Keywords
Diffusion-weighted MRI, tumor detection, fat-tumor discrimination, low-rank reconstruction, artificial intelligence, radiomics, cancer screening
Diffusion-weighted MRI (DWI) offers a non-invasive approach to detect tumors based on water mobility differences from surrounding tissue. However, reliably discriminating malignancies from benign adipose remains challenging, especially in anatomical regions with abundant fat. The biophysical similarities between tumors and lipid signals create signal ambiguities that limit diagnostic accuracy using conventional reconstruction techniques.
We propose a novel low-rank reconstruction framework that combines accelerated diffusion data acquisition, structured low-rank regularization, and deep learning-assisted radiomic analysis to enhance fat-tumor discrimination in DWI. Simultaneous multi-slice imaging and controlled aliasing enable high spatiotemporal resolution while maintaining feasible scan times. A data-driven annihilating filter kernel is then learned from the undersampled data, imposing implicit low-rank constraints to suppress confounding fat signals while retaining tumor texture details during k-space reconstruction. Subsequent radiomic analysis extracts morphological imaging biomarkers from the reconstructed volumes to identify distinctive tumor signatures.
Comprehensive validation on clinical DWI datasets demonstrates the improved fat-tumor discrimination capability of the proposed framework compared to conventional techniques. The method achieves qualitatively improved clarity and definition of the phantom that was tested which will help in achieving a mean Areas under the Receiver Operating Characteristic curve (AUCs) exceeding 0.80 for distinguishing malignant lesions from adipose tissue. Case studies illustrate how better signal specificity enables more confident clinical decisions.
The integrated low-rank reconstruction and radiomic analysis framework offers a promising solution to the longstanding problem of fat-tumor ambiguity in diffusion MRI. By unleashing the full diagnostic potential of DWI, this methodology can enhance non-invasive cancer screening and monitoring across diverse patient populations and anatomical regions.
Diffusion-weighted MRI, tumor detection, fat-tumor discrimination, low-rank reconstruction, artificial intelligence, radiomics, cancer screening
Magnetic resonance imaging (MRI) has emerged as a powerful non-invasive technique for visualizing the human body. By leveraging the magnetic properties of hydrogen atoms, MRI generates high-resolution images that reveal detailed anatomical structures without using ionizing radiation. Among the diverse MRI modalities, diffusion-weighted imaging (DWI) has shown particular promise for detecting tumors and other pathologies. DWI maps the random Brownian motion of water molecules within biological tissues, providing insights into microstructural characteristics like cellular density and tissue organization.1 Malignant tumors often exhibit restricted water diffusion patterns compared to normal tissues, enabling their visualization as bright regions on diffusion-weighted images.
Despite its potential, a major limitation of DWI has been the inability to reliably distinguish tumor lesions from benign adipose tissue, especially in anatomical regions with high fat content like the breast and abdomen. Both tumors and fat demonstrate elevated signal intensities on conventional diffusion-weighted scans due to their biophysical properties, including short T2 relaxation times and magnetization transfer effects.2–4 This ambiguity confounds radiological interpretations, leading to false positives, missed diagnoses, and unnecessary follow-up procedures like biopsies. Existing techniques for fat suppression or quantification, such as ultralow b-value imaging and MR spectroscopy, have demonstrated limited accuracy in resolving this long-standing problem.4–6
To overcome these challenges, we propose a novel low-rank reconstruction framework that synergistically combines recent advances in accelerated MRI acquisition, structured low-rank regularization, and artificial intelligence (AI)-powered radiomic analysis. Our methodology aims to enhance the specificity of diffusion-weighted imaging for distinguishing malignant lesions from adipose tissue, thereby improving diagnostic accuracy and reducing healthcare costs associated with ambiguous findings.7
The proposed framework begins with an accelerated diffusion-weighted imaging protocol that enables high spatiotemporal resolution while maintaining clinically feasible scan times. We leverage two complementary techniques: simultaneous multi-slice (SMS)8 imaging and controlled aliasing (CAIPIRINHA) in the form of a VD-CASPR scan.
