjects combine attention, perception, and logical analysis to cope with a task. These strategies are also applied in daily life and can be considered as the expression of the personality “in action.”
Specifically, the test provides information on both structure and dynamic functioning of personality; the term “structure” indicates the combination of personality states (temporary and situation-dependent set of characteristics, attitudes and moods) and traits (stable characteristics and attitudes). The concept of “functioning” describes how states and traits guide one’s perception of the world and behavior, consistent with one’s own beliefs and needs (Exner, 2003; Exner & Erdberg, 2005).
The test consisted of 10 cards, each showing an inkblot for which the subject was asked to answer the question “What could it be?” (Exner, 2003). The goal was to detect personality features through the answers provided in the test. The purpose, therefore, is to observe what the person “is”, instead of what the person “says he is”. In other words, explicit behaviors measured by the test are assumed to reliably reflect the implicit dimensions of psychological functioning.
In 1970, J. E. Exner began a systematic investigation to make Rorschach method a test: standardize and objective the Rorschach method. The first version of the Comprehensive System was published in 1974, and over the years, it will undergo numerous revisions, the last of which dates back to 2005. Exner, following Rorschach’s thought, emphasizes the role of perceptual processes, which are clearly distinct from associative ones. A projective approach considers the content of a response as a product of the unconscious. According to Exner, projection plays a core role only in some phases of the response process: at the beginning, when the stimulus is “scanned,” and at the end, when the subject personalizes the answer. In all other phases, cognitive and perceptual mechanisms, such as attention or the processing of inkblots, predominate (Exner, 2003; Exner & Erdberg, 2005). After the answer phase, there is an inquiry that aims to investigate the response process to understand how the subject got to the answer (Exner, 2003). Finally, the collected data are transformed into a series of indices reported in the Structural Summary (SS) (Exner & Erdberg, 2005).
Exner’s work has provided instruments with good psychometric properties. In particular, the test has a high degree of inter-rater reliability; excellent test-retest fidelity; and adequate convergent, divergent, and predictive validity (Exner, 2003; Exner & Erdberg, 2005).
Most of the brain’s energy is directed to support its activity at rest (Raichle, 2011). Spontaneous activity that occurs in the brain when it is not engaged in any specific task consumes more than 80% of the brain’s energy (Rosazza & Minati, 2011). These data suggest that spontaneous brain activity is associated with what is called “present state” or “mental state” (Raichle, 2011). In the last 20 years, resting-state functional Magnetic Resonance Imaging (rs-fMRI) has been used to investigate physiological correlates of spontaneous brain activity (Canli & Amin, 2002; Chen et al., 2020). The goal of neuroimaging studies that measure brain activity at rest is to identify spontaneous neuronal oscillations in large areas of the brain when the person is not engaged in any specific task (Buckner & Vincent, 2007). Furthermore, the tool allows us to study functional connectivity, that is, synchronous neural activation, among brain regions from an anatomical point of view, which may not even be close to each other (Rosazza & Minati, 2011).
Despite the growing interest in large-scale resting-state brain networks, many aspects of their function (DeYoung et al., 2010). Nevertheless, there are several hypotheses, such as the idea that spontaneous activity reflects cognitive processes and that they reflect a combination of conscious activity and internal neural dynamics that could be essential for the emergence of behaviors, although they appear even in the absence of a behavioral correlate (Rosazza & Minati, 2011). However, two aspects are well known: 1) these patterns have a high test-retest reliability, which means that they are stable over time (DeYoung et al., 2010; Teeuw et al., 2021; Zuo et al., 2010); and 2) resting-state connectivity is strictly linked to one’s history and experience (Sporns, 2013). Therefore, it is reasonable to assume that brain activity during REST reflects individual differences in several healthy and pathological psychological variables.
Over the years, several brain networks associated with functional connectivity during resting conditions have been identified. Despite methodological differences, the most supported across different studies are the following networks: the default mode network (DMN), sensorimotor network, executive control network, two lateralized frontoparietal networks (FPN), visual network, temporoparietal (auditory) network, dorsal attention network (DAN), and salience network (SN) (Lund et al., 2022; Razi et al., 2017; Rosazza & Minati, 2011).
Nowadays, the more studied more because it is more active when people are at rest than when they are engaged in a task (Rosazza & Minati, 2011), although the exact cognitive meaning of its activity is not yet fully clear (Mancuso et al., 2022). While it is generally described as a unitary network, three main sub-components can be identified, each possibly underlying a slightly different cognitive domain. The first encompasses the medial prefrontal cortex (mPFC), which is crucial for receiving sensory information coming both from the external world and from inside the body, which is then conveyed to structures such as the hypothalamus, amygdala, and periaqueductal gray (PAG). This pattern suggests a role of the DMN in the regulation of social behavior and mood and in motivational drive, three aspects that play an important role in shaping one’s personality (Raichle, 2015). The second sub-component consists of the dorsomedial prefrontal cortex (dmPFC) and is associated with self-referential judgments (Gusnard et al., 2001). The third sub-component includes the activation of the posterior cingulate cortex (PCC), adjacent precuneus, and lateral parietal cortex. The last module plays an important role in the evocation of previously formed memory. In fact, it is associated with hippocampal formation, a structure long known for its involvement in memory processes (Shannon et al., 2013; Yeshurun et al., 2021). The activity of the DMN has also been observed in non-human primates, which may suggest on the one hand that it is a stable component across various species and, on the other, that it is an intrinsic property of the brain (Rosazza & Minati, 2011).
If the DMN is the most studied component, the sensorimotor network will be the first to be identified using resting-state fMRI (Biswal et al., 1995, 2010). It complies with the pre-motor gyrus, post-motor gyrus, and supplementary motor areas. The overlap between this network and the sensory and motor components is not only anatomical but also functional; indeed, this network seems to be activated during the execution of (active) motor tasks. The question, therefore, is how the same pattern that is activated during the execution of a task is consistently activated in resting conditions. One hypothesis is that regions that are activated together during active tasks tend to be activated together during resting stages (a type of procedural neural memory (Rosazza & Minati, 2011).
Another resting-state network is the visual network, for which there is no agreement on the number and components of the network. The most solid findings are in line with defining this network as formed by mesial visual areas, that is, the striate cortex and typically mesial extra-striate regions (e.g., the lingual gyrus), and lateral visual areas (e.g., occipital pole and occipito-temporal regions). Stevens et al. (2010) observed that fluctuations in this component were modulated by a visual task that was completed before data acquisition. This result seems to support the hypothesis that resting states have experience-dependent dynamic components that may play a role in memory consolidation.
The executive control network comprises three components that are generally involved in tasks that require the intervention of executive functions, such as working memory tasks: the medial frontal gyrus, superior frontal gyrus, and anterior cingulate cortex (Rosazza & Minati, 2011; Seeley et al., 2007).
The FPN is defined by two highly lateralized components: one in the left hemisphere and the other in the right hemisphere. This circuit includes the inferior frontal gyrus, medial frontal gyrus, precuneus, inferior parietal lobule, and the angular gyrus. In addition, the brain areas implicated in this circuit represent the neural correlates of a plethora of cognitive processes ranging from memory to language (Rosazza & Minati, 2011).
The temporoparietal network is composed of the inferior frontal gyrus, medial and superior portions of the temporal gyrus, and the angular gyrus. The anatomical structures involved in this circuit are usually associated with language and, although the functions are not yet completely clear (Rosazza & Minati, 2011), it seems that the activation of this ls-rsbn network corresponds to the functional organization of language processes (Rosazza & Minati, 2011; Dronkers et al., 2004; Koyama et al., 2010; Turken & Dronkers, 2011).
DAN is an important mediator of goal-directed and selective attentional processing. It includes the frontal eye fields, the posterior and anterior intra-parietal sulci, and the middle temporal visual area (Lanssens et al., 2020; Allan et al., 2019; Raichle, 2011; Razi et al., 2017).
The SN is involved in the interoception of feelings associated with gratification and consists of synchronous activation of the insula, anterior cingulate cortex, and ventral striatum (Pace-Schott & Picchioni, 2017). Its connection to other networks suggests that the SN also contributes to a wide variety of mental functions such as communication and social behavior (Menon, 2015). Although the results are not yet fully clear, some studies have shown activation of this network in response to an experience of social rejection (Redcay & Warnell, 2018).
Finally, the last network is represented by the cerebellum, whose functions are not limited only to the motor field but also expand to cognitive processes (Guell et al., 2018; Tang et al., 2022).
Cognitive psychologists have used neuroimaging techniques to study brain connectivity underlying functions such as memory or language. The study of neurobiological correlates of complex psychological constructs, such as human personality or psychopathology, is complicated. Recently, however, a growing number of studies have investigated the ancestral neuronal roots of personality and the biological correlates of many psychiatric disorders (Panksepp & Davis, 2020).
Based on the literature presented so far, it is possible to ask whether neuroimaging techniques can identify the neural correlates of personality, as measured by the Rorschach CS. Otherwise, can the Rorschach CS predict some neural parameters?
Currently, only a few studies have investigated the relationship between Rorschach responses and their neural correlates. For instance, Giromini and colleagues (2017b) found greater cortical activations of temporo-occipital and fronto-parietal areas in individuals that were looking at ten Rorschach cards compared to simple fixation cross conditions. Furthermore, specific associations between the activity of the mirror neuron system and human movement responses have been found among non-clinical subjects (Giromini, Viglione, Pineda et al., 2017a), as well as specific associations between dependency-related Rorschach responses and brain activity of the reward neural system (Giromini et al., 2019). The response style of the entire Rorschach protocol was related to specific neural activation. Indeed, Vitolo et al. (2021) investigated how the general complexity of given Rorschach responses (conceptualized as the psychological effort that and individual could adopt during the Rorschach task: Meyer et al., 2011) could be related to specific attentional brain networks and found significant associations between these responses and the activity of delimited brain areas concerning the dorsal attention network. More recently, Vecchio et al. (2023) summarized these and other results in a systematic review, highlighting how the involvement of the Rorschach task could be reflected by the activity of specific visual, attentional, social, and emotional brain areas.
Despite the relevance of these recent studies, some problematic issues remain. First, scanner noise could compromise the ecological validity of the Rorschach test. Furthermore, the context of the scanner makes it impossible to follow standardized test procedures. Moreover, it has been suggested that neural correlates associated with different personality traits are not limited to specific brain areas but are related to large-scale dynamic interactions, including brain regions responsible for lower-level sensory functions and those responsible for higher-order cognitive functions (Adelstein et al., 2011). Given these considerations, it seems that the existing literature does not cover the aforementioned issues, focusing only on specific brain areas or administering the Rorschach during MRI scans (even if the study participants were not able to talk).
