Keywords
Platelet-Rich Plasma, PRP, Erectile Dysfunction, ED
Platelet-rich plasma (PRP) is a novel treatment for erectile dysfunction (ED) that is considered to be a cost-effective option. It has demonstrated positive outcomes and minimal adverse effects. By injecting PRP into the intracavernous tissue, it has the potential to promote angiogenesis and neuroregeneration, leading to a potential permanent improvement in erectile performance. The potency and safety as well as the variety of PRP administration protocols in the treatment of ED are the objectives of this paper.
Using certain terms, a systematic search was done on three different databases: Science Directs, Scielo, and PubMed. Papers about PRP therapy for EDs within the last decade, be randomized controlled trials (RCTs), and be original studies with freely available full-text content were included in this study. This article omitted letters to the editor, reviews, and editorials regarding PRP and ED.
Three RCT were included in this studies. The improvement is observed at one month, three months, and six months after the treatment, compared to the baseline. PRP group participants met the Minimal Clinically Important Difference (MCID) in the International Index of Erectile Function-Erectile Function (IIEF-EF) at a higher percentage than those in the placebo group at both one month and six months. Injection of PRP leads to a superior enhancement in the IIEF-EF score, compared to a placebo, in patients suffering from ED at one month, three months, six months.
PRP is a proven therapeutic technique that has been shown to greatly enhance ED. Yet, to establish uniform guidelines for PRP in treating erectile dysfunction, further investigation into PRP, particularly in protocol development, is imperative.
Platelet-Rich Plasma, PRP, Erectile Dysfunction, ED
Erectile dysfunction, abbreviated as ED, refers to the incapacity to achieve and maintain a sufficiently strong erection for sexual activity.1 The incidence of erectile dysfunction is steadily rising, highlighting its potential influence on the overall health of sexually active males worldwide. Estimates indicate that the global prevalence of ED in 1995 was approximately 152 million men. It is anticipated that the aforementioned figure would increase to around 322 million by the year 2025, reflecting an almost 170 million growth.2 ED is classified by International Society of Impotence Research (ISIR) as organic and psychogenic. Organic ED is then further divided into vasculogenic (which consists of arteriogenic, cavernosal, and mixed), neurogenic, anatomic, and endocrinologic. We divide psychogenic into two categories: generalized and situational.3
The etiology of erectile dysfunction is influenced by multiple factors, and its prevalence tends to rise with advancing age, particularly beyond the age of 60.4 Inactivity, obesity, poor nutrition, and smoking are all associated with ED. Additional contributing factors include mental and emotional issues, insulin resistance, heart disease, high blood pressure, hypercholesterolemia, metabolic syndrome, and reduced testosterone levels. Management of erectile dysfunction initially ranges from lifestyle changes to oral phosphodiesterase-5 (PDE-5) inhibitors, intracavernous injections, vacuum-assisted erectile Device (VED) and implantation of penile prostheses. However, all of these treatments mentioned above do not attempt to treat the underlying pathophysiologic mechanism of vasculogenic erectile dysfunction. Out of the novel treatments, PRP is considered a financially viable alternative for ED therapy with favorable results and relatively negligible adverse effects.5,6
PRP, characterized by a platelet concentration that exceeds the initial level observed prior to the centrifugation process, is classified as a biological entity found in an individual’s blood plasma fraction. Therefore, PRP consists of a substantial quantity of platelets as well as a complete range of clotting factors, with the latter generally maintaining their usual physiological levels. The composition of the material is influenced by a variety of growth factors (GFs), chemokines, cytokines, and plasma protein molecules. Prior to centrifugation, platelet-poor plasma (PPP) is extracted from the blood of patients, resulting in the separation of blood constituents including PPP, red blood cells, and PRP, which have distinct density gradients.7 Administering PRP to intracavernous tissue may promote new vascular formations as well as neural regeneration, with the hope of increasing erectile functions permanently since the PRP is full of growth factors.8 Administration of PRP via intracavernous injection has demonstrated enhanced erectile function in animal models afflicted with neurogenic erectile dysfunction.5 The objective of this investigation is to evaluate the potency and precaution of a variety of PRP administration protocols in the treatment of ED.
The registration number for this study protocol in PROSPERO is CRD42024560467.
A systematic search was conducted on three separate databases—Science Directs, Scielo, and PubMed. A targeted inquiry was conducted utilizing particular keywords, such as “Platelet Rich Plasma” in conjunction with “Erectile Dysfunction”. All datasets displayed consistency with the identified keywords. This review only included academic research articles that were published within the past decade. In this review, articles had to meet particular criteria to be deemed eligible for inclusion. They needed to be written in English, focus on PRP therapy for EDs, be randomized controlled trials (RCTs), and be original studies with freely available full-text content. Independently, the two investigators (V and IAD) had examined the titles and abstracts for the preliminary screening. Then, abstracts of potentially relevant articles or those lacking sufficient information to warrant a conclusion were examined in their entirety.