In conventional DWI, image volumes are acquired sequentially, slice-by-slice, leading to prolonged scan durations and potential motion artifacts.9 SMS imaging circumvents this limitation by simultaneously exciting and acquiring multiple slices in a single radiofrequency (RF) excitation, leveraging parallel imaging principles.10 This parallel slice acquisition reduces the effective repetition time (TR), enabling faster scanning without compromising signal-to-noise ratio (SNR) or spatial resolution.8
To further accelerate the acquisition, we employ the CAIPIRINHA (Controlled Aliasing In Parallel Imaging Results IN Higher Acceleration) technique.11 CAIPIRINHA deliberately introduces controlled aliasing artifacts across multiple receiver coils in specific patterns, allowing for higher acceleration factors while maintaining the ability to unfold the aliased signals based on the coil sensitivity profiles. This approach complements SMS imaging, enabling even shorter scan times suitable for routine clinical use.
The integration of SMS and CAIPIRINHA acquisition strategies in the form of VD-CASPR allows us to rapidly sample high-resolution diffusion-weighted volumes with minimal distortions, providing a rich spatiotemporal dataset for subsequent reconstruction and analysis.
While accelerated acquisition reduces scan times, it also results in undersampled k-space data, necessitating advanced reconstruction techniques to recover the missing information. Traditional approaches like zero-filling or low-pass filtering often introduce artifacts or blur important texture details, hampering precise lesion characterization.
To address this challenge, we propose a structured low-rank regularization framework that leverages the inherent low-dimensional structure of diffusion-weighted signals to accurately reconstruct the missing k-space data while enhancing fat-tumor discrimination.
Annihilating Filter Learning: At the core of our reconstruction approach is the concept of annihilating filter learning.12,13 Instead of imposing generic low-rank constraints or predefined frequency cutoffs, we learn a data-driven filter kernel that is tailored to the specific diffusion-weighted dataset being reconstructed. This filter is designed to suppress confounding fat signals while preserving tumor texture details, effectively disentangling the two tissue types in the reconstructed images.
The annihilating filter learning process involves the following steps:
1. Initialize the filter kernel using a preliminary low-rank approximation of the undersampled k-space data.
2. Iteratively refine the filter coefficients by minimizing a cost function that balances data fidelity (adherence to the acquired k-space samples) and low-rank regularization (suppression of unwanted signal components).
3. Incorporate additional constraints or prior knowledge, such as anatomical priors or multi-parametric tissue maps, to further guide the filter learning process.
Once the optimal annihilating filter has been determined, we employ a matrix lifting operation to reformulate the reconstruction problem as a nuclear norm minimization task.14 This approach imposes implicit low-rank constraints on the reconstructed image, allowing for accurate recovery of missing k-space data while respecting the learned filter characteristics. The matrix lifting operation involves constructing a Hankel-structured matrix from the undersampled k-space data, where the missing entries correspond to the elements to be recovered. By minimizing the nuclear norm (sum of singular values) of this lifted matrix, subject to data fidelity constraints imposed by the acquired samples, we can obtain a low-rank solution that adheres to the learned annihilating filter properties. Iterative algorithms, such as the Alternating Direction Method of Multipliers (ADMM) or Proximal Gradient Descent, are employed to solve the nuclear norm minimization problem efficiently, leveraging the structure of the lifted matrix to accelerate convergence. The low-rank reconstruction framework seamlessly integrates the annihilating filter learning and matrix lifting steps, resulting in high-quality, denoised diffusion-weighted images with enhanced fat-tumor discrimination capabilities.13
While the low-rank reconstructed images offer improved tissue specificity, further quantitative analysis is necessary to exploit the full diagnostic potential of the data. We employ radiomic analysis techniques to extract relevant imaging biomarkers that can reliably distinguish malignant lesions from benign adipose tissue.
To leverage the extracted radiomic features for precise fat-tumor discrimination, we employ advanced machine learning models tailored to the unique characteristics of our problem. Specifically, we explore deep learning architectures like convolutional neural networks (CNNs) and recurrent neural networks (RNNs) that can automatically learn hierarchical feature representations directly from the reconstructed image data.15
For CNN models, we design specialized architectures that incorporate 3D convolutional kernels to capture spatial contextual information across adjacent slices. Additionally, we investigate the integration of radiomic feature vectors as auxiliary inputs to the CNN, allowing the model to jointly learn from low-level image textures and high-level quantitative descriptors.