To overcome these issues, we examined the relationship between the Rorschach test and brain states in resting conditions using a particular approach. Specifically, the aims are 1) to ascertain the existence of a relationship between the CS variables and RSN, and 2) to develop a new clustering of the CS variables that reflects their association with RSNs activity.
In our research design, participants underwent functional magnetic resonance imaging (fMRI) under resting conditions. In particular, they were asked to rest with their eyes open and to passively start at a point. The Rorschach test is performed outside the scanner. In this way, it was possible to obtain a more reliable measure of personality since the Rorschach test was administered in an ecological context and in full compliance with the standardization rules. Furthermore, it was possible to detect active brain patterns at rest and not during task performance.
In this study, secondary data provided anonymously were used.
The sample consisted of 24 healthy and right-handed participants who were recruited using the “snowball” sampling method. The sample was balanced by sex (12 males and 12 females) and age (mean = 29.6; sd = 6.82), with an average education level of 18 years (sd = 3.03).
The inclusion criteria were as follows: a) absence of brain accidents; b) absence of neurological and/or psychiatric pathologies; c) no use of psychotropic drugs; d) absence of pregnancy (or suspected ongoing pregnancy); e) absence of anxiety symptoms, panic attacks, and claustrophobia; f) absence of pacemakers, vascular stents, metal prostheses in the brain, heart, and large blood vessels to comply with MRI safety standards; and g) age between 20 and 40 years (to ensure comparability at the level of brain morphology).
Each participant signed a self-certification as part of the informed consent, in which they stated that they had not previously received a psychiatric diagnosis and that they had never been hospitalized for this type of disorder. All procedures presented in the current study were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
The experimental procedure is divided into three parts. During the first phase (pre-scan phase), the following psychological tests were conducted to exclude subjects with clear psychopathological symptoms. These tests, protected by copyright, were made available by the Psychology Department of the University of Turin, for which it holds the necessary licenses.
• Beck Depression Inventory - II (BDI)
• State-Trait Anxiety Inventory (STAI)
• SCL-90-R (Symptom Check-List - 90 Revised)
In the second part (scan phase), participants underwent resting-state fMRI scanning.
Finally, the third phase (post-scan phase) took place one week after the fMRI session, which consisted of the administration of the Rorschach CS test, which is not protected by copyright.
Beck Depression Inventory - II (BDI - II). The BDI-II is a self-administered questionnaire that detects the presence, severity, and intensity of depressive symptoms in individuals over 13 years of age. The Italian version was administered and validated by Ghisi et al. (2006).
State-Trait Anxiety Inventory (STAI). The STAI-Y is a self-administered questionnaire that is useful for detecting the presence and intensity of anxiety symptoms in adolescents and adults. In particular, the Italian version validated by Pedrabissi and Santinello (1989) was administered.
SCL-90-R (Symptom Check-List - 90 Revised). The SCL-90-R is a self-administered questionnaire that investigates a wide range of psychological problems and symptoms in individuals over 12 years of age. In particular, the Italian version validated by Sarno et al. (2011) was administered.
Rorschach CS. The Rorschach was administered and signed according to the Comprehensive Exner System (CS) by expert clinicians (at least five years of practice). To test the reliability of the instrument, the degree of agreement between different judges was assessed. In particular, 20 tests were randomly selected and resigned by a second independent judge. The Intraclass Correlation Coefficient indicates reliability from good (0.68 for Color-Shape responses) to excellent (1 for R). On an exploratory basis, the Rorschach variables were selected considering the trait variables most representative of the test and supported by the literature: 27 CS variables (Exner & Andronikof-Sanglade, 1992); 65 CS variables (Mihura et al., 2013). Table 1 summarizes the Rorschach variables considered in this study.
| CS Variables (Exner, 1991) | Variables Interpretations (Mihura et al., 2013) |
| PTI | Disturbed thinking and distorted perceptions |
| M- | Distorted perceptions of others, including psychotic perceptions |
| DEPI | Depressive tendencies |
| S-CON | Suicide risk |
| D Score | Current level of coping abilities |
| Xy | Preoccupations with body vulnerability or its functioning |
| Ay | Minimizing emotional experiences by intellectualizing |
| r | Narcissistic tendencies |
| FQ- | Distorted perceptions |
| WSumC | Emotions influence on thoughts and experiences |
| Art | Minimizing emotional experiences by intellectualizing |
| (2) | Egocentricity, either narcissistic or distress related (high) or negative self-image (below low cut point) |
| WSum6 | Thought disturbance |
| PHR | Disturbed and maladaptive understanding of others |
| AdjD | Level of coping abilities regardless of current stressors |
| F | Avoidance vs attentiveness to complexity, subtlety, or nuance |
| AB | Minimizing emotional experiences by intellectualizing |
| R | The ability or tendency to respond with many ideas |
| GHR | Healthy and adaptive understanding of others |
| HVI | Interpersonal vigilance |
| M | Mental abilities, including planning, imagination, and empathy |
| CDI | Interpersonal and/or emotional deficits |
| An | Preoccupations with body vulnerability or its functioning |
| CritCont | (An, BI, Ex, Fi, Fd, Sx, Xy, AG, MOR) |
Neuroimaging data analyses were performed using archival data collected for other research purposes (unpublished data). Despite procedures and data analyses referring to archival data, the findings are discussed in light of the most recent scientific literature.
2.4.1 MRI data acquisition
In line with standard resting-state procedures, participants were instructed simply to keep their eyes closed, think of nothing in particular, and not to fall asleep. After each run, participants were asked if they had fallen asleep during the scan. Data from subjects with positive or doubtful answers were excluded from the study. Images were gathered using a 1.5 T INTERA™ scanner (Philips Medical Systems) with a SENSE high-field, high-resolution (MRIDC) head coil optimized for functional imaging. Two functional resting states (T2*-weighted) were acquired using echoplanar (EPI) sequences, with a repetition time (TR) of 2000 ms, an echo time (TE) of 50 ms, and a 90° flip angle. The acquisition matrix was 64x64, with a 200 mm field of view (FoV). A total of 850 volumes were acquired for the first run and a total of 450 volumes were acquired for the second run, with each volume consisting of 19 axial slices, parallel to the anterior posterior (AC–PC) commissure; slice thickness was 4.5 mm with a 0.5 mm gap. In addition to resting-state runs, 3D high-resolution T1-weighted structural images were acquired using a Fast Field Echo (FFE) sequence, with a 25ms TR, an ultrashort TE, and a 30° flip angle. The acquisition matrix was 256x256, and the FoV was 256 mm. The dataset consisted of 160 contiguous sagittal images covering the entire brain. The in-plane resolution was 1 mm × 1 mm, and the slice thickness was 1 mm (1 × 1 × 1 mm3 voxels).
2.4.2 Data preprocessing
Analyses of the resting-state data were performed using the software package FSL 5.0 (Woolrich et al., 2009; Smith et al., 2004; Jenkinson et al., 2012), which is an open source comprehensive library of analysis tools for functional and structural magnetic resonance imaging, and diffusion brain imaging data ( https://fsl.fmrib.ox.ac.uk/fsl/fslwiki). The data were corrected for motion artifacts using the MCFLIRT function and spatially smoothed using a Gaussian kernel of 8-mm FWHM. High-pass temporal filtering (50s) was also performed to remove low-frequency scanner-drift artifacts. (Jenkinson et al., 2002).
Preprocessed data were first co-registered to the native T1 images and then normalized into the MNI152 standard space using the FSL-FLIRT function (Jenkinson & Smith, 2001; Jenkinson et al., 2002).
2.4.3 Independent component analysis (ICA)
Analyses of resting-state fMRI data were performed using independent component analysis (ICA) as implemented in the FSL MELODIC function (Beckmann & Smith, 2004). Briefly, this technique allows us to identify independent sources of signals in the brain, thereby grouping voxels showing a similar temporal profile during the resting state (i.e., the RSN). In this study, the number of independent components (ICs) to be extracted was set to 35. Seventeen of these were considered to represent noise and were discarded after visual inspection. Finally, 10 out of the 18 remaining components were retained for further analyses, as they were deemed to represent the following RSN: Default Mode Network (DMN), salience (SAL), Left Frontoparietal (LFP), Right Frontoparietal (RFP), sensorimotor (Smot), low-order visual (VIS), high-order visual (V+), cerebellar (cerebellum), temporoparietal (temporoparietal), and Supplementary Motor Area (SMA).
When applied to multisubject data, ICA produces a unique set of independent components (ICs) for the entire sample. To obtain subject-specific ICs related to individual Rorschach responses, a dual-regression approach (Beckmann et al., 2009; Nickerson et al., 2017) was used to bring the 10 ICs (and therefore the 10 RSN) observed at the group level back to the topography of each subject.
Table 2 summarizes these brain networks.
2.4.4 Partial Least Square regression (PLS) analysis
Patrial Least Square regression (PLS) is a machine learning technique that can be used to investigate the relationship between two sets of observations pertaining to the same group of subjects but based on different variables. This is achieved by means of a dimensionality reduction technique that allows to identify the so called “latent factors” that can explain both two sets of variables used (Chen et al., 2019). In more technical terms, the goal of the Partial Least Square Regression (PLS) is to predict from and to describe their common structure. In this specific case, is a matrix in which each row represents a different participant and each column represents the activity in a given voxel of the brain. is again a subject × variable matrix, with the variables being CS Rorschach scores.
Therefore, the aim of PLS in our study is to predict Rorschach variables on the basis of resting-state activity and to describe their common structure. Besides producing the latent factors, the output also returns the so called “factors loading.” These represent the strength of the association between the computed latent factors and the variables originally measured for the sample and used as inputs for the analysis.
The first calculation step is the mean centering of the data then the relationship between the columns of and is obtained by a cross-product as:
The SVD decompose into three matrices:
The latent variables of X are given by:
This matrix shows how the brain activity relates to each observation.
Then, the loading of (the projection of onto the space of ) is given by
describe the voxels as explained by the latent variables.
In the same manner for the behavioral variables stored in we have:
where is a diagonal matrix representing the regression weights used to predict from . The predicted scores are given bywhere is the Moore-Penrose pseudo inverse of .Statistical inference consists of two separate analyses. Fixed effects and random effects models. For the fixed effects model, the quality of the regression of the L latent variables is obtained by computing the predicted matrix and then measuring the similarity with the original data . The most popular measure of similarity is the residual sum of squares, defined as:
the smaller the value of RESS, the better the prediction.To generalize the results to the population of interest, the random effects model, it is necessary to estimate the generalization capacity of the PLSR model. In this case, a standard parametric approach can be used; therefore, the model was evaluated using the jackknife method.