For all statistical analyses, the application Review Manager (RevMan) version 5.3 was utilized. For continuous variables, WMD and the associated 95% CI were used in this meta-analysis. The diversity of the selected studies was analyzed utilizing tools such as Cochran’s Q chi-square test and I2 statistics. A fixed-effects model was implemented when there was minimal variation (p≥0.05 and I2≤50%). Applications of a random-effects model were implemented when there was substantial heterogeneity (p<0.05 or I2>50%). A p-value of less than 0.05 was used to determine statistical significance for all test data. The potential publication biases were evaluated using forest plots.
From the aforementioned three databases, this search strategy produced 74 results. Each abstract was examined by the author in order to identify articles. Consequently, 71 articles were excluded from the evaluation process, leaving three articles for further examination within the context of this review. The method of doing a literature search is depicted in Figure 1, which is a flow diagram.
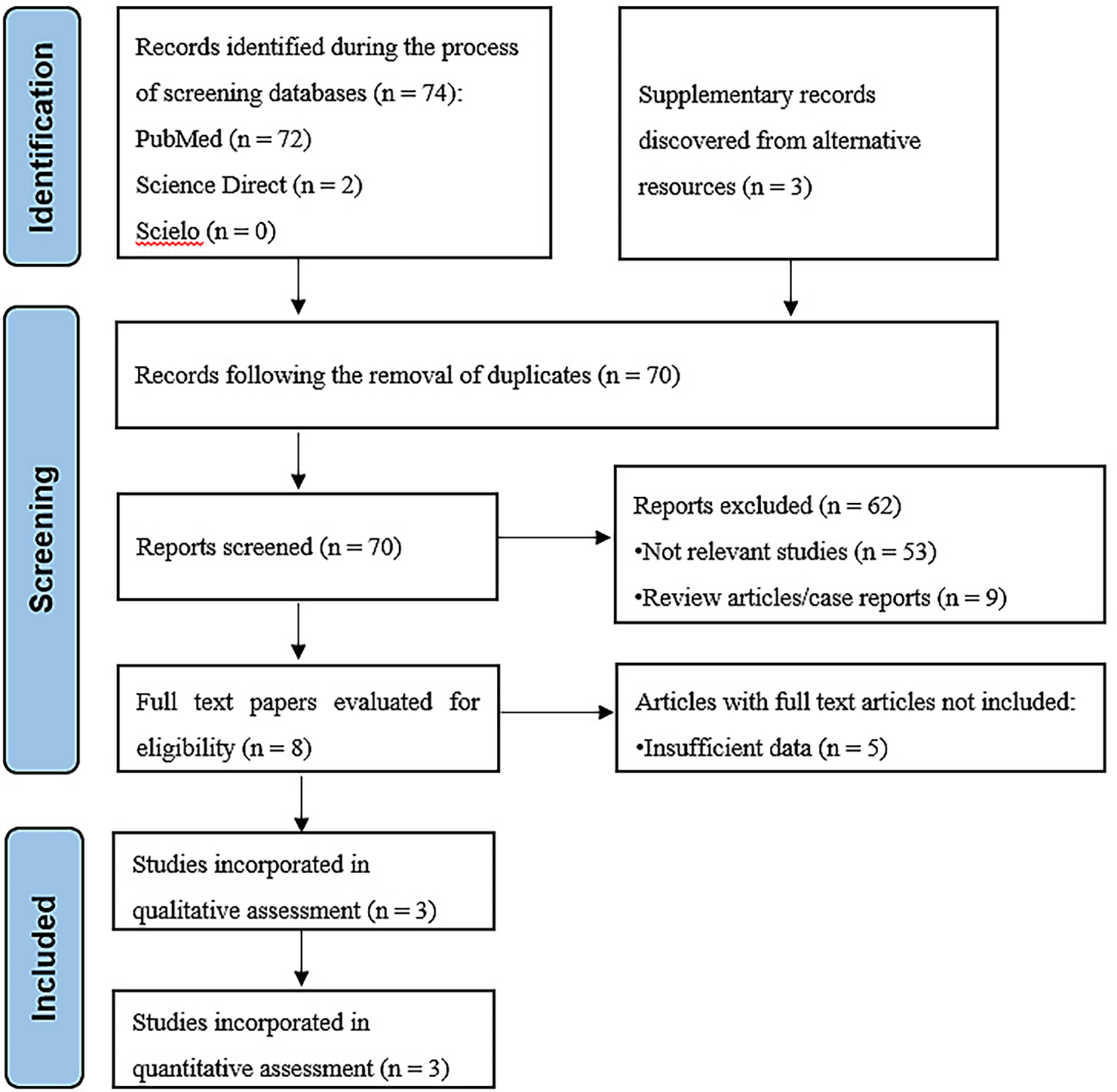
Figshare URL: https://figshare.com/articles/figure/PRISMA_study_flow_diagram_png/26046988.
