Keywords
Antibiotic resistance,Epigenetics,Epitranscriptomics,Regulatory mechanisms,Therapeutic targeets
This article is included in the Antimicrobial Resistance collection.
Antibiotic resistance is the leading cause of death globally, with a higher possibility of the emergence of highly resistant pathogens, leading to epidemics. Several antibiotic resistance mechanisms have been discovered, such as enhanced efflux of antibiotics, reduced influx of antibiotics, alteration of antibiotics or their targets, and adaptation to antibiotics. However, this mechanism cannot fully explain the development of antibiotic resistance because the genes associated with this mechanism have been elucidated. However, the factors governing their regulation are not yet fully understood. Recent studies have highlighted the epigenetic and epitranscriptomic roles of antibiotic resistance development-associated genes. Epigenetic modification is associated with DNA modification, whereas epitranscriptomic modification is associated with RNA modification to control gene expression by regulating various biological phenomena such as splicing, translation, and stability. Therefore, this review will focus on the discovery of epigenetic modifications, particularly by DNA methyltransferases, such as restriction-modification (R-M) systems associated with methyltransferases, orphan DNA methyltransferases, and nucleoid-associated proteins that contribute to the development of antibiotic resistance. This scrutinization further expands to epitranscriptomic modification of non-coding RNA, which has a role in the regulation of antibiotic resistance. Epitranscriptomic modification of ribosomal RNA (rRNA), which is a major target of antibiotics, has been well explored. while non-coding RNA such as cis and trans small non coding RNA, and riboswitches are poorly explored. This epigenetic and epitranscriptomic modification will help to understand the regulation of antibiotic resistance-associated genes, which will help to identify key regulators of antibiotic resistance, paving the way for new antibiotic discovery, leading to decreased antibiotic mortality globally.
Antibiotic resistance,Epigenetics,Epitranscriptomics,Regulatory mechanisms,Therapeutic targeets
Bacterial infection is the most common infection in humans and other mammals owing to the abundance of bacteria living around us. Antibiotics have been developed to combat bacterial infections. Antibiotics are compounds that inhibit antibacterial activity by inhibiting cell wall synthesis, such as β-lactams (including cephalosporins, carbapenems, penicillins, glycopeptides, and monobactams), depolarization of the cell membrane via lipopeptides, inhibition of protein synthesis through binding to the 30S ribosomal subunit seen in tetracyclines, and aminoglycosides or binding to the 50S ribosomal subunit, as observed in lincosamides, chloramphenicol, macrolides, streptogramins, and oxazolidinones; inhibition of nucleic acid synthesis, such as fluoroquinolones and quinolones; and the disruption of metabolic pathways, including trimethoprim and sulfonamides.1
Antibiotic resistance hinders effective treatment, causing more than 1.2 millions deaths in 2019. In the absence of intervention, the annual global deaths attributed to antibiotic resistance could reach 10 million by 2050.2 Overuse, misuse, and global travel are major causes of accelerated antibiotic resistance. Therefore, antibiotic resistance represents a significant challenge to public health, putting the efficacy of current treatments at risk, and emphasizing the pressing requirement for inventive therapeutic approaches. As bacteria are exposed to various antibiotics over time, antibiotic resistance has emerged as a new problem and started a new journey of antibiotic resistance mechanism research, resulting in the identification of various types of antibiotic resistance, including alterations to the antibiotic molecule achieved through chemical modifications, destruction of the antibiotic molecule, reduced antibiotic penetration, and efflux facilitated by diminished permeability or efflux pumps. Additionally, changes in the target sites may occur through mechanisms such as target protection, modification of the target site via mutations, enzymatic alterations of the target site, complete replacement or bypass of the target site, or global cell adaptations to confer antibiotic resistance.3
Conventional understanding of antibiotic resistance primarily revolves around genetic mutations and horizontal gene transfer. However, recent advances in epigenetics and epitranscriptomics have illuminated a previously underappreciated layer of complexity in the regulatory mechanisms governing bacterial responses to antibiotics. As we venture into the realm of epigenetic and epitranscriptomic modification-mediated regulation of antibiotic resistance, this investigation seeks to unravel the molecular intricacies that govern these processes. The implications of such an understanding extend beyond theoretical insights, offering potential avenues for therapeutic interventions that could redefine our approach to combating antibiotic-resistant bacterial infections. Hence, there is a need to scrutinize recent studies related to epigenetics and epitranscriptomic-mediated antibiotic resistance.