In the jackknife method, each observation is excluded from the data, and the remaining set of data constitutes the learning set. Each of these sets is used to perform a PLSR that is used to predict the left-out observation. The predicted observations were stored in a matrix called . The quality of the model was evaluated as the similarity between and . In this case, the best measure is the residual sum of squares defined as
For a random model, it is critical to determine the optimal number of latent variables for analysis. A good approach (Tenenhaus, 1998) starts by adding a latent variable, one at a time, and computing the ratio for the l-th latent variable defined as
where PRESS is the value for the l-th latent variable, and RESS is the value for the (l-1)-th latent variable. A latent variable was maintained if the was greater than 0.05.When the number of correct latent variables was obtained, the confidence intervals of the predicted values were derived using a bootstrap statistic. In a bootstrap statistic, a large sample is obtained by drawing observations with replacements from the set of original data. The PLS distribution obtained using these observations was used to estimate the sampling distribution of the parameters.
2.4.3 Cluster analysis
As explained before, PLS allowed us to understand the relationship between resting state activity in 10 RSN and 27 CS scoring variables of the Rorschach signature. However, resting-state information could also be useful to better elucidate the relationship between CSs. Clustering methods can be used to group items that show a high degree of similarity with a given dimension (Cauda et al., 2010; Manuello et al., 2022). In this specific case, the similarity among the CSs was based on the variance explained by them for the activity profile of the RSNs. In practice, a vector called “network profile” was created for each CS. Each value of this vector represents the variance explained by the specific CS for each of the ten RSN. The obtained network profiles were then organized into a CSs × RNS matrix (i.e., 27 rows for 10 columns) that was used as the input for the hierarchical clustering analysis. The between-row distance (i.e., between CSs) was computed using Euclidean distance as a metric, whereas clusters were created using Ward’s linkage (Ward, 1963).
PLS regression showed that by means of three latent factors, the 27 considered Rorschach variables could explain the activity observed in ten ICs identified through ICA, which had been retained for further analyses as they were deemed to represent some canonical RSNs.
Latent factor 1 was found to explain the variance in each of the considered RSN, with a peak at approximately 40% for the SMOT network. Latent factor 2 explained a much reduced amount of variance for the SAL, LFP, and CEREB networks. This was also the only network that showed some contribution from latent factor 3 (Figure 1).
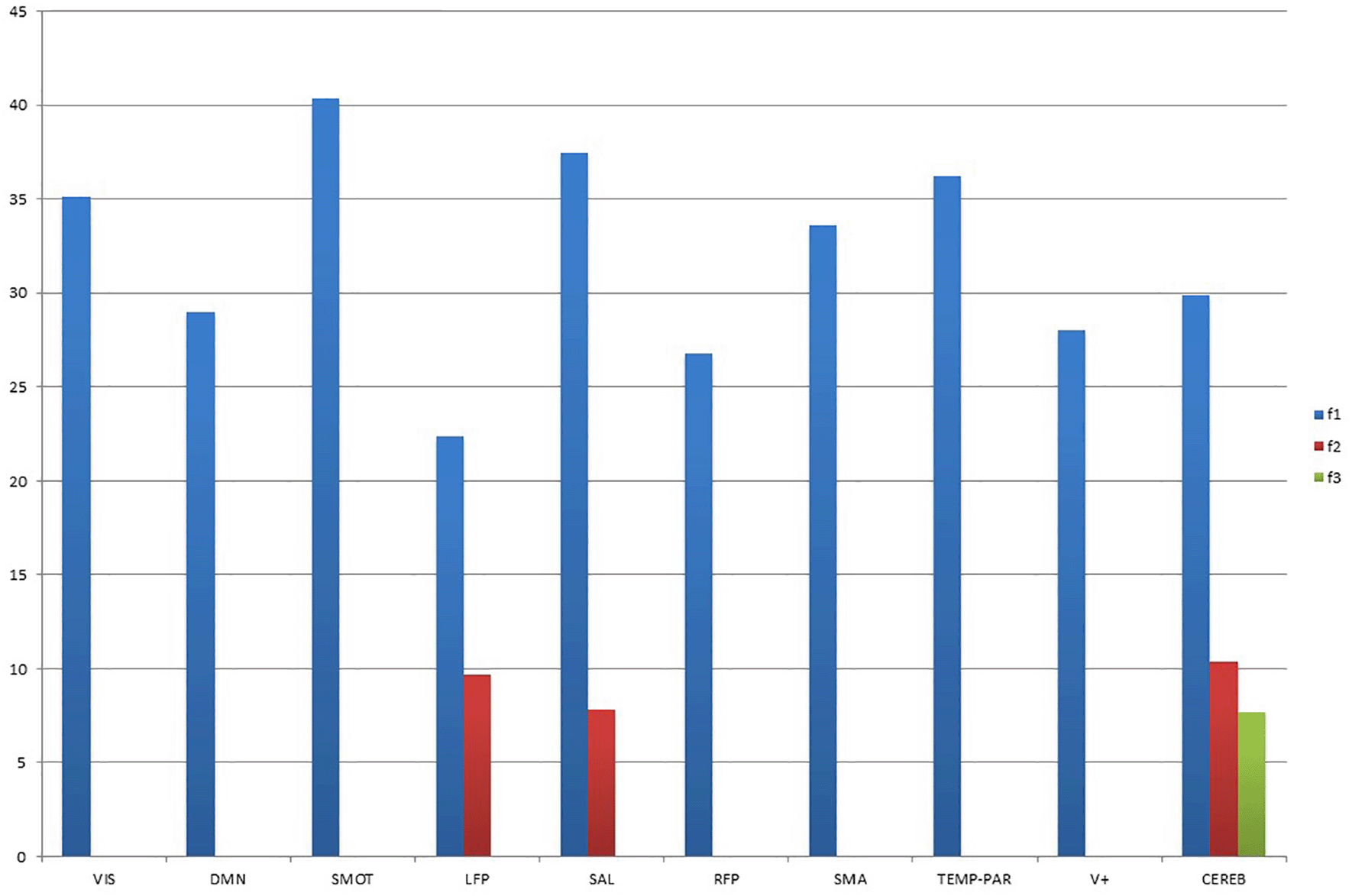
Note: Values on the y-axis represent the percentage of explained variance; VIS = low-order visual; DMN = default mode network; SMOT = sensorimotor; LFP = left frontoparietal; SAL = salience; RFP = right frontoparietal; SMA = supplementary motor area; TEMP-PAR = temporo-parietal (auditory); V+ = high-order visual; CEREB = cerebellar.
In addition to estimating the variance explained by each of the three latent factors, PLS regression allowed the quantification of the relationship between each variable and the obtained latent factors. It was therefore possible to observe how the 27 CSs were related to each of the three latent factors, and at the same time, which voxels across the brain each factor is relevant for (Figures 2 to 11). Notably, for all ten RSNs considered, latent factor 1 showed a loading pattern that closely resembled the original brain network. This means that the relationship between a given RSN and the 27 CSs captured by the latent factor involved the whole RSN in a way comparable to how it had been observed during the resting state and not only by the contribution of a few sparse voxels across the brain. Moreover, even when latent factors 2 and 3 were involved, the significant voxels were still localized in a coherent manner with the expected spatial pattern for canonical RSNs.
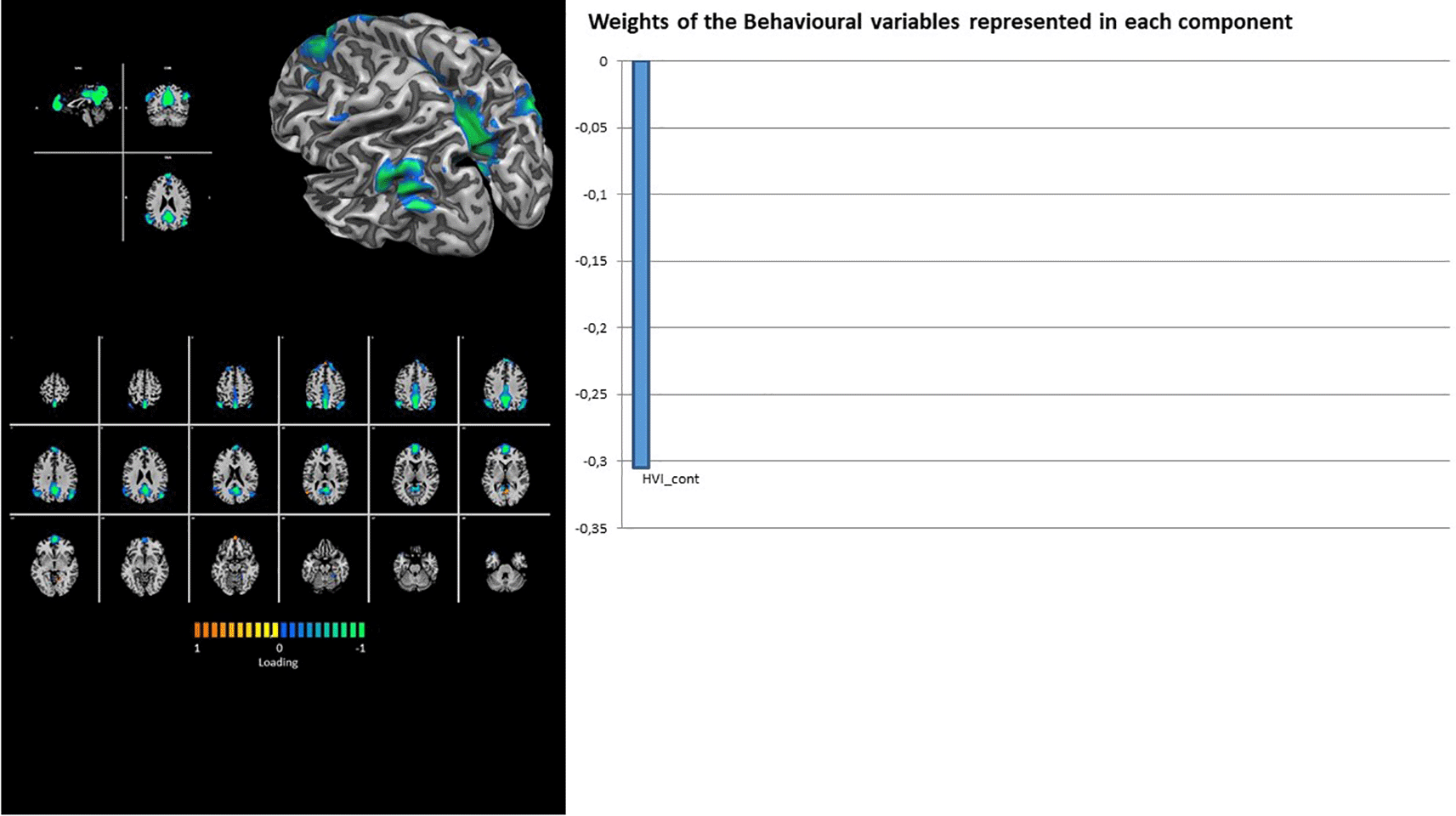
Regarding the loadings on CSs, the latent factors showed that most of the considered RSNs were associated with only a few of them.