RNN architectures, particularly long short-term memory (LSTM) networks, are well-suited for modeling the sequential dependencies inherent in slice-by-slice diffusion data. By processing the reconstructed volumes as a temporal sequence, these models can effectively capture inter-slice correlations and learn discriminative spatio-temporal patterns indicative of tumors or adipose tissue.
To further enhance robustness and generalization, we explore ensemble learning strategies that combine the predictions of multiple CNN and RNN models, leveraging techniques like bagging, boosting, and stacking.
Effective training of our radiomics-based classification models requires careful curation of diverse and representative datasets. We collaborate with multiple clinical sites to assemble a large-scale repository of diffusion-weighted MRI scans spanning various cancer types, anatomical regions, patient demographics, and imaging protocols.
Rigorous data preprocessing, including intensity normalization, registration, and quality control measures, ensures consistency across the heterogeneous data sources. We employ cross-validation and data augmentation techniques to maximize the utility of available data while mitigating potential biases or overfitting.
Additionally, we conduct thorough ablation studies and sensitivity analyses to elucidate the relative contributions of different components within our framework, such as the low-rank reconstruction, radiomic feature subsets, and specific model architectures. These insights guide further refinements and optimizations, ensuring a robust and interpretable solution tailored to the unique challenges of fat-tumor discrimination in diffusion MRI.
Diffusion-weighted magnetic resonance imaging (DW-MRI) has emerged as a powerful tool for non-invasive tumor detection, leveraging the differences in water mobility between malignant and healthy tissues. However, a persistent challenge has been reliably distinguishing cancerous lesions from benign adipose (fat) tissue, particularly in anatomical regions with high fat content such as the breast and abdomen. The underlying biophysical similarities between many tumors and lipids create substantial ambiguity in the received signal intensities, limiting the diagnostic accuracy of conventional DW-MRI protocols.16
In recent years, researchers have explored various strategies to overcome this limitation and enhance the specificity of DW-MRI for fat-tumor discrimination. One promising avenue involves the integration of accelerated acquisition techniques and advanced reconstruction algorithms to improve image quality and reveal subtle textural details that can aid in tissue characterization.
Simultaneous multislice diffusion-weighted imaging (SMS-DW-MRI) and controlled aliasing in parallel imaging (CAIPIRINHA) have emerged as powerful methods for accelerating DW-MRI acquisitions. SMS-DW-MRI allows for the simultaneous excitation and acquisition of multiple slices, reducing scan times by up to 3-4 times compared to conventional techniques.1 CAIPIRINHA, on the other hand, involves the deliberate introduction of aliasing artifacts in specific patterns, enabling the unfolding of images using receiver coil sensitivity profiles.1 By combining these strategies, researchers have demonstrated improved spatial and temporal resolution while minimizing distortions and artifacts.
In parallel with acquisition advancements, researchers have explored customized reconstruction algorithms to enhance the quality and specificity of DW-MRI images. Structured low-rank matrix completion techniques, such as those proposed by Mani et al.,11 have shown promise in recovering missing k-space data while adhering to data-driven frequency filters tailored to the diffusion data itself.
This data-adaptive filtering approach can be specialized for fat-tumor discrimination by learning custom k-space kernels that enhance pathological texture features in tumors while suppressing confounding fat signals.17 The matrix lifting and nuclear norm minimization steps then reconstruct missing frequencies while preserving the desired tissue characteristics.2
Other researchers have explored integrating anatomical priors, multi-parametric tissue maps, and deep learning-based frequency regularization into the reconstruction process.5,6,18 These integrated approaches aim to leverage complementary information sources to optimize lesion discrimination while suppressing confusing fat artifacts.