The quality of the randomized controlled trial studies was evaluated with the help of the Cochrane Risk of Bias assessment tool in this study. The overall assessment of a randomized controlled trial study was determined using an algorithm, which classed the studies as having low, moderate, or high risk of bias (Figure 2a).
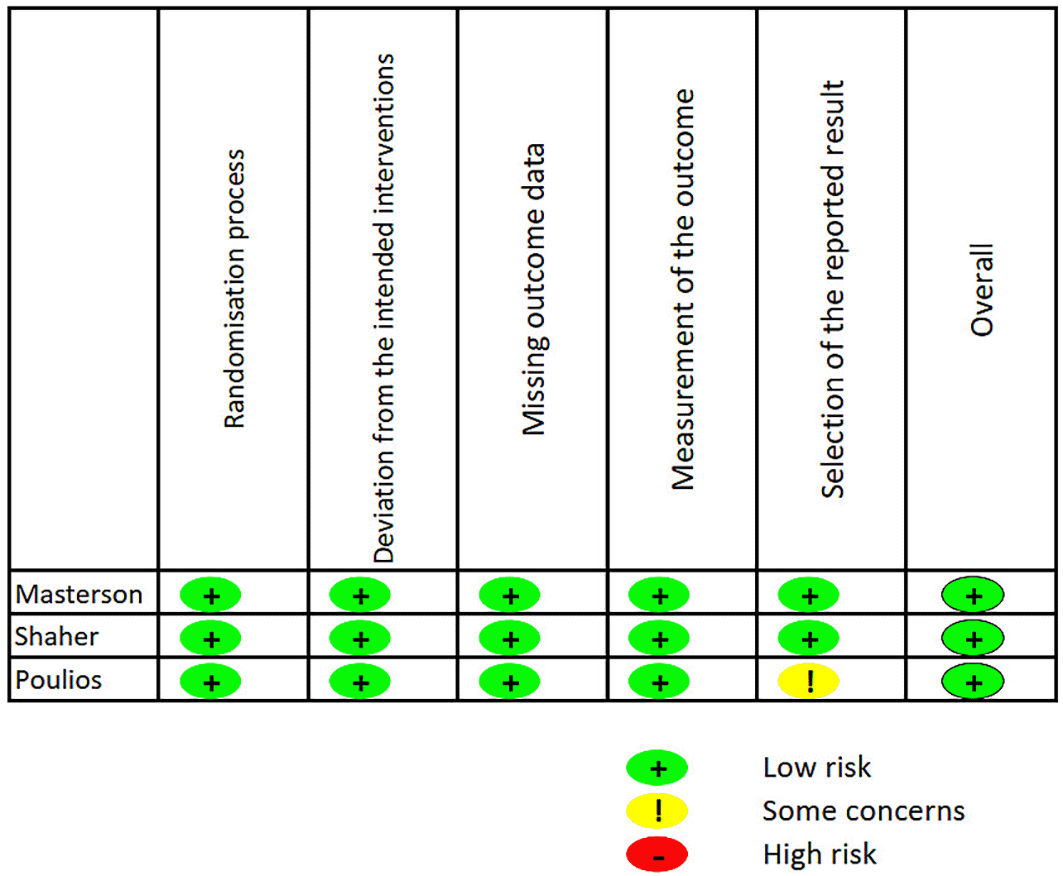
Risk of Bias URL: https://figshare.com/articles/figure/Risk_of_Bias/26047030.
Our search approach identified three journals that satisfy the specified criteria for inclusion and exclusion from three search engines. Out of the 8 studies that were included, the paper originated from 3 distinct countries, including the USA, Greece, and Egypt. Upon doing the risk of bias evaluation, it was determined that none of the studies exhibited a significant risk of bias. The review exclusively enrolled male participants aged 30 to 75 years who had received a diagnosis of erectile dysfunction.