Epigenetic modification refers to the modification of DNA to regulate gene expression without altering its genetic code. Bacterial epigenetics focuses on DNA modifications, particularly methylation. Bacterial genomes lack a membrane-bound nucleus, and organization into nucleoids is facilitated by nucleoid-associated proteins (NAPs). Prominent NAPs include heat-unstable (HU) and histone-like nucleoid-structuring (H-NS), which influence bacterial virulence and pathogenesis.4 Epitranscriptomic modifications involve the modification of RNA, such as methylation, to dynamically regulate gene expression to control various biological phenomena. Approximately 60 modifications in ribosomal RNA (rRNA) and transfer RNA (tRNA) have been identified in bacteria. These modifications include base alteration, base isomerization, methylation of the ribose 2′-hydroxyl group, and intricate modifications that involve the sequential addition of various chemical groups or alterations.5 RNA modifications, found in tRNA, mRNA, rRNA, and small ncRNAs, crucially regulate transport, stability, splicing, localization, translation, gene regulation, and biological processes.4
This review delves into the diverse forms of DNA methylation. Highlighting the involvement of DNA methyltransferases in the addition of methyl groups to specific DNA positions emphasizes the significance of these alterations in relation to antibiotic resistance. Furthermore, the role of nucleoid-associated proteins in antibiotic resistance is also discussed. This exploration extends to the emerging field of epitranscriptomics, specifically the methylation of non-coding RNA. Since Cis and trans small non-coding RNA, along with riboswitches, are recognized as crucial contributors to the control of genes associated with antibiotic resistance, this exploration delves into the role of non-coding RNA in antibiotic resistance regulation and scrutinized related studies in (see Figure 1 and Table 3), which provides a detailed examination of specific ncRNAs implicated in antibiotic resistance, involving their functions and possibilities as targets for therapeutic interventions.
Epigenetics in bacteria refer to heritable changes in gene expression or phenotypes that occur without altering the underlying DNA sequence. Unlike classical genetics, which deals with changes in the DNA sequence itself, epigenetic changes involve modifications to the structure of DNA or associated proteins, thereby influencing gene expression.6
Epigentic mechanisms in bacteria include:
Bacterial epigenetic modifications mainly occur via the addition of a chemical moiety to DNA without altering its sequence, such as methylation, DNA phosphorothioate modification, and nucleoid-associated protein. In bacteria, DNA methylation occurs primarily at cytosine residues within specific sequence motifs. For example, in many bacteria, adenine methylation is associated with the GATC sequence, whereas cytosine methylation is often found in sequences such as CC(A/T)GG.6,7 DNA methylation mainly occurs via the restriction-modification R-M system and orphan methyltransferases. DNA methyltransferases, similar to those found in the restriction-modification (R-M) system, attach methyl groups to specific bacterial DNA sequences. Like those in the restriction-modification (R-M) system, add methyl groups to specific bacterial DNA sequences. This defense mechanism recognizes unmethylated foreign DNA, leading to its degradation by the endonucleases. Orphan methyltransferases, not part of R-M systems, exist independently of categories such as DNA adenine methyltransferase and DNA cytosine methyltransferase.8 RM systems, categorized into four types (I-IV), differ in structure and cleaving processes and include methyltransferases such as EcoRV, CfrBI, M. NgoAV, modA13, and ModS2. Methyltransferases such as cell cycle-regulated methyltransferase (CcrM), DNA cytosine methyltransferase (Dcm), and DNA adenine methyltransferase (Dam) are known as orphan DNA methyltransferases.4,8 DNA phosphorothioate modification refers to the replacement of a non-bridging oxygen atom in the phosphate backbone of DNA with a sulfur atom.9