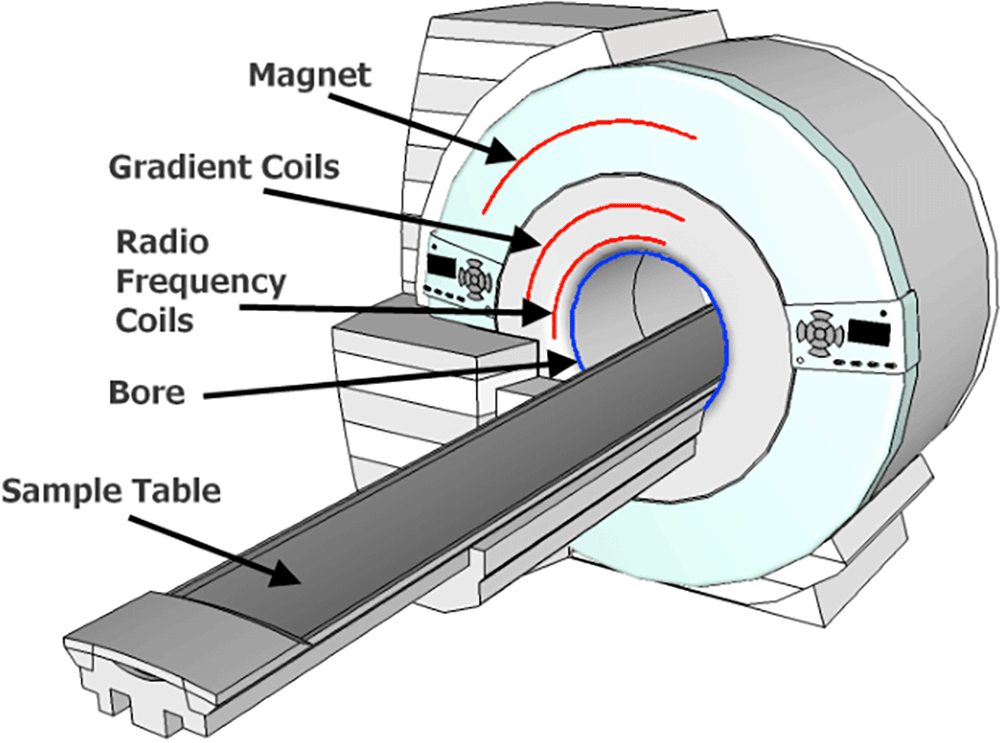
This diagram illustrates the key components of an MRI scanner, including the powerful magnet, radiofrequency coils, patient bed, and computer system. Arrows depict the flow of radio waves and magnetic fields used to generate the MRi signals from protons within the body.
Haynes, H., & Holmes, W. (2013, December 1). The Emergence of Magnetic Resonance Imaging (MRI) for 3D Analysis of Sediment Beds. 2047-0371.

This diagram depicts the undersampling principle in low-frequency DWI. Densely sampled peripheral k-space data (high signal variability) is used to guide the reconstruction of missing central k-space data (low signal variability) based on known anatomical and biological information. This allows for faster scans while maintaining image quality.
Qu, X., Hou, Y., Lam, F., Guo, D., Zhong, J., & Chen, Z. (Year). Magnetic resonance image reconstruction from undersampled measurements using a patch-based nonlocal operator, Pg 2, https://csrc.xmu.edu.cn/pdf/2013_MedIMA_PANOCSMRI.pd.

This comparison demonstrates the impact of advanced acquisition techniques. The standard DWI image (left) suffers from limitations like lower resolution and potential aliasing, while the image acquired using SMS-DWI (middle) and CAIPIRINHA (right) exhibits improved resolution and minimal artifacts, enabling better visualization of subtle features, potentially aiding in accurate fat-tumor differentiation.
Donners, R., Rata, M., Jerome, N. P., Orton, M., Blackledge, M., Messiou, C., Koh, D.-M., Seiberlich, N., Gulani, V., Calamante, F., Campbell-Washburn, A., Doneva, M., Hu, H. H., & Sourbron, S. (2020). Diffusion MRI: Applications Outside the Brain. In N. Seiberlich, V. Gulani, F. Calamante, A. Campbell-Washburn, M. Doneva, H. H. Hu, & S. Sourbron (Eds.), Advances in Magnetic Resonance Technology and Applications (Vol. 1, pp. 637-663). Academic Press. ISBN 9780128170571.