There are varying protocols used in this paper. Masterson’s protocol of PRP administration initiates with the extraction of 120 mL of autologous blood from the brachial vein via phlebotomy. Following this, the blood undergoes processing using the Arthrex Angel PRP system in a separate room, resulting in the extraction of approximately 5 mL of PRP from the 120 mL of whole blood. The PRP is subsequently divided into two 5-mL syringes, each containing 2.5 mL of PRP, and infused into both corpus cavernosum. Poulios et al, on the other hand, employs a 60-mL syringe to extract blood, which is then processed with a Magellan Autologous Platelet Separator to produce approximately 10 mL of PRP. The syringe contains 8 mL of anticoagulant. Subsequently, 5 mL of platelet-rich plasma (PRP) is injected slowly into each corpus cavernosum over 2 minutes to avoid harming platelet cells. The needle is carefully removed to improve the spread of PRP within the erectile tissue.10 Shaher’s method commences with the withdrawal of 30 mL of the patient’s blood, which is subsequently transferred to tubes that contain an anticoagulant citrate-dextrose solution. The plasma is subsequently isolated by centrifuging the cylinders at a speed of 2500 RPM for 5 minutes. The separated plasma is thereafter transferred into a separate tube. The gathered plasma is further centrifuged for 10 minutes at a speed of 3500 RPM. The resulting plasma, which has been separated, amounts to a total of 6 mL and is intended for injection. Administer 3 milliliters of platelet-rich plasma (PRP) into each corpora cavernosa using an insulin syringe. Administer the injections at three precise locations: 1 centimeter towards the corona, 1 centimeter away from the lower portion of the penis, and at the middle of the shaft’s midpoint.11