Bacterial epigentic modification increases the virulence and antibiotic resistance of diverse pathogens, and bacteria-mediated host epigenetic modification is a major cause of high mortality and bacterial infection.6,10–13 While bacterial antibiotic resistance is primarily associated with genetic mutations and the acquisition of resistance genes, emerging research suggests that epigenetic mechanisms can also contribute to antibiotic resistance.8 Epigenetic modifications can influence gene expression patterns without altering the underlying DNA sequence, thereby affecting the bacterial responses to antibiotics. Epigenetic modification is mainly orchestrated by the R-M system; orphan DNA methyltransferases and NAPs are the leading causes of antibiotic resistance (see Figure 2 & Table 1).
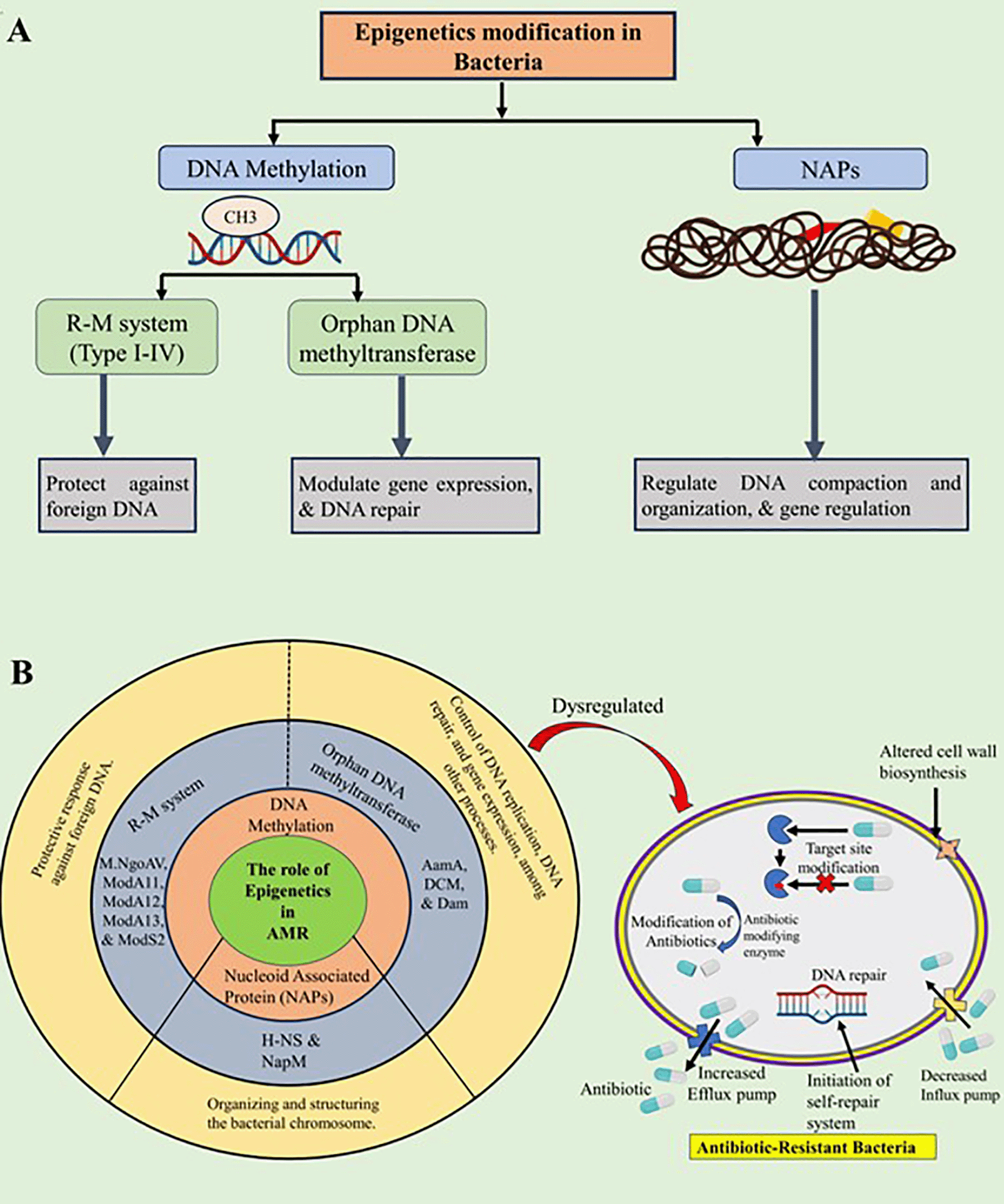
(A) Detailed depiction of various epigenetic components in bacteria; (B) Summary of the diverse epigenetic components contributing to antibiotic resistance. Created with Biorender.com.
| Categories | Effector (Differential expresssion) | Antibiotics | Target | Organism | Pathway | Ref. |
|---|---|---|---|---|---|---|
| R-M System | M. NgoAV | Bacitracin | RplA, & Envc | N. gonorrhoeae | Cell membrane sythesis | 14 |
| AamA | Polymyxin B, Erythromycin, & Kanamycin A | TrmD | A. baumannii | Efflux Pump | 15 | |
| ModA11 | Doxycycline, Nalidixic acid, Cloxacillin, Ciprofloxacin, & Ceftazidime | - | N. meningitidis | - | 16 | |
| ModA12 | Cloxacillin, Rifampin, & Cephalothin | - | N. meningitidis | - | 16 | |
| ModA13 | Triton-X | MtrF | N. gonorrhoeae | - | 17 | |
| ModS2 | Ampicillin | - | S. suis | - | 18 | |
| Orphan DNA Methyltransferases | Dam | Ciprofloxacin | - | E. coli | - | 19 |
| Dam or Dcm | β-lactams, Tetracyclines, Quinolones, Aminoglycosides, & macrolides | - | E. coli | - | 20 | |
| Dam | Quinolones | E. coli | 21 | |||
| Dam (Up) | Nalidixic acid | Csg and Mar | E. coli | Efflux pumps | 22 | |
| Dcm | EtBr | SugE | E. coli | Efflux pumps | 23 | |
| Dcm (Down) | Gentamicin | - | E. coli | - | 24 | |
| NAPs | H-NS | Colistin | EptA | A. baumannii | 25 | |
| H-NS | β-lactams | AcrEF | Salmonella enterica serovar Typhimurium | Efflux pump | 26 | |
| NapM | Rifampicin, & Ethambutol | - | M. smegmatis | - | 27 |
The methyltransferase M. ngoAV, found in Neisseria gonorrhoeae, regulates antibiotic resistance. Examination of the mechanism revealed that the knockout mutant, which is more susceptible to bacitracin, demonstrated decreased sensitivity to imipenem and cefotaxime in comparison to the wild-type strain. Altered gene expression included upregulated rlpA (peptidoglycan lytic transglycosylase) and downregulated envC in the mutant. -peptidoglycan.14 AamA methyltransferase of Acinetobacter baumannii has been reported to modulate antibiotic resistance. Mechanistic studies have shown that mutations result in decreased trmD operon expression, lower antibiotic minimum inhibitory concentrations (MICs) (Polymyxin B, Erythromycin, and Kanamycin A), and diminished ethidium bromide (EtBr) efflux pump activity.15 The ModA11_ON 1R variant displayed twice the susceptibility to doxycycline, nalidixic acid, and cloxacillin and four times more susceptibility to ciprofloxacin and ceftazidime compared to the modA11::kan variant. Similarly, the ModA12_ON variant exhibited a two-fold increase in sensitivity to cloxacillin, rifampin, and cephalothin compared with the modA12::kan variant.16 N. gonorrhoeae regulates antibiotic resistance against triton-X through DNA methyltransferase modA13. The mutant modA13 showed upregulation of mtrF, suggesting that it is regulated through modA13.17 The deactivation of ModS2 in Streptococcus suis resulted in a two-fold increase in ampicillin resistance compared to the activation of ModS2.18 In Escherichia coli K-12(E. coli K12), removing the dam gene increased ciprofloxacin sensitivity. Similarly, deleting the dam gene in a clinically obtained highly ciprofloxacin-resistant UPEC strain with several mutations conferring resistance to quinolones substantially reduced the ciprofloxacin MIC by more than 50% and MBC90 by 4.6-fold, albeit not fully restoring sensitivity.19 The lack of Dam or Dcm results in decreased half-maximal effective concentration (EC50) values for a broad range of antibiotics, including β-lactams, tetracyclines, quinolones, aminoglycosides, and macrolides, against Escherichia coli MG1655(E. coli MG1655).20