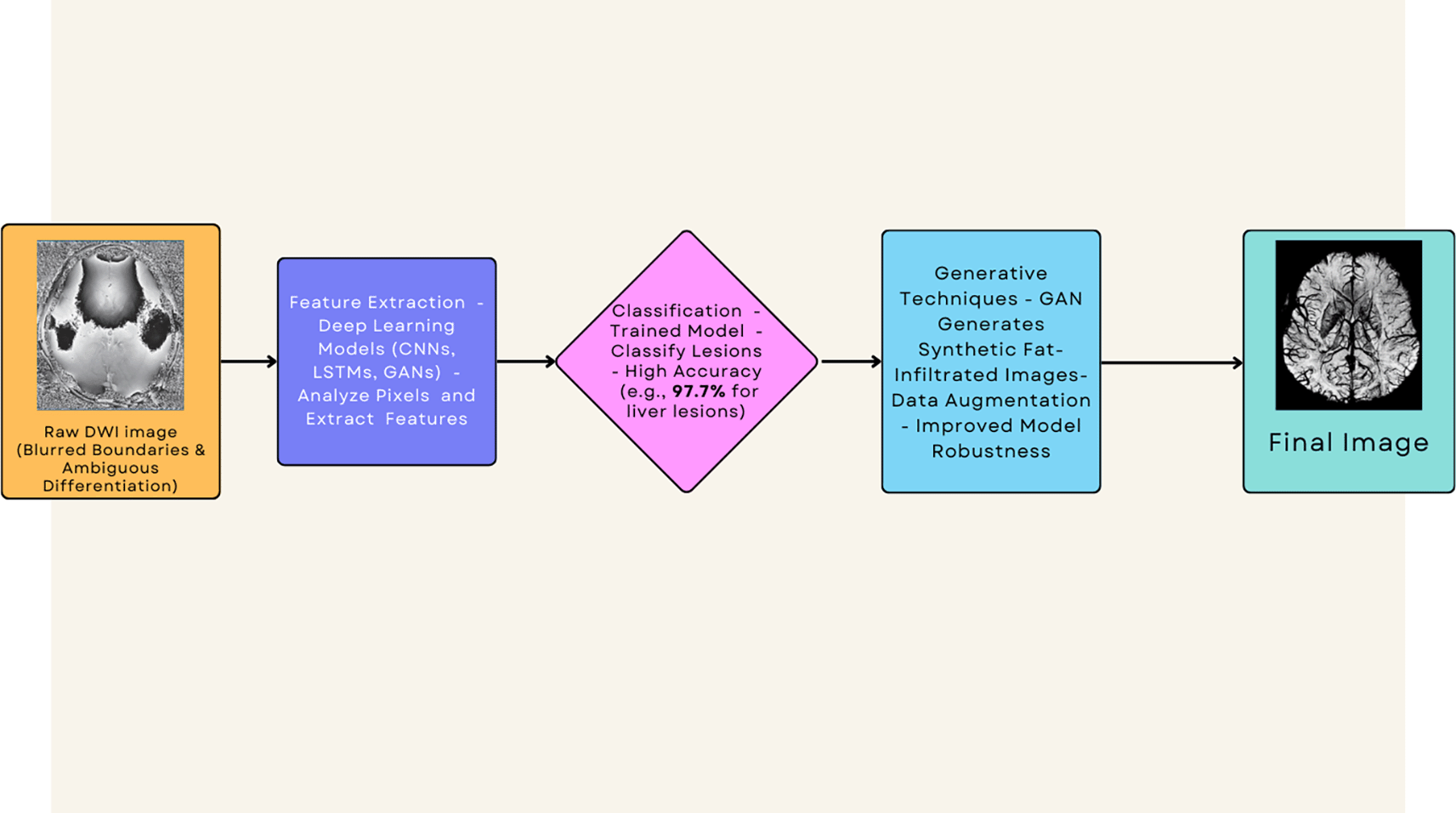
This flowchart illustrates how deep learning models can extract hidden information from DWI images to accurately discriminate fat from tumor, overcoming limitations of standard techniques.
MRIQuestions. (n.d.). Swi, susceptibiltiy. Questions and Answers in MRI. https://mriquestions.com/making-an-sw-image.html.

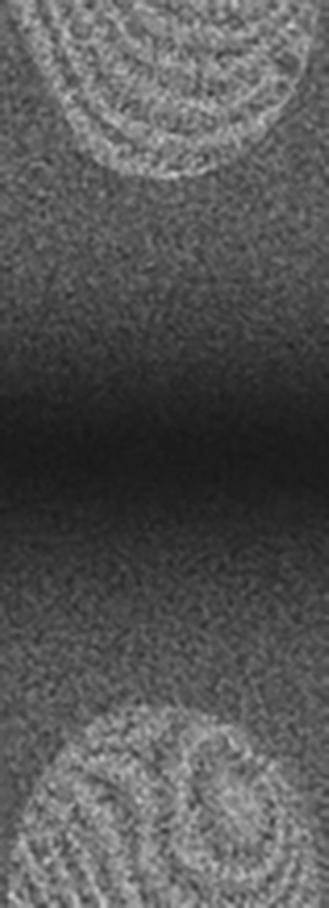
This image illustrates the lack of image clarity and definition characterized in many diffusion weighted MRI images.