As listed in the Table 1, among the research conducted,10–12 two of them10,11 found no negative effects resulting from the injection of PRP. These effects include discomfort, hematoma, bruising, ecchymosis, fibrous plaques, penile abnormalities, or symptoms of infection. A single study12 documented the presence of new plaque in only one patient from the PRP group, while one patient from the placebo group experienced a hematoma.
| Study | Country | Sample Size (n) | Protocol | Brand of Plasma Separator | Complication or Side Effect | Age (years) | Outcome | |
|---|---|---|---|---|---|---|---|---|
| PRP | Placebo | |||||||
| Masterson, 202312 | USA | 61 | The Platelet-Rich Plasma (PRP) was divided equally into two 5-mL syringes, with 2.5 mL of PRP in each syringe. | A total volume of 5 mL was injected, with approximately 2.5 mL administered into each of the right and left corpus cavernosum using normal saline. | An optical sensor in the automated PRP system Arthrex Angel helps to distinguish the platelet-rich layer. | A new plaque was developing in one participant of the PRP group, while one individual in the placebo group experienced a hematoma. | 30-75 | IIEF-EF |
| Poulios, 202110 | Greece | 60 | 10 mL PRP injections (5 mL in each corpus). | 10 mL normal saline. | Magellan Autologous Platelet Separator. | No complications or side effects reported. | 40-70 | IIEF-EF |
| Shaher, 202311 | Egypt | 100 | The administration consisted of three injections, with each injection containing 3 mL of the substance. The time period between each injection was 15 days. | 3 mL saline in the same injection site as PRP. | Not mentioned (5 minutes at 2500 RPM ➔ 10 minutes at 3500 RPM). | No complications or side effects reported. | 45-65 | IIEF-EF |
Our comprehensive examination of three randomized controlled trials (RCTs) (Figure 2b) has shown that administering platelet-rich plasma (PRP) directly into the penis leads to a substantial enhancement in the IIEF-EF score among patients suffering from erectile dysfunction. The improvement is observed at one month (mean difference [MD] 2.57 [95% confidence interval (CI) 1.02-4.13], p=0.001, I2=54%, random-effect models), three months (MD 2.47 [95% CI 0.81-4.12], p=0.003, I2=53%, random-effect models), and six months (MD 3.01 [95% CI 2.06-3.96], p<0.00001, I2=0%, fixed-effect models) after the treatment, compared to the baseline.