The double mutant Δdam ΔrecA showed either no growth or delayed growth after 24 hours in the presence of quinolones, contrasting with the control strain. Spot tests indicated that the Δdam ΔrecA double mutant exhibited increased sensitivity compared to both the ΔrecA single mutant and the wild type in both resistant and susceptible genetic backgrounds.21 Escherichia coli XL1-Blue (E. coli XL1-Blue) strains, when exposed to nalidixic acid, exhibited a five-fold enhancement in bacterial survival due to elevated dam expression. This increased resistance was correlated with two-fold upregulation of efflux pumps.22 Cells lacking the dcm gene exhibit increased expression of the drug resistance transporter SugE, which is associated with resistance to ethidium bromide (ETBR). Additional investigation revealed that cells with Dcm knockout exhibited greater resistance to EtBr compared to wild-type cells, and the reintroduction of a plasmid-borne dcm gene reinstates EtBr sensitivity. Conversely, cells without SugE displayed higher sensitivity to EtBr than wild-type cells.23 Genes trmJ, rlmH, and rlmB exhibited increased expression in ampicillin- and gentamicin-resistant E.coli respectively. Conversely, the gentamicin-resistant line showed downregulation of dcm. This finding suggests that methyltransferases play a role in antibiotic resistance.24
Nucleoid-associated proteins, such as H-NS, have also been found to regulate antibiotic resistance, and NapM regulates various antibiotic resistance genes (see Figure 2, and Table 1). In the A. baumannii AB5075 Δhns strain, there was upregulation of genes related to resistance against aminoglycosides, β-lactams, quinolones, trimethoprim, colistin, sulfonamides, chloramphenicol, and colistin. Conversely, compared to the parental strain, genes associated with tetracycline resistance were downregulated in the Δhns strain. Additionally, there was an upregulation of efflux pump-coding genes in the AB5075 Δhns strain.28 Mutation of H-NS in colistin-resistant A. baumannii led to high colistin resistance. Mechanistic studies revealed that in colistin-resistant A. baumannii with an H-NS mutation, there was an increase in the expression of eptA, which is responsible for a second lipid A-specific pEtN transferase. Simultaneously, expression of pmrC, another relevant gene, remained unchanged.25 H-NS regulates multidrug resistance by affecting gene expression in E. coli. Deleting H-NS in DeltaacrAB increased the resistance to erythromycin, oxacillin, doxorubicin, acriflavine, crystal violet, novobiocin, ethidium bromide, methylene blue, tetraphenylphosphonium bromide, sodium dodecyl sulfate, sodium deoxycholate, rhodamine 6G, and benzalkonium chloride. Dual acrEF and mdtE deletion suppressed Δhns-facilitated resistance, suggesting derepression of drug exporter genes.29 Regulation of the AcrEF multidrug efflux pump by H-NS has also been reported in Salmonella enterica serovar Typhimurium.26 NapM, an NAPs that binds to AT-rich DNA of the major groove to protect DNA from DNase I digestion, was found to confer resistance in Mycobacterium smegmatis against rifampicin and ethambutol. Mechanistic studies have shown that the ABC transporter operon is responsible for the napM-dependent ethambutol resistance. NapM regulates anti-tuberculosis drug resistance in Mycobacterium tuberculosis.27 Various NAPs are still poorly explored and require urgent investigation for a more comprehensive understanding of antibiotic resistance. Exploration of epigenetic modifications will unravel a new key regulatory cascade for the regulation of antibiotic resistance, offering a new opportunity to decrease antibiotic resistance mortality.
Epitranscriptomics is a field of study that focuses on the chemical modifications of RNA molecules, which can dynamically regulate various aspects of RNA biology, including stability, splicing, localization, and translation.5 Several mechanisms underlie epitranscriptomic modifications in bacteria (see Figure 3).