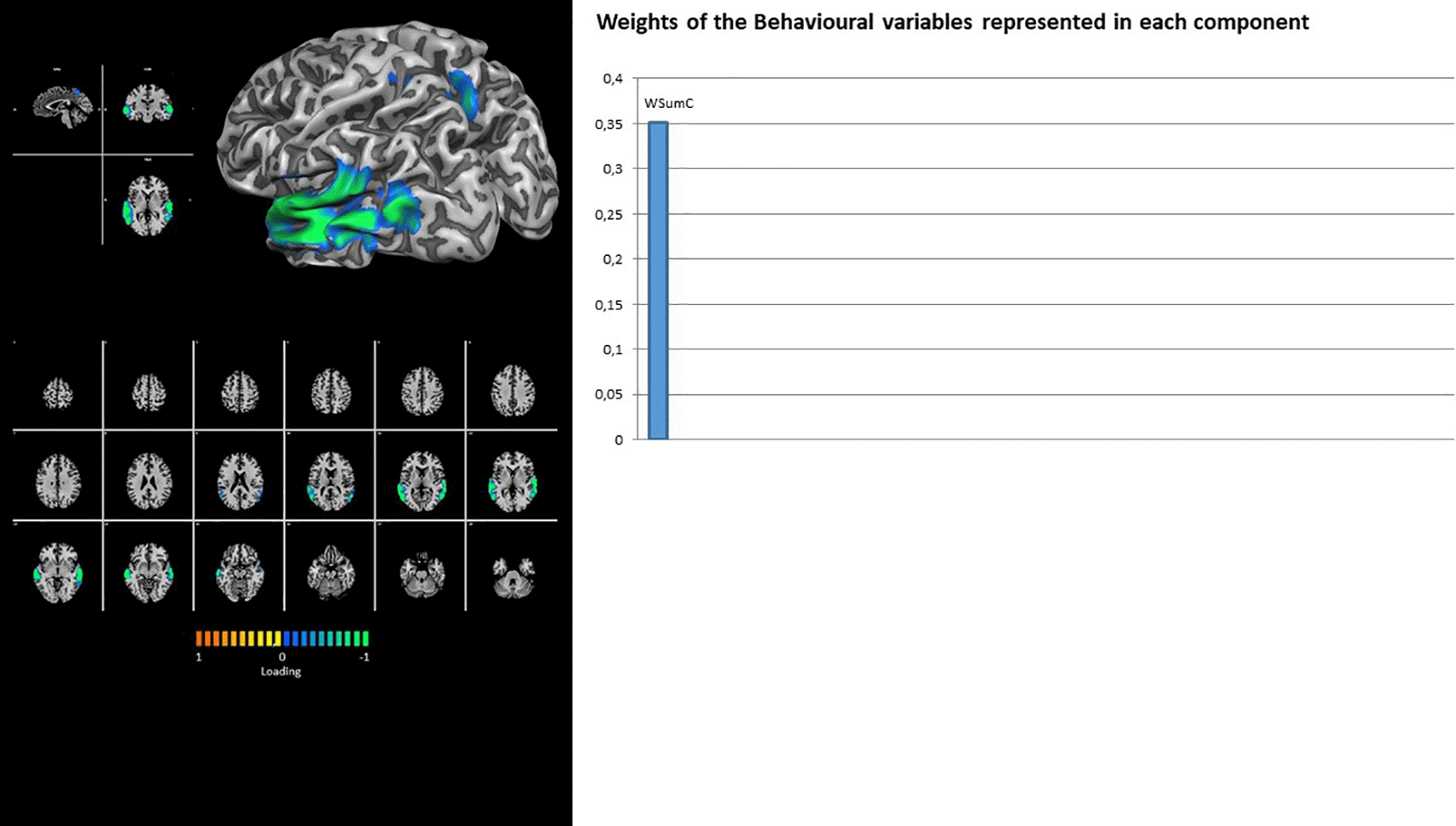
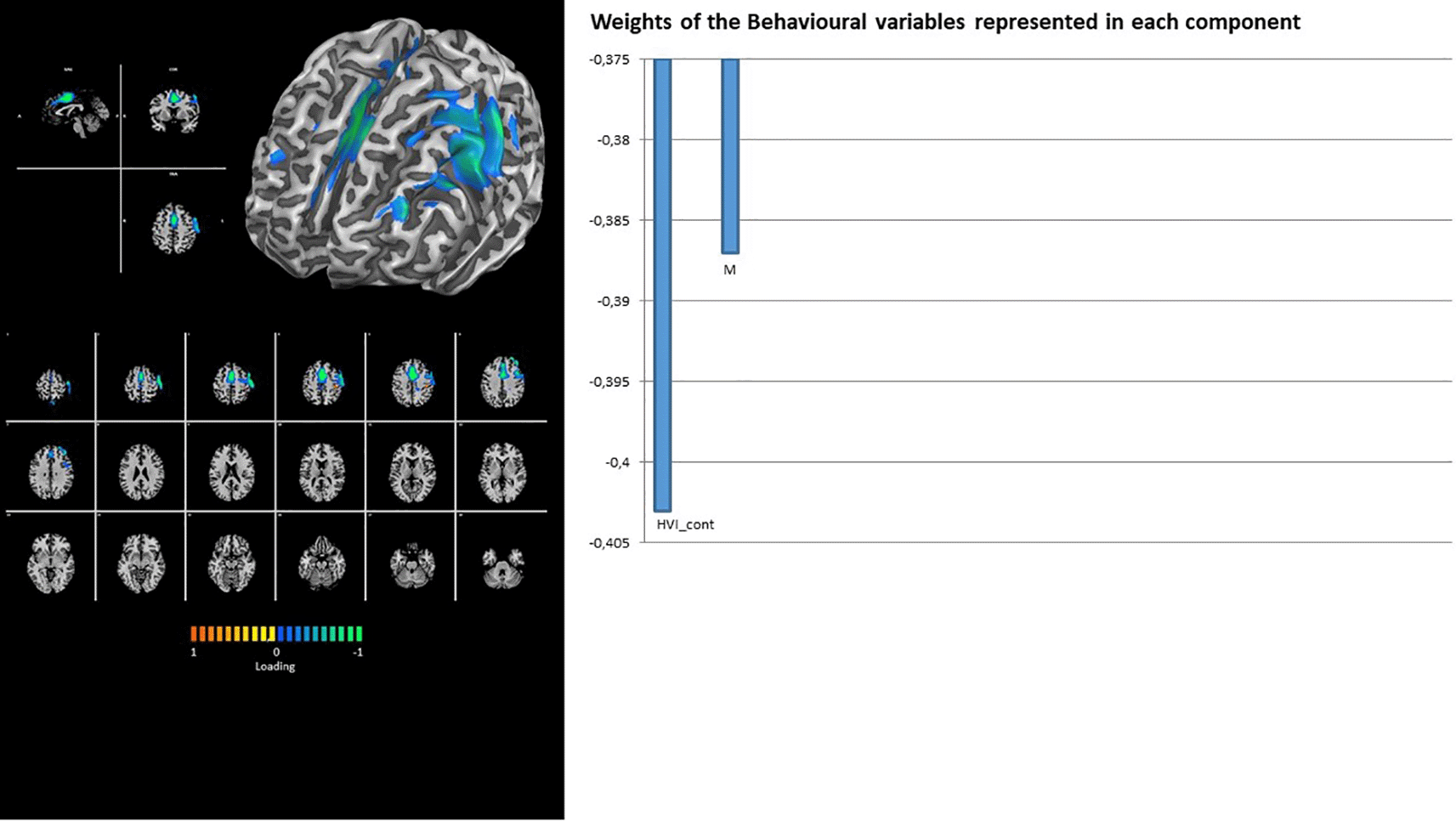
In fact, the DMN showed the contribution of latent factor 1 only, with a unique loading on the Rorschach variable HVI_cont (Figure 2). Similarly, the TEMP-PAR (auditory) network showed the contribution of latent factor 1 only, with a unique loading on the Rorschach variable WSumC (Figure 3).
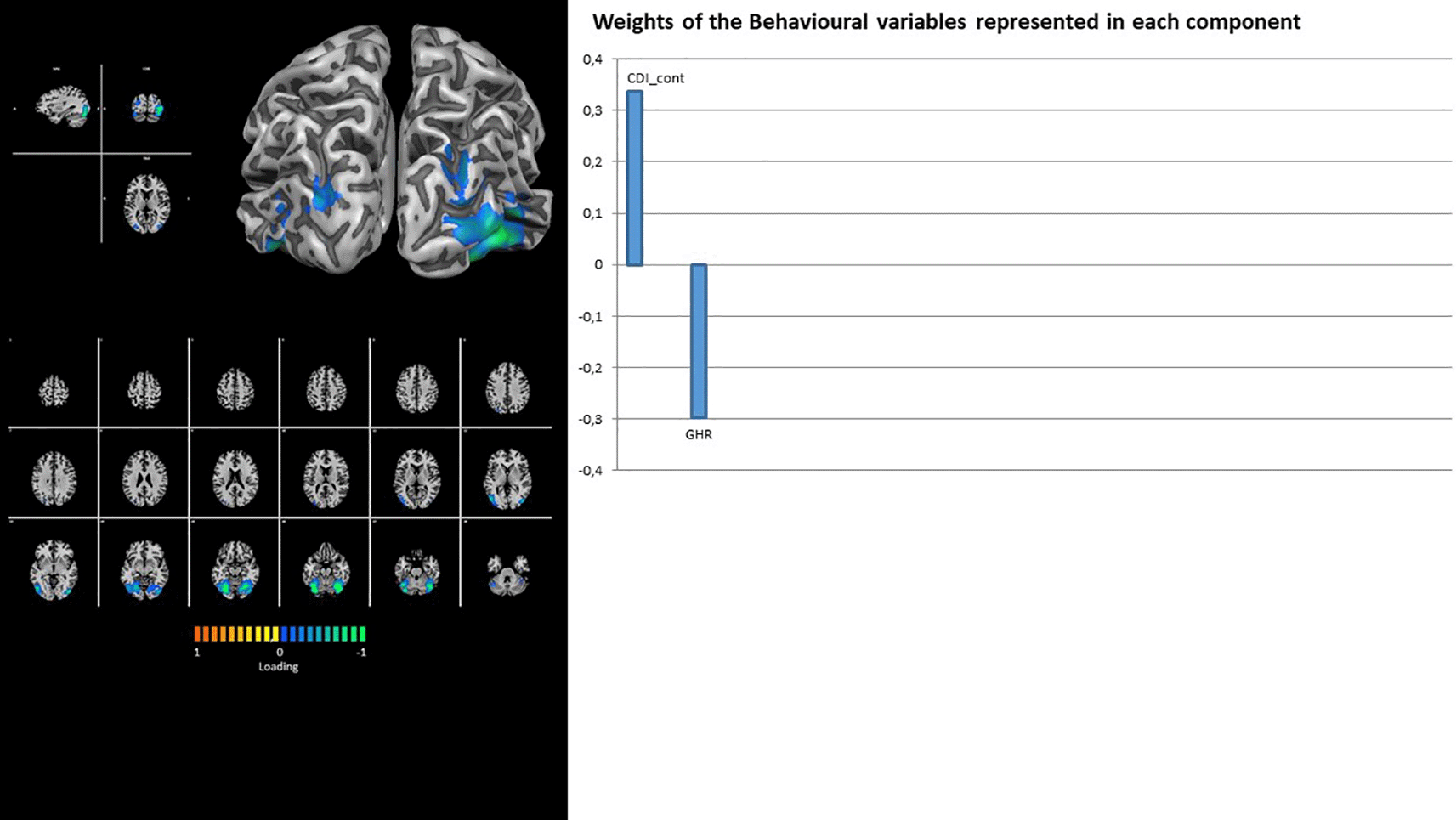
Both visual RSNs (i.e., VIS and V+) show the contribution of latent factor 1 only, with a unique loading on Rorschach variables DEPI_cont for the former (Figure 4) and loadings on CDI_cont and GHR for the latter (Figure 5).