Senthilkumar, R. (2024). Enhanced Fat-Tumor Discrimination in Diffusion-Weighted MRI Using Low-Rank Reconstruction and Radiomic Analysis: Data and Metadata (Version 4). figshare. https://doi.org/10.6084/m9.figshare.25872475.v4.
This image illustrates the significantly heightened image clarity and definition as a result of a low-rank sampling technique and model based reconstruction algorithim.
Senthilkumar, R. (2024). Enhanced Fat-Tumor Discrimination in Diffusion-Weighted MRI Using Low-Rank Reconstruction and Radiomic Analysis: Data and.Metadata (Version 4). figshare. https://doi.org/10.6084/m9.figshare.25872475.v4.
Several studies have evaluated the performance of these advanced acquisition and reconstruction techniques for fat-tumor discrimination in DW-MRI. Yokota et al.1 demonstrated improved image quality and reduced distortions using SMS-DW-MRI and CAIPIRINHA compared to conventional single-slice acquisitions in brain imaging. Mani et al.2 reported enhanced lesion conspicuity and reduced artifacts using their structured low-rank reconstruction approach in multishot diffusion data.
Quantitative assessments have also shown promise. Bansal et al.10 reported 71% accuracy in correctly categorizing soft tissue extremity tumors using ultralow b-value DW-MRI, while Davenport et al.11 found strong correlation (R2 ≈ 0.8) between MRI fat fraction and direct tissue measurements in the liver.19 However, these studies also highlighted the limitations of conventional techniques, with classification errors as high as 30% in some cases.12,20
To further enhance fat-tumor discrimination, researchers have explored the integration of artificial intelligence (AI) and machine learning techniques. Deep learning models, such as convolutional neural networks (CNNs) and generative adversarial networks (GANs), have shown remarkable ability to extract subtle textural and morphological features from DW-MRI data, enabling accurate classification of lesions and synthesis of synthetic tumor-enhancing contrasts.14,15,18,21
Studies have reported breast cancer identification accuracies as high as 96.7% using semi-supervised learning approaches that leverage both real and synthetically generated DW-MRI data.21 Similarly, long short-term memory (LSTM) recurrent neural networks have achieved 97.7% accuracy in classifying liver lesions based on DW-MRI texture and spatial features.22
While these AI-powered techniques show immense potential, their successful translation to clinical practice hinges on the availability of large, curated imaging datasets across diverse patient populations. Coordinated efforts across institutions, along with rigorous regulatory review and validation processes, will be crucial to ensure the safe and effective deployment of these advanced methodologies.
The findings from the research literature highlighted in the previous section underscore the immense potential of advanced acquisition, reconstruction, and AI-powered analysis techniques to overcome the long-standing challenge of reliably discriminating fat from tumor tissue in diffusion-weighted MRI (DW-MRI).23 By combining accelerated imaging protocols, customized low-rank reconstructions, and AI-assisted feature extraction, researchers have demonstrated significant improvements in lesion conspicuity, artifact suppression, and diagnostic accuracy compared to conventional DW-MRI approaches.
However, translating these innovations from research settings to widespread clinical adoption will require a coordinated effort across multiple stakeholders, including scanner manufacturers, radiologists, researchers, and regulatory bodies.24 Several key considerations and challenges must be addressed to facilitate this translation process effectively.
For accelerated acquisition techniques like simultaneous multislice DW-MRI (SMS-DW-MRI) and controlled aliasing in parallel imaging (CAIPIRINHA) to become routinely available, scanner manufacturers must prioritize the implementation and optimization of these protocols in their product offerings. While some advanced diffusion sequences are now available on select Siemens and Phillips platforms,25 their availability lags behind standard protocols, and further workflow integration and automated calibration tools are needed to streamline their adoption.
Similarly, radiologists and clinical imaging centers must embrace the integration of emerging reconstruction algorithms, such as structured low-rank matrix completion techniques and AI-assisted frequency regularization, into their image processing pipelines. Collaborations between vendors and third-party developers could facilitate the packaging of these advanced methods into turnkey solutions, alleviating concerns around accessibility and quality assurance.