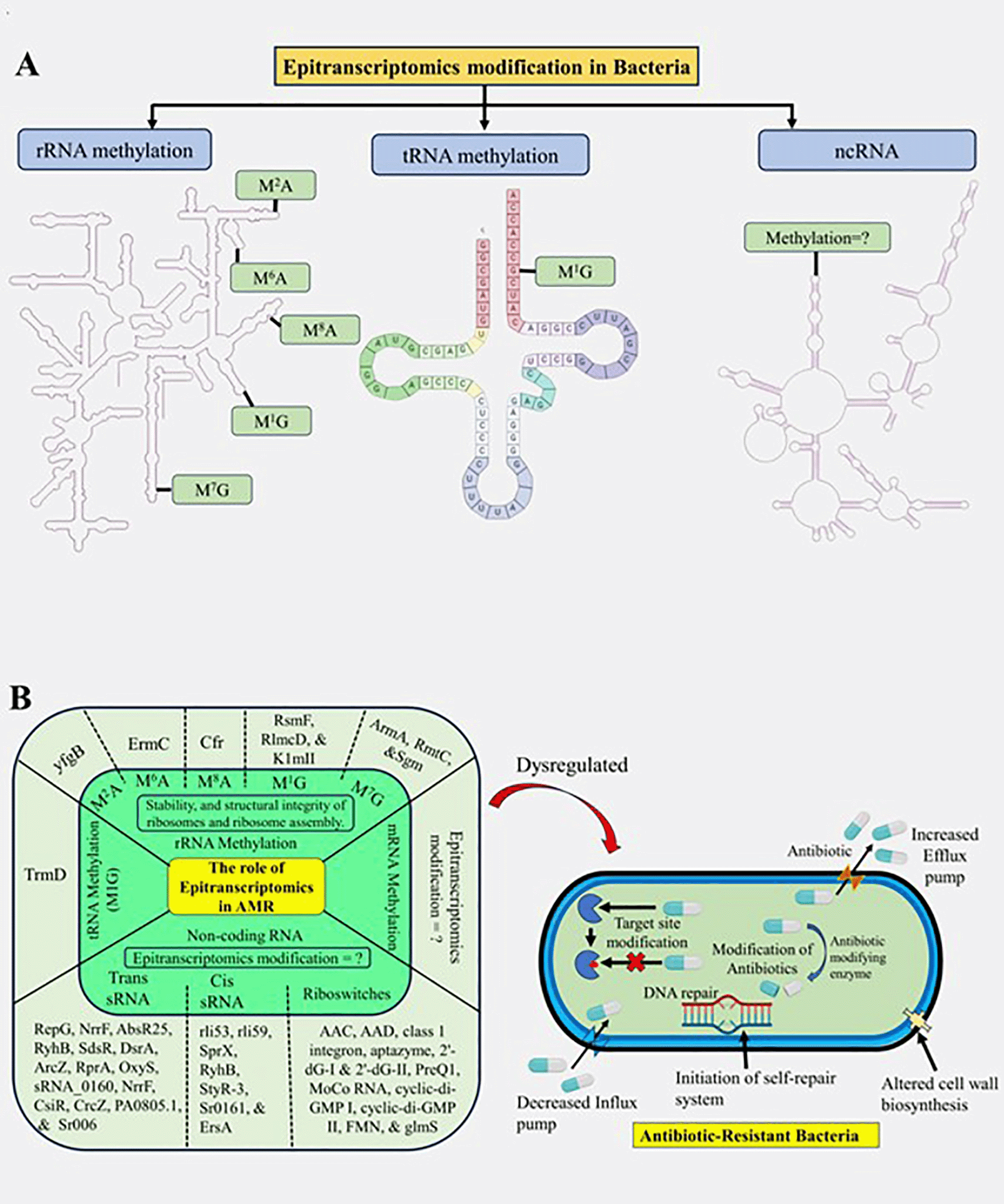
(A) Detailed representation of various epitranscriptomic components in bacteria; (B) Summary of the diverse epitranscriptomic components influencing antibiotic resistance. Created with BioRender.com.
• RNA Methylation: m6A, m5C, m7G, m1G, m2A, and m8A
For example, methylation of RNA nucleotides is a prevalent epitranscriptomic modification in bacteria, and N6-methyladenosine (m6A) has been observed in mRNA derived from Escherichia coli (E. coli) and Pseudomonas aeruginosa (P. aeruginosa). Additionally, N5-methylcytosine (m5C) has been mapped in mRNAs from Sulfolobus solfataricus (S. solfataricus). In addition, N7-methylguanosine (m7G), 1-methyl guanosine (m1G), 2-methyladenosine (m2A), and 8-methyladenosine (m8A) modifications have been reported.5
• Capping of RNA: nicotinamide adenine dinucleotide (NAD), and nucleoside tetraphosphate (Np4)
Similarly, epitranscriptomic modifications such as Np4 and NAD capping are also involved in mRNA stability, thus altering gene regulation to modulate various biological phenomena, such as stress response.30,31 Epitranscriptomic modifications have been shown to modulate infectivity, oxidative stress, antibiotic resistance, and temperature regulation in bacteria.32,33 Understanding the impact of epitranscriptomic modifications on antibiotic resistance mechanisms can provide insights into novel therapeutic strategies to combat multidrug-resistant bacteria (see Figure 3). Targeting enzymes involved in RNA modification or utilizing epitranscriptomic information to design RNA-targeting antibiotics may offer promising approaches for overcoming bacterial resistance and improving treatment outcomes.