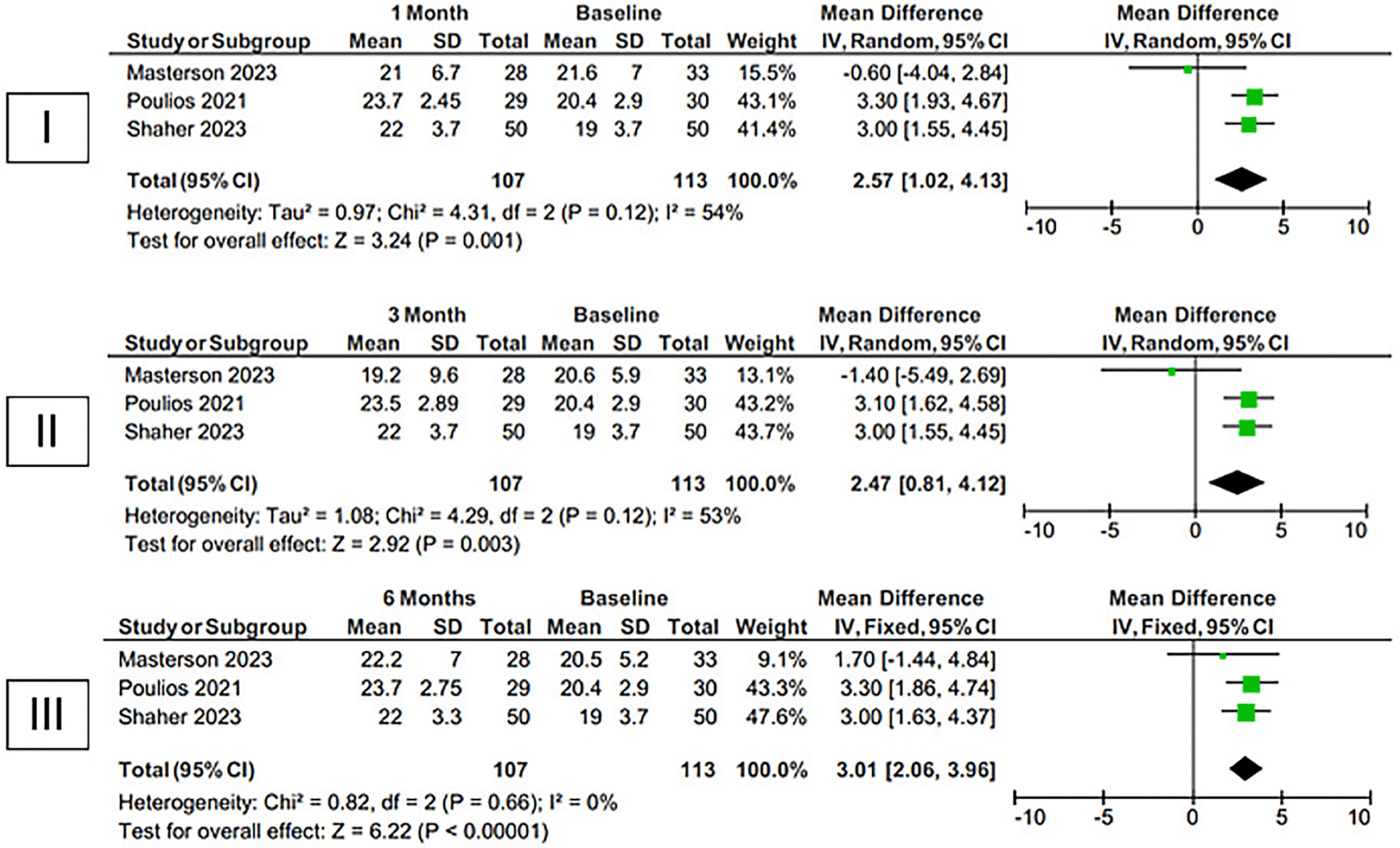
The analysis focuses at the one-month (I), three-month (II), and six-month (III) intervals compared to the initial measurement. PRP vs Baseline URL: https://figshare.com/articles/figure/Forest_Plot_of_PRP_vs_Baseline/26047048.
The group of PRP were at a higher percentage than those in the placebo group who achieved the MCID in the IIEF-EF, according to our meta-analysis, which included three randomized controlled trials at both one month (OR 5.52 [95% CI 1.20–25.42], p = 0.03, I2 = 83%, random-effect models) and six months (OR 5.64 [95% CI 2.05–15.55], p = 0.0008, I2 = 60%, random-effect models) (Figure 3).
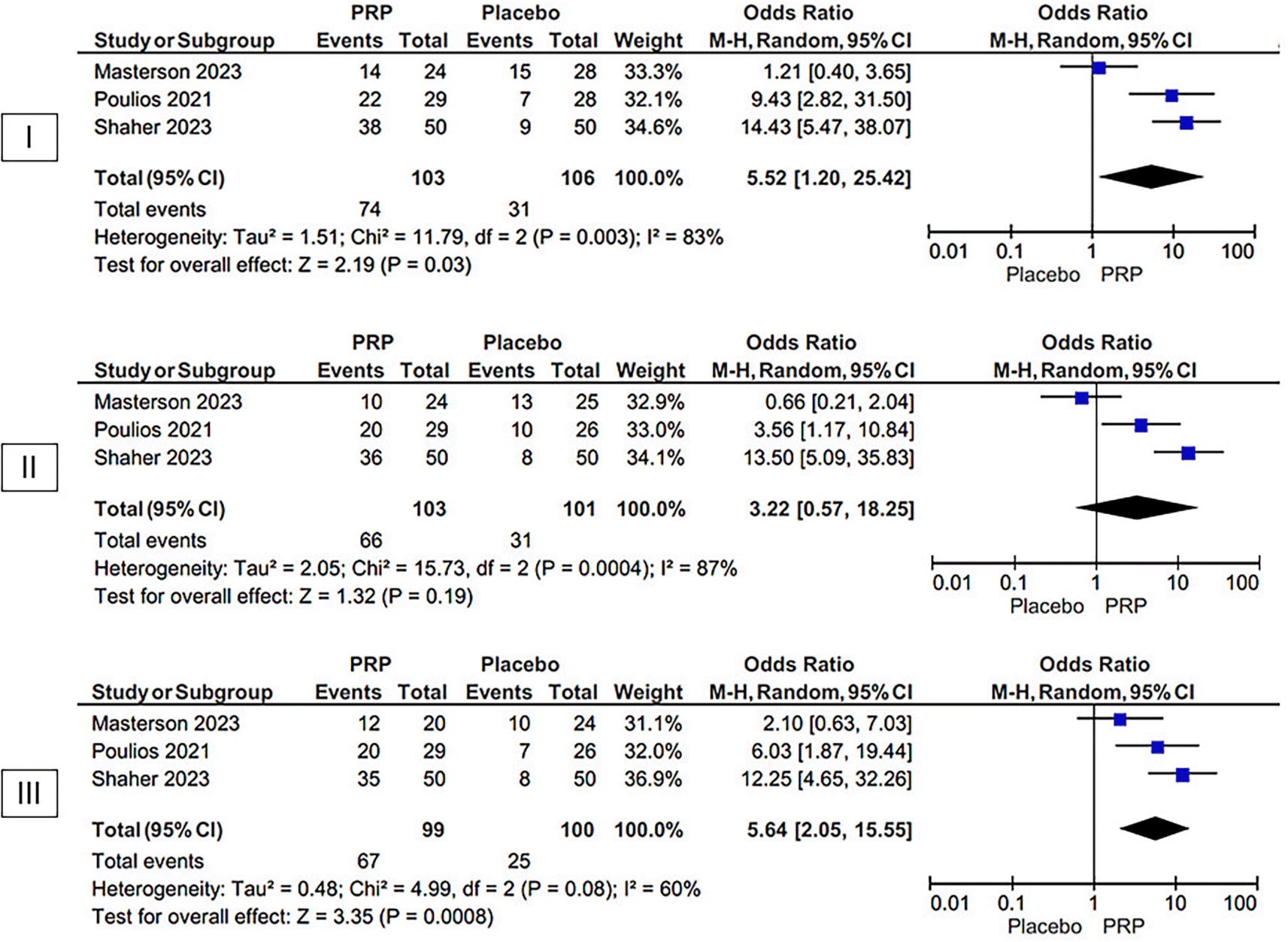
MCID URL: https://figshare.com/articles/figure/MCID/26047069.
The findings of our meta-analysis, as shown in Figure 4, demonstrate that the intracavernosal injection of PRP leads to a superior enhancement in the IIEF-EF score, compared to a placebo, in patients suffering from ED. This conclusion is based on the inclusion of three randomized controlled trials (RCTs) in our analysis. The mean difference in the IIEF-EF score between PRP and placebo was 2.53 [95% CI 1.27-3.79] at 1 month, with a p-value<0.0001 and an I2 value of 0% using fixed-effect models. At 3 months, the MD was 2.30 [95% CI 0.84-3.75], with a p-value=0.002 and an I2 value of 44% using fixed-effect models. At 6 months, the MD was 3.15 [95% CI 1.79-4.52], with a p-value<0.00001 and an I2 value of 0% using random-effect models.
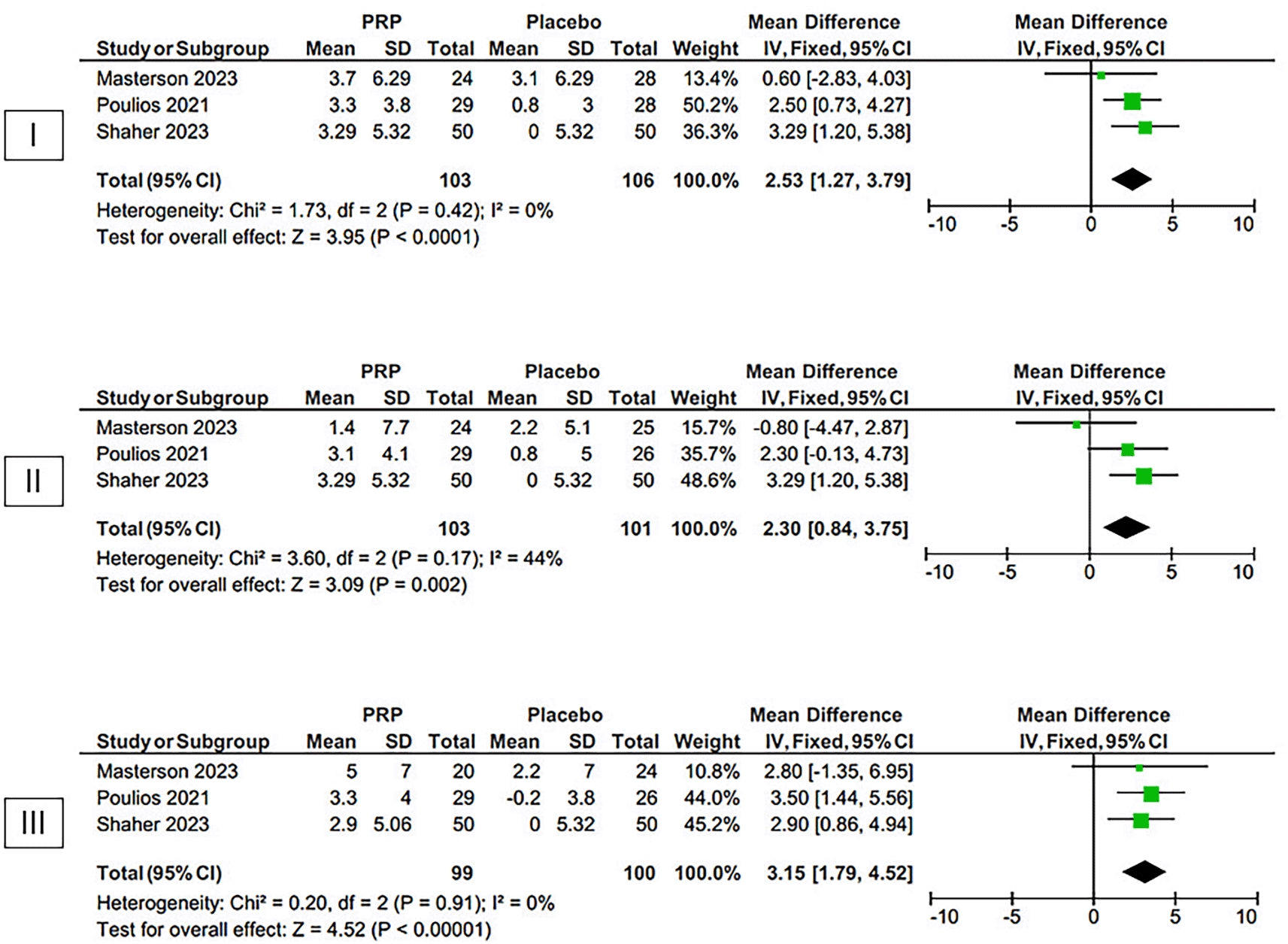
The plot focuses on the changes of the IIEF-EF score after one month (I), three months (II), and six months (III).
Potency of PRP URL: https://figshare.com/articles/figure/PRP_vs_Placebo/26047084.