The networks involved in sensory-motor processing (i.e., SMOT and SMA) again show the contribution of latent factor 1 only, with loadings on Rorschach variables PTI_cont and M- for the former (Figure 6) and HVI_cont and M for the latter (Figure 7).
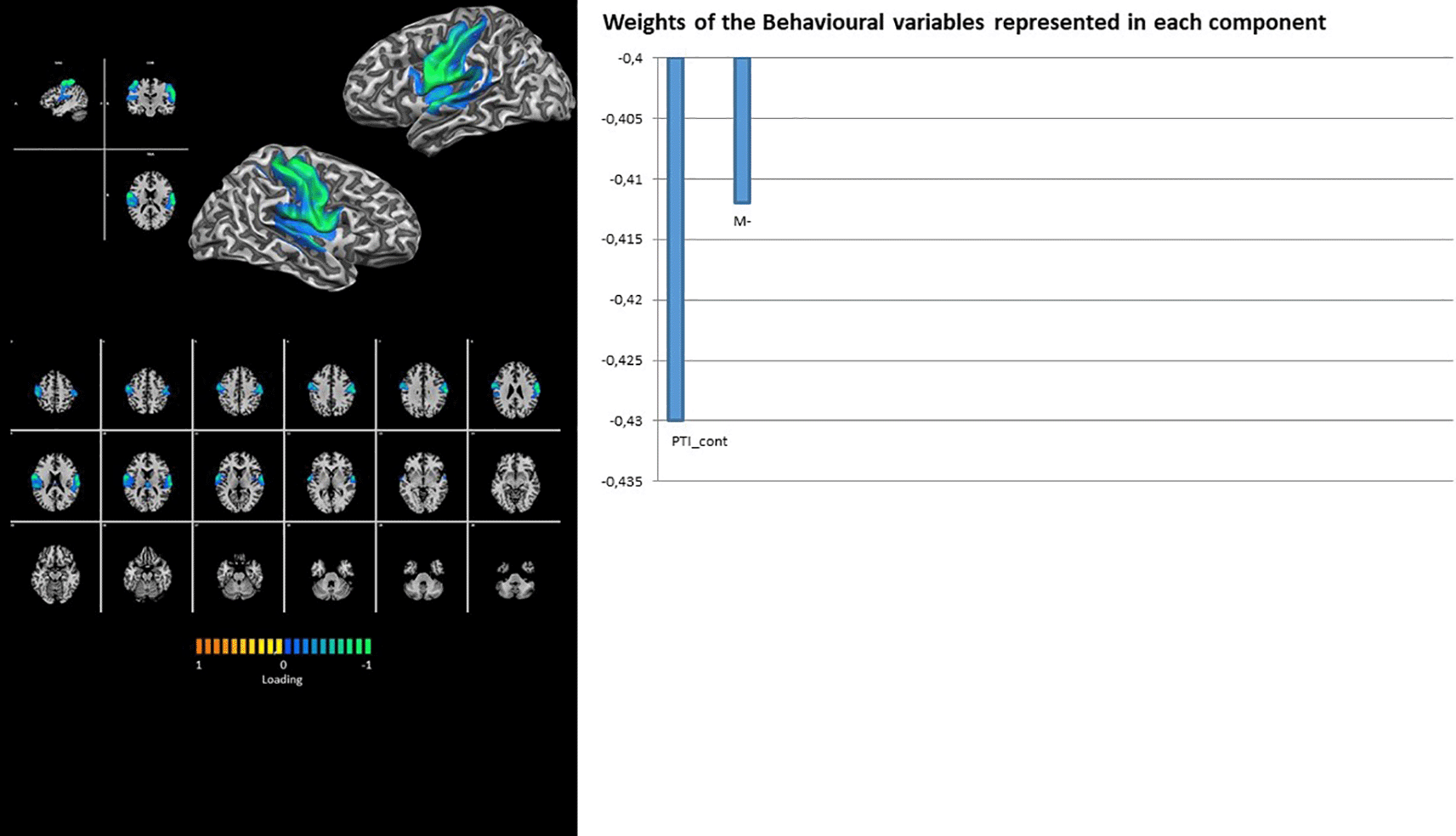
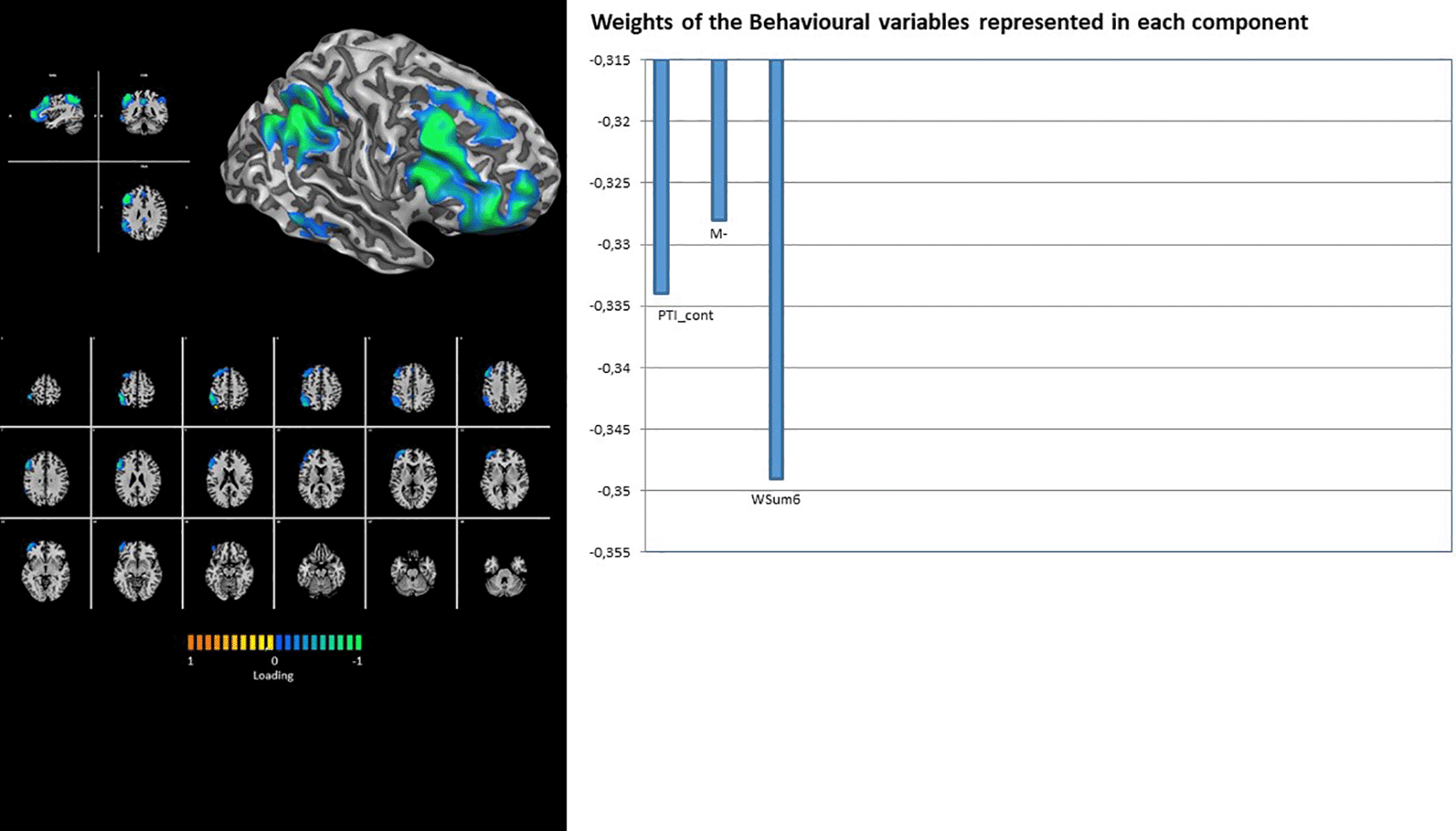
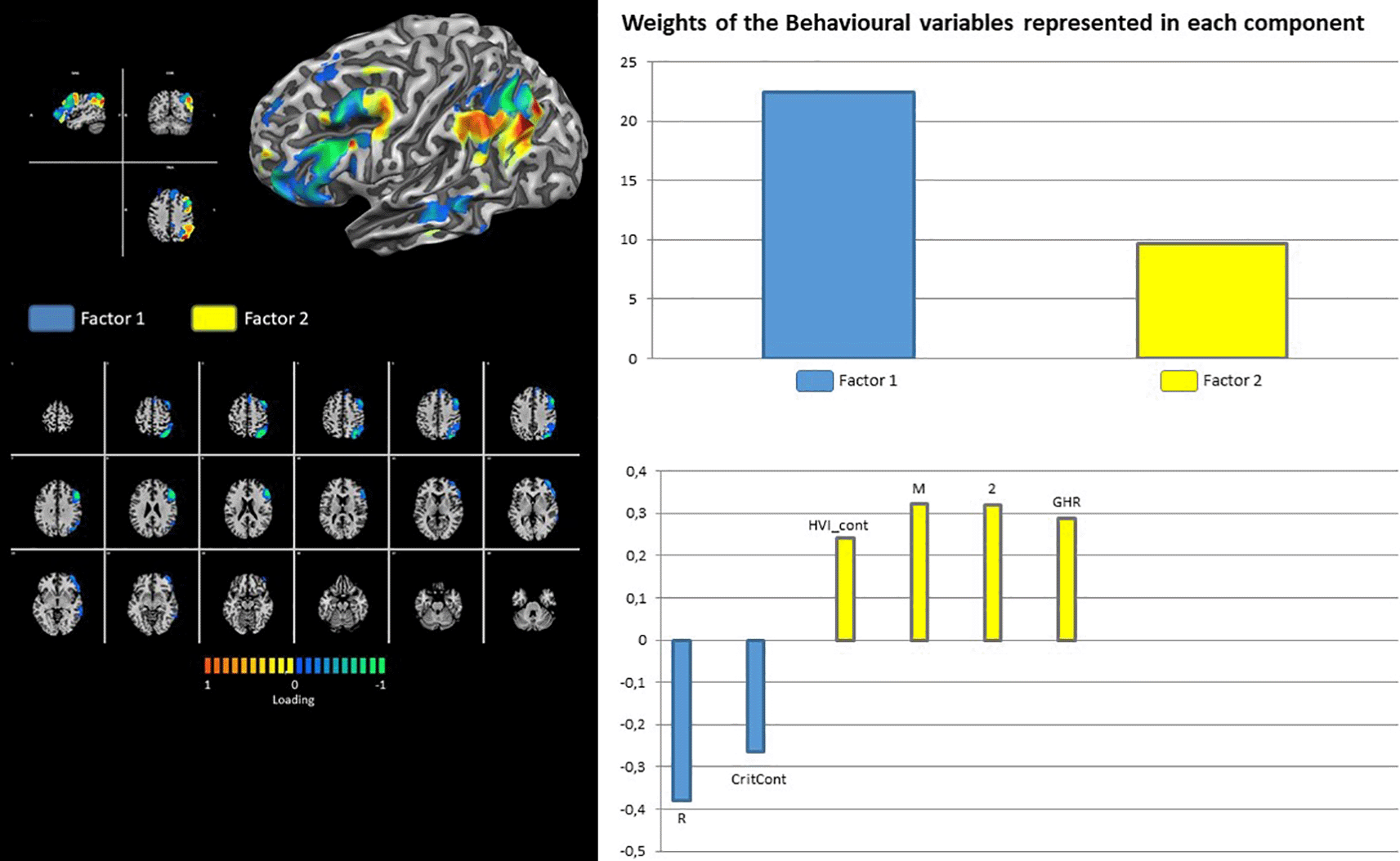
Finally, RFP shows the contribution of latent factor 1 only, with loading on the Rorschach variables PTI_cont, M-, and WSum6 (Figure 8). Contrary to its right counterpart, the LFP showed the contributions of both latent factors 1 and 2. The loadings for factor 1 were on Rorschach variables R and CritCont, whereas HVI_Cont, M, 2, and GHR were associated with latent factor 2 (Figure 9).