Ribosomes is one of major target of antibiotics, and rRNA methylation are emerging as a crucial epitranscriptomic modification to regulate antibiotics resistance along with dysregulation of non-coding RNA expression which epitranscriptomic modification is poorly explored (see Figure 2 & Table 2).1,4 Ribosomal RNA (rRNA) is a central target of aminoglycoside antibiotics, such as streptomycin, amikacin and 16 srRNA methylase producing A. baumannii and P. aeruginosa, which were found to regulate aminoglycoside resistance.34 Of the 60 amikacin-resistant isolates, the presence of the armA gene, responsible for 16S rRNA methylation, was the sole prevalent gene identified.35 Aminoglycoside-resistant 16S rRNA (m7G1405) methyltransferase RmtC confers aminoglycoside resistance.36 sgm methyltransferase imparts resistance to aminoglycosides by adding m(7)G1405 to 16S rRNA’s A site. Escherichia coli’s 16S rRNA has methylations, such as m(5)C1407 and m(4)Cm1402. Further studies showed that RsmF faces obstacles modifying m(5)C1407 due to sgm accessing adjacent G1405 on the 30S subunit.37 The methylation of U747 and/or U1939 by RlmCD enhances subsequent G748 methylation by RlmAII, promoting effective binding of telithromycin (TEL) to the ribosomes in S. pneumoniae to modulate TEL resistance.38 Erythromycin-resistant methyltransferases such as ErmC, belonging to methyltransferases with a Rossmann fold dependent on S-adenosylmethionine (SAM), methylate at N6 of A2058 in 23S rRNA, which obstructs the binding of various antibiotic classes to 23S rRNA, to confer an MDR phenotype such as erythromycin resistance in bacteria expressing the enzyme.39 Clostridium bolteae 90B3 and Clostridium difficile T10 with Cfr rRNA methyltransferase confer oxazolidinones, phenicols, lincosamides, streptogramin A (PhLOPSA), and pleuromutilin resistance through the alteration of 23S rRNA by m8A2503 methylation.40 Similar results were also noted for both Escherichia coli and Staphylococcus aureus.41 E. coli protein methyltransferase yfgB (rlmN) was found to modify A2503 of the 23S rRNA to m2A. The yfgB knockout resulted in the absence of an alteration in 23S rRNA at nucleotide A2503. Further study showed that the E. coli rlmN-deficient strain exhibited a consistent two-fold increase in susceptibility to sparsomycin, hygromycin A, and tiamulin in comparison to the wild-type strain. Similarly, inactivation of rlmN in S. aureus resulted in a two-fold heightened vulnerability to linezolid.42
| Categories | Methylation location | Effector | Antibiotics | Organism | Ref. | |
|---|---|---|---|---|---|---|
| rRNA | 16S rRNA | m7G1405 | RmtC, & Sgm | Aminoglycoside | E. coli | 35,36 |
| 23s rRNA | m5U747, & m5U1939 | RlmCD | Telithromycin | S. pneumoniae | 38 | |
| m1G748 | RlmAII | |||||
| m6A2058 | ErmC | Erythromycin | - | 39 | ||
| m8A2503 | Cfr | PhLOPSA | C. bolteae, & C. difficile | 40 | ||
| m2A2503 | RlmN (yfgB) | Sparsomycin, hygromycin A, & tiamulin | E. coli | 42 | ||
| RlmN | Linezolid | S. aureus | ||||
| tRNA | m1G37 | TrmD | Ampicillin, carbenicillin, kanamycin, gentamicin, paromomycin, rifampicin, & ciprofloxacin | E. coli, & Salmonella | 43 | |
Transfer RNA (tRNA) is a fundamental type of non-coding RNA that assists in the translation of mRNA into proteins by attaching the correct amino acids to the growing polypeptide chain.44 New tRNA-modifying enzymes have been identified, shedding light on their significance in bacterial physiology. For instance, truD is involved in Ψ13 modification, dusA (also known as yjbN) catalyzes D16 and D17 modifications, Um32 modification is mediated by trmJ (yfhQ), and trmJ (yfhQ) is responsible for Cm32 modifications.45 tRNA modification is an emerging critical component of bacterial physiology. Several modifications have been reported to regulate biological processes. For example, a decrease in Mg2+ levels lowers TrmD activity, resulting in a reduced modification of tRNAPro. This reduction prompts the attenuation of the MgtL leader peptide, facilitating the expression of the mgtA transporter gene. The ratio of mcmo5U to cmo5U notably increased during growth. Low iron levels decrease MiaB activity, leading to a decreased modification of tRNASer. This diminishes the translation of uof and fur, which act as negative regulators of the low-iron response.46 Recent research has broadened our understanding of how epitranscriptomic modifications of tRNA contribute to antibiotic resistance so we scrutizes the role of epitranscriptomic modification in tRNA in antibiotic resistance in this section (see Figure 2 & Table 2).