Erectile dysfunction, abbreviated as ED, refers to the incapacity to achieve and maintain a sufficiently strong erection for sexual activity. PRP is a new treatment for ED, along with stem cells. The underlying foundation for this notion is that PRP may improve crucial aspects of the pathophysiological processes that contribute to ED by exerting vasculogenic, neuroprotective, neurotrophic, reparative, and anti-inflammatory effects.6 However, PRP might be seen as a more affordable option compared to stem cell therapy.
Our comprehensive research and statistical synthesis showed the IIEF-EF test was much better after intervention compared to the baseline scores. The IIEF-EF scores after the intervention always showed much better results than the placebo. Our research showed that the PRP injection into the cavernosum was safe. There were no reports of serious or significant side effects. Additionally, Geyik et al.13 reported several side effects in previous studies, including pain experienced at the site of PRP injection. Tas et al.14 observed the presence of bruises and fibrotic plaque but noted that these did not result in curvature. The research revealed that from 1 to 6 months post-intervention, there were higher occurrences of MICDs in the group of PRP as compared to the group of placebo. Following a duration of 3 months, there was no significant disparity. The potential effect of integrating research participants who were lost during the follow-up period in Masterson et al.’s study12 on the outcome should not be overlooked. The protocols examined in this analysis included the amount of PRP injected, the type of PRP separator utilized, the frequency of therapy, and the duration of treatment. All three studies were done with subjects ranging in age from 30 to 75 years old with erectile dysfunction without prior urology surgery. Each paper compares the outcomes of a PRP injection with a placebo, where the PRP injections administered varied from 2.5 mL to 10 mL.