In addition, the SAL network showed the contribution of both latent factors 1 and 2: the only loading for the former was on the Roarshach variable Art, while the loadings for Factor 2 were on the Rorschach variables An, CDI, CritCont, GHR, and M. (Figure 10).
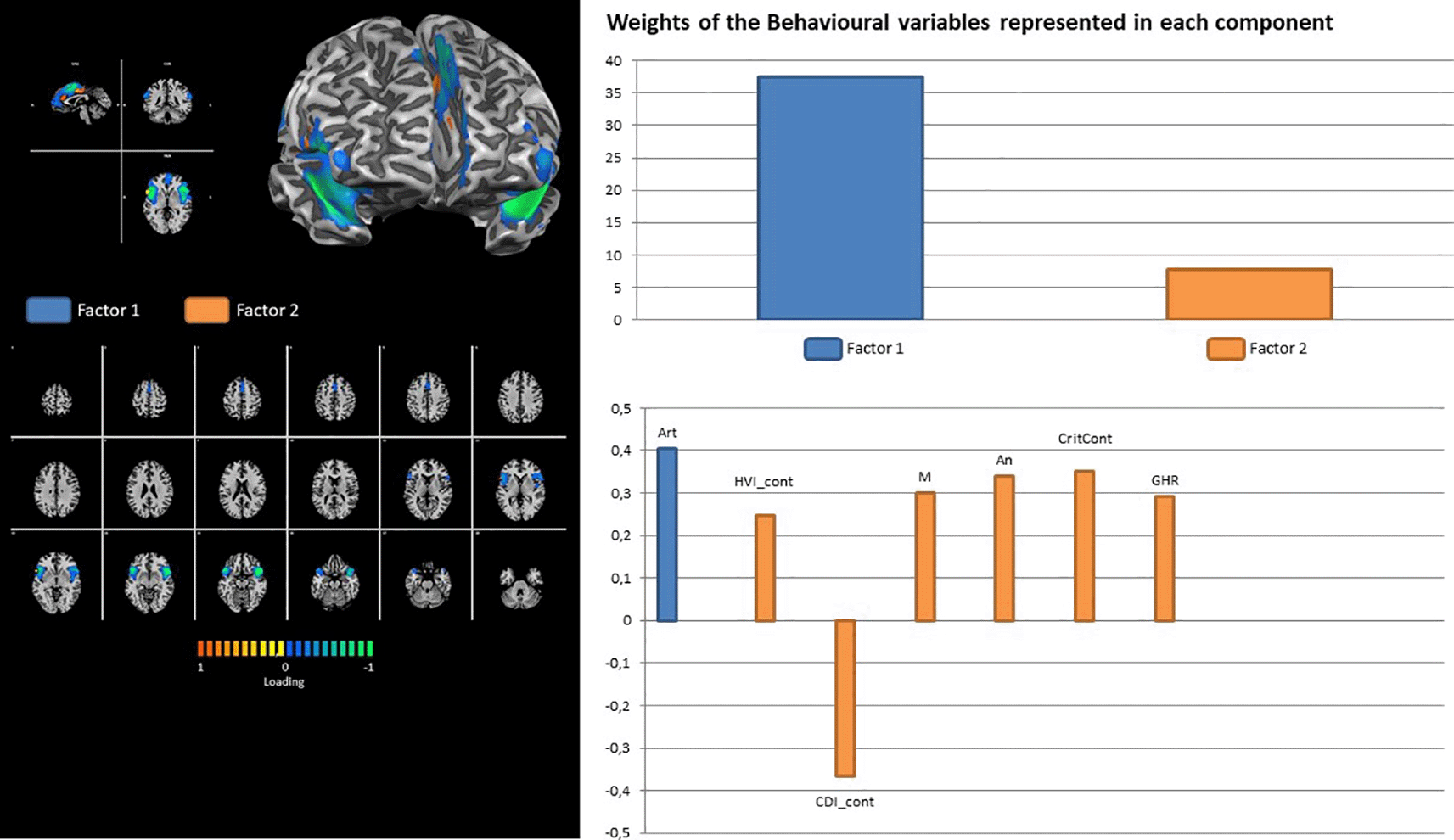
The cerebellar network is the only network that shows the contribution of all three latent factors. The loading on Rorschach variables was onCDI_cont for latent factor 1; AdjD_cont, R, F, AB, and GHR for latent factor 2; and HVI_cont, M, 2, Wsum6, and PHR for latent factor 3 (Figure 11).
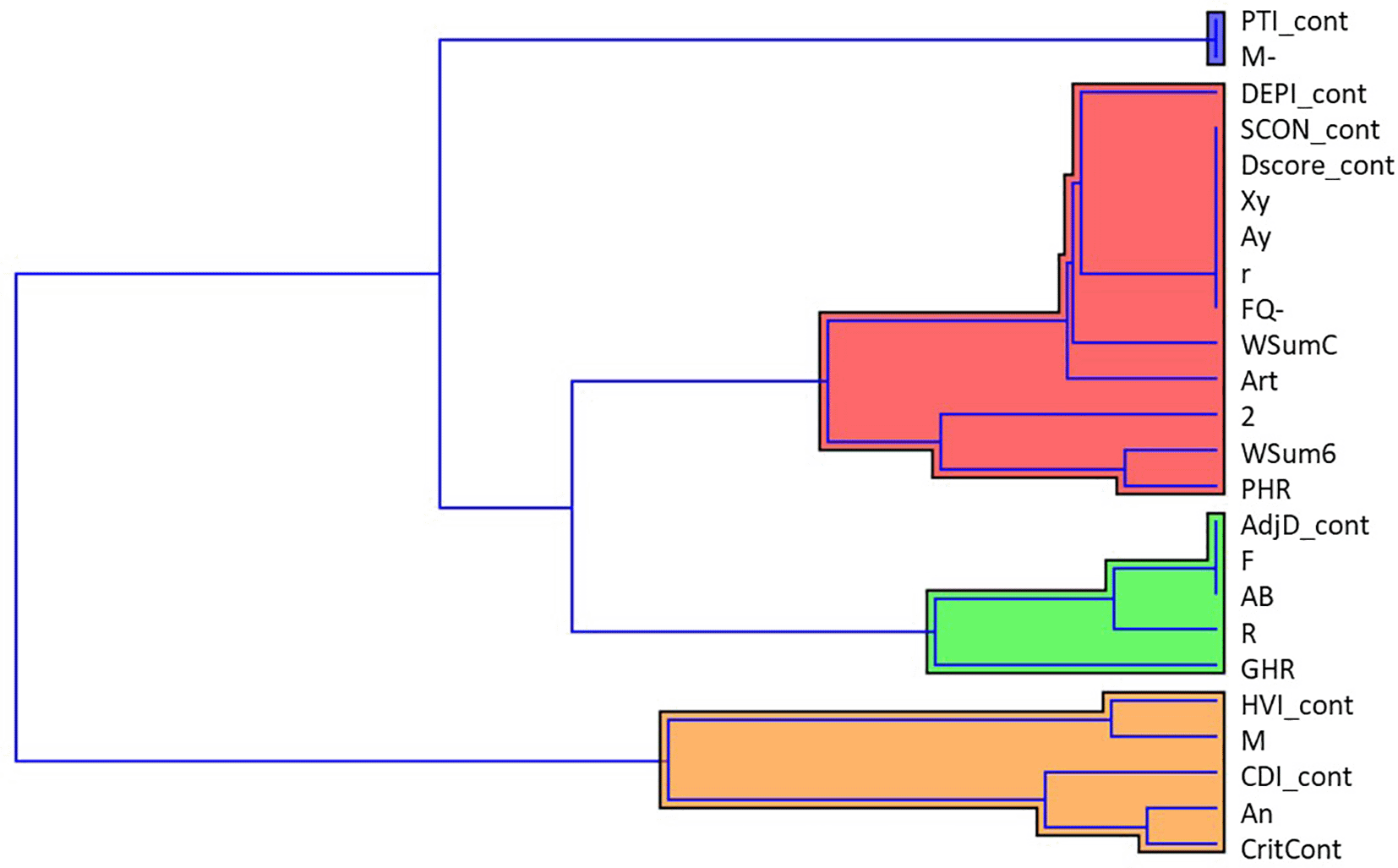
The dendrogram obtained through cluster analysis clearly suggested the existence of four clusters across the 27 CSs (Figure 12). The widest cluster contained 12 CSs, whereas PTI_cont and M- comprised the smallest cluster.