Methylation of m1G37-tRNA controls proline codons near the open reading frame, boosting bacterial drug resistance via trmD. Reduced m1G37 levels in Escherichia coli and Salmonella disrupt membrane integrity, hindering the development of ampicillin, carbenicillin, kanamycin, gentamicin, paromomycin, rifampicin, and ciprofloxacin resistance.43 Further studies have shown that TrmD inhibitors could be used to overcome antibiotic resistance.47 tRNA modification genes, such as Tgt, DusB, TruA/B/C, TrmA/B/E/H, and RlmN, were found to modulate responses to several antibiotics, such as aminoglycosides, fluoroquinolones, β-lactams, chloramphenicol, and trimethoprim in Vibrio cholerae suggesting that their role needs to be further explored to elucidate the role of tRNA modification in antibiotic resistance.48 In addition, several tRNA modifications have been associated with bacterial survival. For example, m7G tRNA methyltransferase (TrmB) catalyzes m7G tRNA modification, which is crucial for stress responses and pathogenesis in Acinetobacter baumannii baumannii,49 while the enzyme m1A22-tRNA methyltransferase (TrmK) transfers a methyl group from SAM to adenine 22 in tRNAs, which is crucial for Staphylococcus aureus survival during infection.50 This finding suggests that epitranscriptomic modification of tRNA orchestrating key players can be targeted to develop new antibiotics or reverse antibiotic resistance to overcome the global threat of antibiotic resistance.
It is imperative to acknowledge that, while rRNA methylation has been a focal point in understanding epitranscriptomic modifications and their role in antibiotic resistance, a vast expanse of uncharted territory remains. The intricate world of non-coding RNA, encompassing cis and trans small non-coding RNA, as well as riboswitches and their respective targets, presents a rich tapestry of regulatory elements that are yet to be explored in terms of their methylation status. As we navigate the complex landscape of epitranscriptomics, it becomes evident that our current understanding is the tip of the iceberg. The myriad roles played by non-coding RNA in controlling antibiotic resistance are tantalizing, with profound implications for therapeutic interventions. Our exploration has led us to compile a comprehensive summary of non-coding RNA entities that orchestrate the intricate dance between RNA modification and antibiotic resistance, as presented in Table 3. However, the absence of detailed insights into the methylation status of these non-coding RNA elements underscores the need for further investigations. Unraveling the specific modifications that these regulatory elements undergo will undoubtedly provide a more nuanced understanding of their function in the context of antibiotic resistance. This crucial information could unveil new targets for intervention, shaping the landscape of antibiotic development and refining our strategies to overcome bacterial resistance. While our current understanding has shed light on the interplay between rRNA methylation and antibiotic resistance, it is a mere prelude to the broader symphony orchestrated by non-coding RNA. The journey into the epitranscriptomic regulation of antibiotic resistance is far from over, and the unexplored realms of non-coding RNA have motivated researchers to delve deeper into the intricacies of their methylation dynamics. Within this uncharted territory, the next chapter of epitranscriptomics in antibiotic resistance regulation awaits the promise of transformative insights for the future of antimicrobial strategies.
| sRNA | Organism(s) | Resistance and/or inducer | Mechanism | Ref. | |
|---|---|---|---|---|---|
| Tran s-encoded small non coding RNA | RepG | Helicobacter pylori | Polymyxin B (PxB), a membrane-targeting antibiotic and surrogate for host CAMPs. | RepG sRNA regulates expression of hp0102 (in addition to tlpB) and in turn LPS biosynthesis | 51 |
| NrrF | Neisseria gonorrhoeae | Sulfonamides | Shortens the lifespan of mtrF mRNA which promote mRNA degradation | 52 | |
| AbsR25 | Acinetobacter baumannii | Fosfomycin | Negative control over the MFS superfamily efflux pump gene abaF | 52 | |
| RyhB | E. coli | Levofloxacin, Cefotaxime, and Gentamicin | sRNA RyhB to persistence occurs independently of the sRNA-binding protein Hfq, yet it acts synergistically with the ppGpp and Fur proteins | 53 | |
| SdsR | E. coli | Fluoroquinolones | Suppressing the activity of the drug efflux pump, TolC | 54 | |
| DsrA, ArcZ, RprA, and OxyS | E. coli | Resistant to oxacillin, cloxacillin, erythromycin, rhodamine 6G and novobiocin | Control of the MdtEF efflux pump (by ArcZ, RprA, and OxyS), directly regulate the expression of a phosphoethanolamine transferase and LPS modifications (by ArcZ), resistance by DsrA to all mentioned Antibiotics controls MdtEF efflux pump | 55 | |
| sRNA_0160 | Enterococcus faecium | Daptomycin | Response to antibiotic stress and resistance | 56 | |
| NrrF | Neisseria gonorrhoea | Penicillin and erythromycin | Hindering the production of MtrF and elevating resistance to hydrophobic antimicrobials | 55 | |
| SdsR | E. coli | Fluoroquinolones | Inhibiting the TolC drug efflux pump | 54 | |
| SdsR | Shigella sonnei | Norfloxacin (decrease sensitivity) | Elevated binding stability to tolC mRNA exhibited the greatest rate of growth | 54 | |