A study conducted on human such as Geyik et al.13 outlined the procedure for preparing and administering PRP. The methodology involved collecting 30 ml of whole blood and placing it in two self-gelled and citrated PRP sets (Ycellbio PRP, Ycellbio Medical Co. Ltd.). The specimen underwent centrifugation for a duration of 10 minutes at the acceleration due to gravity (3,700 ×g). Two kits generated 12–16 milliliters of injectable PRP. The injections were administered using minuscule needles within a timeframe of 3 to 5 minutes after the preparation of PRP. As part of a single treatment plan, three PRP shots were given, with 10 to 14 days between each one. Three places were chosen to inject 3–4 ml of PRP: one in the intraavernosal space, one under the skin (in the right and left lateral neural lines), and one in the dorsal balanic submucosal space.13 Protocol used by Zaghloul et al.15 were the patient received three injections of RegenKit Platelets (RegenLab®) containing PRP between 7 and 9 mL beginning, 4 weeks, and 8 weeks into their treatment. Autologous PRP was generated using PRP Regen Blood Cell Therapy® (BCT) tubes, which entailed processing 24 ml of venous blood with sodium citrate serving as an anticoagulant. Through the elimination of the top 2 ml of each tube, a cumulative amount of 9 ml of PRP was obtained, featuring a concentration of platelet five times greater than that found in whole blood. The penis was injected intracavernosally at four specific sites, with two injections on each side. A 5/16-inch 28G needle was used to give the shots in the proximal third and middle third of the penis.15 Francomano et al.16 stated the protocol used were by centrifuging 6 vials of autologous venous blood (20 ml) at 1500 rpm for 15 minutes, following the manufacturer’s instructions (regenlaB®, Budron b2, 1052 Mont-sur-lausanne, Switzerland). For administering the PRP, a 10 ml syringe equipped with a 25-gauge needle was employed to inject 5 ml of PRP into both corpora cavernosa, approximately 1 cm apart from the midpoint of the penile shaft.16
According to the protocol of Matz et al.,17 There were two containers that were filled with nine milliliters of whole blood. After being centrifuged at 6,000 revolutions per minute for six minutes, the samples were taken out. Subsequently, they were isolated from the blood sample. By adding a 10% calcium chloride solution to PRP in a 1:10 ratio, the fibrinogen in the PRP was converted into fibrin. Patients usually receive either one or two vials of injectable PRP, with each vial containing around 5.5 mL. The volume of PRP injections administered per session varied between 4 and 9 mL.17 Study by Khodamoradi et al.18 used protocol such as phlebotomy to collect 60 mL of whole blood from participants, which was spun into PRP using the Arthrex Angel. The Arthrex Angel equipment is an automated machine that produces consistent PRP concentrations. This technique uses flow cytometry, which measures cell light absorption. The equipment was utilized in the investigation done by Masterson et al.12 and Saltzman et al. Saltzman et al.19 stated each person’s 120 ml of peripheral blood is used to make autologous PRP. A blood processing device called the Arthrex Angel, manufactured by Arthrex in Naples, FL, USA, utilizes an optical sensor to separate platelets from blood samples. In each session, a combined total of 5 ml of PRP is administered through injection into both corpus cavernosum. 2.5 ml is put into each side.19 Schirman et al.,20 Every patient was administered a 2 mL solution of sodium citrate with a concentration of 3.13%, which was then combined with 23 mL of recently collected blood. In accordance with the kit instructions, Transferring the 25 mL mixture to the hourglass, it was centrifuged at 3000 rotations per minute for 3 minutes. Each corpus cavernosum received three milliliters of PRP, with an additional six milliliters administered to the patient subcutaneously. Every session, 12 mL of PRP were given to each subject. Three shots, 15 days apart were given to all patients. To prepare and administer PRP by Tas et al.,14 1.5 ml of citrate dextrose solution was combined with the participant’s blood in a tube in order to prevent the blood from clotting. Centrifugation was done on the tubes at a speed of 2,800 turns per minute for 8 minutes. After being segregated from red blood cells for twenty minutes, the layer of plasma was spun in a centrifuge at a rate of 3,500 rotations per minute. Each corpora cavernosa received 3 mL PRP injections using a 25 gauge needle. Patients were administered intravenous autologous PRP on three occasions, with a 15-day gap between each administration. Mid-penile injection locations vary by 1 cm.14 These varying protocols prove that the PRP currently doesn’t have any consensus or standardization, There is an expectation that in the future, a protocol with defined recommendations will be developed to facilitate the administration of the PRP.
There were various constraints in this investigation. The included studies were limited to Egypt, Greece, and the United States, which may compromise their representativeness of the worldwide population. Furthermore, the study sample size was limited, consisting of only three randomized controlled trials (RCTs). Furthermore, the construction of a funnel plot was not possible due to the constraints imposed by the investigations. Larger, more carefully planned RCTs should be the main focus of future research to confirm these findings and give more accurate ratings of how well and safely PRP works to treat ED.
All data underlying the results are available as part of the article and no additional source data are required.
Reporting guidelines
1. Figshare: The potency and safety of platelet-rich plasma and its therapy protocol for erectile dysfunction: A systematic review and meta analysis of randomized controlled trials. 2024.
This project contains the following underlying data:
• PRISMA Study Flow Diagram, https://figshare.com/articles/figure/PRISMA_study_flow_diagram_png/26046988. 21
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
2. Figshare: The potency and safety of platelet-rich plasma and its therapy protocol for erectile dysfunction: A systematic review and meta analysis of randomized controlled trials. 2024.
This project contains the following underlying data:
• PRISMA 2020 CHECKLIST, https://figshare.com/articles/figure/PRISMA_2020_CHECKLIST/26090929. 22
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
3. Figshare: The potency and safety of platelet-rich plasma and its therapy protocol for erectile dysfunction: A systematic review and meta analysis of randomized controlled trials. 2024.
This project contains the following underlying data:
• PRISMA 2020 Checklist for Abstract, https://figshare.com/articles/figure/PRISMA_2020_Checklist_for_Abstract/26092147. 23
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)