Note: For Rorschach variables abbreviations, please see Table 1.
The Rorschach test is one of the most popular assessment tools used in clinical settings for psychopathology and personality assessments. However, it is not yet clear how the verbal replies offered by patients, and captured through signatures, relate to neural activity. Previous investigations have attempted to use task-fMRI to solve this issue. However, as mentioned above, this approach has technical criticalities that could bias the results. For this reason, the present study adopted the resting state instead to be associated with scoring of the Rorschach test administered at a later time. Notably, resting-state networks are thought to represent a more stable modality of each individual’s brain activity, as this is not affected or guided by the task implemented during the MRI scan.
From a neurobiological standpoint, our results underlined specific brain activity patterns associated with the given Rorschach variables. First, we observed that DMN activity was significantly negatively correlated with HVI. This variable reflects relational and cognitive style, thus representing psychological processes such as control, emotional and relational distance, and alert and distrust towards the environment (Exner, 1991; Exner & Erdberg, 2005; Mihura et al., 2013). In other words, it could represent a sort of hyper-vigilance process towards the inner and external worlds. It is noteworthy that the HVI variable showed a positive correlation with the SAL network activity, which came along with variables that represent a kind of social attention pointed towards representations of self and interpersonal relationships (i.e., An, CritCont, GHR, and M, grouped in the “Factor 2”).
The DMN is involved in the monitoring of somato-emotional and vegetative information, feelings and body posture, free association of thoughts, inner speech, mental images, emotions, planning of future events, interpretation and prediction of environmental requirements, autobiographical memories, working memory, theory of mind, integration, creativity, self-referential thought, and integration between self and environment (Cozolino, 2020). In contrast, the SAL Network is involved in the interoception of feelings associated with gratification and consists of synchronous activation of the insula, anterior cingulate cortex, and ventral striatum (Pace-Schott & Picchioni, 2017). Moreover, it is involved in understanding others’ intentions (Rijpma et al., 2021) and in self-recognition from first- and third-person perspectives, a critical process for the development of self-consciousness (Asakage & Nakano, 2023). According to the Rorschach literature, it seems more appropriate to consider the HVI construct through the DMN-SAL activation axis. Indeed, the mutually excluding activation of the DMN and SAL (Goulden et al., 2014; Sridharan et al. 2008), well describes the circularity of psychic investment of the hyper-vigilant person. Hypervigilance may be represented by two components: the first one is more internally directed and is likely supported by a series of functions in which the DMN is involved; the second one is more other-directed and may be supported by SAL activity (Koch et al., 2016).
Hypervigilant subjects, because of their need to maintain interpersonal control, invest a high amount of energy to ensure that all elements in the field are under their control. The result is that this self-directive cognitive activity, supported by the DMN, is chaotic and inefficient because they are stuck in their flood of thoughts (rumination). DMN activity is associated with high rumination in patients (Boyd et al., 2018). It is likely that depressed subjects have high HVI scores because of their tendency to ruminate in a rigid and persistent way about a world that is perceived as threatening and hostile. In other words, it is likely that hypervigilant subjects respond to their sense of fragility by overcontrolling everything. At the same time, hypervigilant people pay attention to external stimuli, which have to remain under their control (Richards et al., 2014).
Our analysis underlined the involvement of the TEMP-PAR network. Although it has historically been associated with language processes (Rosazza & Minati, 2011), it has recently been associated with emotion regulation processes (Buhle et al., 2014; Kohn et al., 2014; Morawetz et al., 2017; Vitolo et al., 2022). Indeed, it has been outlined how emotion regulation processes, despite their strong association with prefrontal activity (Etkin et al., 2015; Goldin et al., 2008), could recruit large portions of parietal and temporal regions (Morawetz et al., 2017; Ochsner et al., 2012; Sripada et al., 2014), because of the additional attentional and re-interpretative processes involved in modulation processes (Morris et al., 2014; Ochsner et al., 2012). Interestingly, the activity of the TEMP-PAR network was found to be related, in our analyses, to the presence of the Rorschach variable WSumC, which represent the weighted sum of the responses in which the respondents “use” the inkblots’ colors to provide a rationale for their responses (Exner & Erdberg, 2005). During the Rorschach task, using colorful details to provide responses could indicate the way in which the respondents express their emotions, thus performing a sort of self-modulation of themselves (Exner, 2003; Malone et al., 2013). Pushing forward to these concepts, the Rorschach variable WSumC represents the presence of emotional modulation processes (Exner, 2003). Thus, its association with a brain network involved in emotion regulation processes, such as the TEMP-PAR network, as outlined in our analyses, could foster these interpretations.
Moving forward, our analyses revealed an association between certain Rorschach variables and the activity of the visual network in both its lower-order (VIS) and higher-order (V+) components. Although this network is particularly active during visual tasks (Rosazza & Minati, 2011), it has been observed that activation of the visual network could modulate BOLD fluctuations in resting-state activity (Stevens et al., 2010). Our analyses outlined how the resting-state activity of both visual components was associated with the Rorschach variables DEPI, CDI, and GHR. Interestingly, DEPI and CDI were positively associated with both visual components, whereas GHR was negatively associated with the high-order visual component (V+). According to the Rorschach literature, the CDI variable could be present in individuals who might experience a sort of immaturity and frustration in living social situations, strongly centered on themselves with a high need for the other, thus being less able to experience a relationship in a rewarding manner (Exner, 2003; Exner & Erdberg, 2005). In contrast, the GHR variable represents the exact opposite, indicating individuals that might have internalized good representations of social relationships (Exner, 2003; Exner & Erdberg, 2005). Moreover, the DEPI variable was elaborated with the intention of detecting typical depressive functioning (Exner, 2003; Exner & Erdberg, 2005), and along with CDI, it was found to be associated with depressive symptoms (Carlson et al., 1997), thus representing a marker for depressive tendencies (Mihura et al., 2013). Notably, patients with depression have been found to show patterns of decreased functional connectivity in the visual areas that comprise the visual network (Lu et al., 2020). Given that high values of DEPI and CDI and low values of GHR could be associated with depressive tendencies (Mihura et al., 2013), the observed association between resting activation of the visual components and the aforementioned Rorschach variables (i.e., DEPI, CDI, and GHR) could be interpreted in light of the abnormal functional activity patterns of the visual areas observed in depressive patients (Lu et al., 2020).
Other relevant findings concern the SMOT network, the activation of which has been historically associated with covert and overt movement, namely motor imagery and passive movement (Jeannerod, 1994; Naito et al., 2002; Stippich et al., 2002). Interestingly, the resting activity if this network was negatively associated, in our analysis, with the presence of Rorschach variables M- and PTI that, as mentioned above, is linked to abnormal manifestations of thoughts and perceptions distortions (Exner, 1991; Exner & Erdberg, 2005; Mihura et al., 2013). More intriguingly, reduced intrinsic activity of SMOT network was found in patients with schizophrenia (Kaufmann et al., 2015). Our results are in line with these considerations, outlining how a more functionally connected SMOT network is associated with a less severe marker of thought disorders. Moreover, motor areas encompassed in the SMA network showed a significant negative association with the HVI Rorschach variable, which serves as an indicator of hypervigilance (Exner, 1991; Exner & Erdberg, 2005; Mihura et al., 2013). Interestingly, Breckel et al. (2011) reported that decreased activity of motor areas (e.g., the SMA) was associated with prolonged exposure to a vigilance task, suggesting that this decreased activity could be interpreted as a function of the time interaction on vigilance processes. Again, our results concerning the negative association between HVI and SMA activity seem to be in line with the aforementioned findings.
Our analyses revealed involvement of the Frontoparietal Network (FPN) in both the right (RFP) and left (LFP) components. Historically, FPN activity has been associated with different functions such as memory (Damoiseaux et al., 2006), attention (Smith et al., 2009), language (Dosenbach et al., 2007; Fox et al., 2005), and visual processes (De Luca et al., 2006). It is noteworthy that FPN is associated with mentalization and reflective processes of the self and others (Lieberman, 2007; Luyten et al., 2020). Given these findings, our results relying on associations between LFP and Rorschach variables that outlined the presence of attentive and mental processes of representations of self and interpersonal relationships (i.e., HVI, M, 2, and GHR, grouped in the “Factor 2”) were not surprising. In contrast, abnormal functional intrinsic and extrinsic connectivity has been found in the right components of FPN in psychotic patients (Baker et al., 2014), as well as in individuals at high risk for mental illness (Peeters et al., 2015; Schmidt et al., 2014). Our analyses revealed that the right components of FPN were negatively associated with Rorschach variables M-, PTI, and WSum6, which could be considered an indicator of thoughts and perception disorganization, thus representing a marker for the presence of a possible psychotic disorder (Exner, 1991; Exner & Erdberg, 2005; Mihura et al., 2013).
The hypervigilant other-directed component can refer to all assets managed by the SAL, including attention to external stimuli.
Factor 1 (Art) explains most of the variance (38%) of the salience network consistently with the processes of attention to detail and integration of visual-spatial perceptual elements linked to visual-motor coordination in order to integrate salient stimuli. In this sense, we can ask ourselves about the construct of the intellectualization index (2AB + Art + Ay), which indicates the process through which the subject tries to reduce or neutralize the intensity of emotions, focusing on the formal features of the percept.