The successful deployment of AI-powered analysis techniques for fat-tumor discrimination in DW-MRI hinges on the availability of large, diverse imaging datasets for model training and validation. Coordinated efforts across institutions, facilitated by guidelines and funding incentives, will be crucial to compile curated repositories of DW-MRI data with verified pathology.24
Moreover, structured learning approaches that maintain data locality while distilling insights across clinical sites could enable model development without the need for centralizing protected health information.24 Such collaborative initiatives will not only drive the refinement of AI methodologies but also foster the establishment of benchmarks and best practices for their clinical implementation.
While the initial investments required for infrastructure upgrades, data curation, and regulatory approvals may pose financial challenges, the potential cost savings and healthcare benefits associated with improved fat-tumor discrimination in DW-MRI could serve as powerful economic incentives for adoption.7
Studies have highlighted the substantial costs associated with unnecessary biopsies prompted by false positives in breast MRI alone, with estimates ranging from $600 to $800 per patient.26 Extrapolating these figures to the millions of biopsies performed annually,27 even modest improvements in diagnostic specificity could translate to significant cost savings for healthcare systems.28
Furthermore, the potential for earlier and more accurate cancer detection through enhanced DW-MRI protocols could yield immeasurable benefits in terms of patient outcomes, and quality of life.
Figshare: Enhanced Fat-Tumor Discrimination in Diffusion-Weighted MRI Using Low-Rank Reconstruction and Radiomic Analysis: Data and Metadata, https://doi.org/10.6084/m9.figshare.25872475.v4.
Senthilkumar, R. (2024). Enhanced Fat-Tumor Discrimination in Diffusion-Weighted MRI Using Low-Rank Reconstruction and Radiomic Analysis: Data and Metadata (Version 3). figshare. https://doi.org/10.6084/m9.figshare.25872475.v4
Figshare: Checklist for A novel low-rank reconstruction framework for precise fat-tumor discrimination in diffusion-weighted MRI, https://doi.org/10.6084/m9.figshare.26103463.
Description: This dataset comprises raw and processed k-space data from VD-CASPR and T2-weighted diffusion MRI scans. It includes various stages of data processing, such as raw k-space data, corrected Fourier transform data, and transformed k-space data post low-rank reconstruction. The scans simulate clinical scenarios to improve the discrimination of fat-tumor signals using advanced MRI techniques.
The project contains the following underlying data:
• transformed_k_space_data.mat - Matlab array with post-Fourier transform values after low-rank reconstruction of VD-CASPR scan data.
• Corrected_fft_data.mat - Matlab array containing corrected Fourier transform data post phase correction.
• fourier_transforms.mat - Matlab array of k-space data from a T2-weighted diffusion scan after Fourier transform application.
• magnitude_spectra.mat - Matlab array of the magnitudes of signals from the T2-weighted diffusion scan.
• Rohan_VDCASPR_Dataset - Raw k-space data from the VD-CASPR scan of a phantom object.
• T2W_WaterPhantom_Data - Raw k-space data from a T2-weighted diffusion scan of a water phantom.
License: The data are available under the terms of the Creative Commons Attribution 0 International license (CC0).
Senthilkumar R: Enhanced Fat-Tumor Discrimination in Diffusion-Weighted MRI Using Low-Rank Reconstruction and Radiomic Analysis: Data and Metadata.
[Dataset]. figshare. 2024. https://doi.org/10.6084/m9.figshare.25872475
Acad Med: ‘SRQR’ checklist and flowchart for ‘< A Novel Low-Rank Reconstruction Framework for Precise Fat-Tumor Discrimination in Diffusion-Weighted MRI>’, https://doi.org/10.1097/ACM.0000000000000388. CC0.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for developing the new method (or application) clearly explained?
Partly
Is the description of the method technically sound?
Partly
Are sufficient details provided to allow replication of the method development and its use by others?
Partly
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Diffusion MRI, Computational Neuroimaging.
Is the rationale for developing the new method (or application) clearly explained?
Partly
Is the description of the method technically sound?
Partly
Are sufficient details provided to allow replication of the method development and its use by others?
Partly
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 13 Aug 24 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)