| CsiR | Proteus vulgaris | Ciprofloxacin | The interaction between CsiR and emrB mRNA significantly influences the post-transcriptional stability | 57 | |
| CrcZ | Pseudomonas aeruginosa | Aminoglycoside | Global regulation of metabolic genes through carbon catabolite repression (CCR) | 58 | |
| PA0805.1 | Pseudomonas aeruginosa | Gentamicin and tobramycin | PA0805.1 plays a role in the regulation of iron acquisition and motility | 59 | |
| Sr006 | Pseudomonas aeruginosa | Polymyxin | Sr006 upregulates the expression of PagL, independently of Hfq | 60 | |
| Cis-encoded small non coding RNA | rli53 and rli59 | Listeria | Lincomycin | Regulate the expression of lmo0919 and lmo1652 genes | 61 |
| SprX | Staphylococcus aureus | Vancomycin and Teicoplanin | Affects the resistance of the bacterium to glycopeptides where SprX negatively regulates the expression of SpoVG | 62 | |
| RyhB | Escherichia coli | Gentamicin | RyhB exerts negative regulation on the expression of nuo and Sdh, likely by binding to their mRNAs and inhibit the translation | 63 | |
| StyR-3 | Salmonella enterica serovar Typhi | MDR (not provided list of antibiotics) | Specific interaction with RamR, the transcriptional repressor of the ramA gene | 64 | |
| Sr0161 | Pseudomonas aeruginosa | Meropenem | Inhibition of oprD translation | 60 | |
| ErsA | Pseudomonas aeruginosa | Meropenem | Inhibition of oprD translation | 60 | |
| Riboswitches | AAC & AAD | Various species | Aminoglycoside | Riboswitch controls aminoglycoside acetyl or aminoglycoside adenyl gene | 65 |
| class 1 integron | Pseudomonas fluorescens | Aminoglycosides | Regulating the expression of the subsequent aminoglycoside resistance gene | 66 | |
| Aptazyme | E. coli | β-lactamase | Control the expression of β-lactamase through interactions with a small molecule aptamer | 67 | |
| 2'-dG-I & 2'-dG-II | Data from various species | Deoxyguanosine | Recognize both cognate and noncognate ligands which may offer valuable insights for developing antibiotics as potential targets | 68 | |
| PreQ1, MoCo RNA, cyclic-di-GMP I, and cyclic-di-GMP II | Various species | Metabolites | As targets for antibacterial drugs | 69 | |
| FMN | Staphylococcus aureus, Listeria monocytogenes, and Escherichia coli | FMN precursors | Synthesis and import of FMN precursors and could be a viable approach for developing antibacterial drugs against FMN | 70 | |
| glmS | Staphylococcus aureus | Glucosamin-6-phosphate | It undergoes self-cleavage upon detecting glucosamin-6-phosphate | 71 |
The global threat of antibiotic resistance necessitates a comprehensive understanding of its mechanisms to develop innovative strategies to combat this peril. Antibiotic resistance is a major cause of global mortality, and has the potential to induce a widespread crisis with the emergence of highly resistant bacteria. To address this urgency, various resistance mechanisms, including the upregulation of efflux pumps, modification of antibiotic targets, and sequestration of antibiotics, have been elucidated. However, the regulation of genes associated with antibiotic resistance remains poorly understood. Recent studies have underscored the significance of epigenetic and epitranscriptomic factors in the regulation of gene expression. Non-coding RNAs, particularly cis and trans small non-coding RNAs and riboswitches, are emerging as key players in modulating antibiotic resistance-associated genes. Despite their recognition, epitranscriptomic regulation of these elements remains largely unexplored. Epigenetic regulation by methyltransferases (e.g., M. NgoAV, AamA, ModA11, ModA12, ModA13, ModS2, Dam, and Dcm) and histone-associated proteins, such as H-NS and NapM, have been implicated in antibiotic regulation. Similarly, the epitranscriptomic regulation of rRNA by methyltransferases such as ArmA, RmtC, Sgm, RsmF, RlmCD, RlmAII, ErmC, Cfr, and yfgB (rlmN) and tRNA by methyltransferases TrmD play a crucial role in antibiotic regulation. Additionally, trans non-coding small RNAs (RepG, NrrF, AbsR25, RyhB, SdsR, DsrA, ArcZ, RprA, OxyS, sRNA_0160, NrrF, CsiR, CrcZ, PA0805.1, and Sr006), cis non-coding RNAs (rli53, rli59, SprX, RyhB, StyR-3, Sr0161, and ErsA), and riboswitches (AAC, AAD, MoCo RNA, class 1 integron, aptazyme, PreQ1, 2’-dG-I and 2’-dG-II, FMN, glmS, cyclic-di-GMP I, and cyclic-di-GMP II) have been identified as regulators of antibiotic resistance. Further exploration of epigenetic and epitranscriptomic orchestrators is pivotal to understand the regulation of genes associated with antibiotic resistance. This knowledge will prepare the ground for the discovery of novel antibiotics and the development of innovative strategies to overcome antibiotic resistance. Additionally, the role of RNA thermosensors in bacterial virulence has been highlighted, yet their involvement in antibiotic resistance remains insufficiently explored.72 Therefore, delving into the epigenetic and epitranscriptomic-mediated regulation of antibiotic resistance, including non-coding RNA exploration, is poised to revolutionize our understanding and aid in the development of new compounds to mitigate antibiotic resistance mortality.
P. K. G. conceptualized and constructed the outline of the review; all authors (PKG, SA and MD) contributed to the writing of the manuscript.
The authors are thankful to colleagues and researchers for their insightful discussions and constructive feedback.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)