Factor 2 (An, CDI, CritCont, GHR, M) explains approximately 10% of the variance in the salience network. CDI indicates difficulty in the area of control and sociality; it characterizes socially immature subjects who have problems interacting with the external environment in emotionally complex social situations. The An variable indicates concerns related to body image (Mihura et al., 2013). The CritCont variable identifies depressive content, needs, and urgencies that are typically inhibited, minimized, or expressed indirectly through an adaptive way of thinking. GHR is an effective approach for interpersonal relationships. Variable M captures the subject’s ability to empathically identify with others. Overall, the variables that explain the large state brain network of SAL can be referred to as social attention processes linked to self-image (CDI, An, and CritCont) and interpersonal relationships (GHR). These data are coherent with spatial detail attention (art) and top-down processes of primary sensory inputs that are typical of this network. In fact, the Salience System is active during the detection of signals, attention to spatial details, and hand-eye coordination. It is also associated with top-down control processes of primary sensory inputs (Friston & Büchel, 2000; Lachaux et al., 2005).
One of the most valuable results concerns the cerebellar network, the activity of which was found to be associated with several Rorschach variables grouped into three main latent factors. Factor 1 was represented by the CDI variable alone, which, as mentioned before, could be a marker for depressive tendencies (Mihura et al., 2013). Factor 2 comprises five Rorschach variables, namely AdjD, R, F, AB, and GHR, which, grouped together, could represent a sort of tendency towards reality simplification (Exner & Erdberg, 2005). Finally, Factor 3 entails the other five Rorschach variables: HVI, M, 2, WSum6, and PHR. When grouped together, these variables could represent the behaviors of hyper-vigilant individuals, particularly those who are warried about their relationships and the emotional contexts in which they are usually embedded. In these contexts, individuals are typically involved in emotional processing and modulation, thus paying attention to other people and the external environment in general (Exner, 2003; Exner & Erdberg, 2005). Regarding the cerebellar network, it has recently been demonstrated that the cerebellar regions associated with motor functions are distinct from those serving non-motor and cognitive functions (Stoodley & Schmahmann, 2009). Historically associated with coordination processes of motor behaviors (Bastian et al., 1999), the cerebellum has recently been shown to play a role in emotional processes (Adamaszek et al., 2017; Baumann & Mattingley, 2012; Schutter & van Honk, 2005), as well as regulation of emotions (Schutter & van Honk, 2009; Turner et al., 2007). Resting-state functional connectivity studies have shown several associations between cerebellar activity and brain regions involved in emotion regulation processes, such as the salience network brain regions (Seeley et al., 2007), amygdala (Roy et al., 2009; Sang et al., 2012), and insular and cingulate cortices (Allen et al., 2005). These findings could explain the association between cerebellar activity and Factor 2 (positive association) and Factor 3 (negative association). Indeed, given that higher cerebellar activity could be associated with both cognitive and emotional processes (Stoodley et al., 2012; Stoodley & Schmahmann, 2018), associations with factors 2 and 3 could explain these considerations. In contrast, Factor 1 (i.e., the Rorschach variable CDI) showed a positive association with cerebellar activity. Given that CDI could represent a marker of depressive tendencies (Mihura et al., 2013), the positive association with cerebellar activity could assume a counterintuitive meaning, in the extent of emotional and cognitive impairments are often associated to depressivepsychotic manifestations (Drevets, 2001). However, it has been recently found that patients with depression may show increased patterns of intrinsic functional connectivity in the cerebellum (Dai et al., 2023). Although these findings could explain the positive association between CDI and cerebellar activity, it seems to be in contrast with the aforementioned findings. Given these contradictory results, our interpretations should be interpreted with caution, probably considering them only at the speculative level, waiting for further investigations.
The aforementioned results pointed out interesting considerations, first concerning a clinical perspective that could embrace Rorschach’s variable interpretations. Rorschach variables that shared a similar network profile were grouped using cluster analysis. The four clusters of variables have interesting diagnostic meanings. In particular, the first one (composed of PTI and M-) could be central from both psychometric and diagnostic points of view, in that it detects the effects of cognitive processing and possible distortions that may affect the clarity of thoughts. The second (formed by DEPI, SCON, DScore, Xy, Ay, r, FQ-, WSumC, Art, 2, WSum6, and PHR) might refer to depressive features, emotion regulation processes, and representations of self and others. The third one (which comprises AdjD, F, AB, R, and GHR) is more heterogeneous and may be associated with a set of features arranged on a continuum from performance and perception components vs symbolic thought. Finally, the fourth and final clusters (composed of HVI, M, CDI, An, and CritCont) can be traced back to characteristics related to social anxiety, worry, and hypervigilance.
Taken together, these findings provide additional evidence indicating that, in some specific profiles, interesting points of convergence emerge between the Rorschach nomological network and RSN. Within this view, our study can be grouped with other studies that attempt to provide evidence-based findings regarding the validity and reliability of the Rorschach test (e.g., e.g., Giromini, Viglione, Pineda et al., 2017a; Giromini, Viglione, Zennaro et al., 2017b; Giromini et al., 2019; Ishibashi et al., 2016; Vitolo et al., 2021). This could help to shed new light on both the Rorschach test, especially the dialogue between neuroscience and clinical practice, and again between mind and brain. Data from this research could provide a starting point for further exploration of the hypotheses that they have generated.
Finally, this study has some limitations. First, the analyzed sample consisted of only 24 participants. However, while it is generally true that neuroimaging has been shifting in recent years towards a big-data approach, our sample is in line with the median size currently used in the field (Szucs & Ioannidis, 2020). Indeed, similar empirical studies on the neural correlates of Rorschach responses included less than 30 subjects (Giromini et al., 2017a, 2017b, 2019; Vitolo et al., 2021). The second element of concern may be related to the RSN identification. ICA is the most commonly used technique to extract patterns of brain activity at rest, but it does not provide standardized labels for the observed components. Therefore, we followed the general and accepted procedure of manual identification carried out by researchers (F. C. and T.C.) with highly specific experience for resting-state data. Moreover, dual regression allowed for a correct return from the RSNs observed at the group level to the subject-specific spatial pattern.
Our data show a strong relationship between Neuroimaging and Rorschach. These results support the possibility of investigating the psychic functions underlying Rorschach variables by studying the activation of different brain areas under resting conditions. Furthermore, this type of research would make it possible to extend the use of neuroimaging techniques to include further psychological testing. Neuroscience and clinical psychology share the same object of study: the human mind and its manifestations.
The fracture between clinical practice and neuroscience was partly caused by Freud’s lack of attempt to corroborate his theory with the aid of neuroscience, mainly because of the absence of adequate tools (Northoff, 2012). However, today’s neuroimaging techniques have completely revolutionized the way of conceiving the study of the brain, allowing the movement of an anatomical-structural vision of brain mechanisms to a more functional one based on the connecting networks between the various areas of the brain. As pointed out by Northoff (2012), Freud would probably be very interested in neuroscience today, finally having tools to investigate the psyche in a fairly sophisticated way, and therefore, similar to how he imagined it.
Moreover, neuroimaging methods can be employed to investigate psychopathological risk factors in both typical and atypical brain maturation processes (Díaz-Arteche & Rakesh, 2020). Thus, our study could be included in this growing integrative perspective, promoted even in other studies (e.g., Giromini et al., 2017a, 2017b, 2019; Ishibashi et al., 2016; Vitolo et al., 2021), and is useful in clinical practice. Indeed, detecting information about problem-solving and perceptual processes usually activated during the Rorschach task could help future researchers and clinicians better understand the interplay between neurobiological and psychological processes.
The study was approved by the Ethics Committee of the University of Valle d’Aosta (protocol no. 97/2024 dated January 8, 2024). At the time of data collection (2011), the Ethics Committee had not yet been established. It was established by Decree of the President of the University Council No. 2, Protocol No. 7475/II26, on August 28, 2015. However, the research complied with the Codice Etico AIP (Ethical Code for Research in Psychology, Italian Psychological Association) and the provisions of Italian privacy and data protection laws (L. 196/2003). All procedures performed in the present study were thus in accordance with the ethical standards of the institutional and/or national research commission and the 1964 Declaration of Helsinki and its subsequent amendments or comparable ethical standards. In addition, we obtained a favourable opinion from the Ethics Committee of the University of Valle d’Aosta (protocol no. 97/2024 of January 8, 2024) for the analysis of the archival data, and given the observational nature of the study, the same Ethics Committee considered the collection of these data to be compatible with ethical research standards.
The research utilized archive data provided by the Tiarè Association, Mental Health Services. From the data available to us, it is evident that informed consent was requested in written form.
Stefania Cristofanelli:
Roles: Conceptualization, Project Administration, Supervision, Writing – Original Draft Preparation, Writing – Review & Editing
Enrico Vitolo:
Roles: Data Curation, Validation, Methodology, Writing – Original Draft Preparation, Writing – Review & Editing
Alessandro Zennaro:
Roles: Conceptualization, Supervision, Resources, Writing – Original Draft Preparation
Franco Cauda:
Roles: Conceptualization, Data Curation, Formal Analysis, Methodology, Resources, Visualization, Writing – original draft preparation
Tommaso Brischetto Costa:
Roles: Conceptualization, Data Curation, Formal Analysis, Methodology, Resources, Visualization, Writing – original draft preparation
Eleonora Centonze:
Roles: Writing – Original Draft Preparation, Writing – Review & Editing
Giorgia Baccini:
Roles: Writing – Original Draft Preparation, Writing – Review & Editing
Jordi Manuello:
Roles: Validation, Writing – Review & Editing
Laura Ferro:
Roles: Conceptualization, Project Administration, Supervision, Writing – Original Draft Preparation, Writing – Review & Editing
All authors reviewed the results and approved the final version of the manuscript.
The data that support the findings of this study are available from the TIARÉ Association for Mental Health. Restrictions apply to the availability of these data, which were used under license for this study. TIARÉ Association for Mental Health collected these data for clinical and research purposes and has thus formalized agreement and authorization on the following points: “Types of data identified and processed by the research group; Operations and procedures performed during data collection and processing; Scientific dissemination of the emerged data; Relevance of the project as being of public/social interest in addition to scientific interest”. The Ethics Committee has therefore expressed a favorable opinion on the appropriateness of the specific measures identified to protect the fundamental rights and interests of the involved parties. Data are available upon reasonable request from the corresponding authors (Enrico Vitolo, Stefania Cristofanelli) luca.ortolan@associazionetiare.org with the permission of the TIARÉ Association for Mental Health.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Andronikof A: The Theory of Hermann Rorschach. Rorschachiana. 2023; 44 (2): 193-213 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Personality assessment, Rorschach test
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 16 Jul 24 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)