Keywords
melasma, Cassipourea species, Network pharmacology, Molecular dynamics simulation, Signalling pathways
Melasma is a common dyschromia, mainly found in women with darker skin types. Although asymptomatic, melasma significantly impacts patients’ quality of life. Due to this complex pathogenesis, melasma is difficult to treat. Plant and plant-derived products have been explored as alternatives for the treatment of melasma.
This study utilized network pharmacology coupled with molecular docking and molecular dynamics simulations to investigate the molecular mechanisms of three selected Cassipourea metabolites in the treatment of melasma.
Of the 202 genes obtained from the 14 profiled metabolites, only PTGS2, TYR, ESR2, and ESR1 were common among metabolites and targets implicated in melasma. From this, The gene ontology highlighted the intracellular steroid hormone receptor, signalling pathway, macromolecular complex, and estrogen receptor activity as the top enriched functional annotations, while the KEGG pathway analysis identified five signalling pathways, from which the prolactin signalling pathway, endocrine resistance, and estrogen signalling pathway were implicated in the pathogenesis of melasma. These pathways were further connected by their linkage to ESR2 and ESR1., Of all Cassipourea metabolites and standards, with afzelechin having the highest docking score for both gens. Further binding interaction analysis showed that ESR2-bound tamoxifen had the highest binding free energy of -47.68 kcal/mol, however, among the interacting Cassipourea metabolites, sitosterol-glycoside exhibited the highest negative binding affinity for both ESR2 (-40.50 kcal/mol) and ESR1 (-78.97 kcal/mol) over 150 ns simulation, suggesting its potential as a dual modulator. Altogether, the metabolites presented remarkable binding stability and thermodynamic compactness with the apo-genes.
The finding that the selected Cassipourea metabolites are associated with the genes and enzymes implicated in melasma pathogenesis, together with their significant binding effects on the enriched genes, suggests their regulatory potential on the profiled targets and, consequently, in the treatment of melasma.
melasma, Cassipourea species, Network pharmacology, Molecular dynamics simulation, Signalling pathways
The skin is the largest organ in the human body. It forms a significant anatomical barrier between internal and external environments.1 The body is continuously exposed to various physical and chemical exogenous polluting substances.2 Ultraviolet radiation (UVR) from excessive sun exposure is the primary exogenous factor that harms the skin. This process has various harmful effects on the skin as it alters the composition of the skin, causing elastic fiber accumulation, collagen reduction, and degeneration leading to wrinkles, sagging, and glycosaminoglycan deposition, resulting in premature aging, known as photoaging.3 Moreover, overexposure to ultraviolet (UV) rays stimulates melanin synthesis owing to the rapid proliferation of melanocytes. Furthermore; to stimulated melanin synthesis, excessive exposure to sunlight, especially UVA and UVB, causes overexpression of reactive oxygen species (ROS) that harm lipids, proteins, and deoxyribonucleic acids.4
Melanin is produced in the epidermis of the skin via a pathway known as melanogenesis, with tyrosinase playing a key role as the rate-limiting enzyme.5 The enzyme catalyzes three steps in melanin biosynthesis: hydroxylation of tyrosine to 3,4-dihydroxyphenylalanine (DOPA), oxidation of DOPA to DOPA quinone, and conversion of 5,6-dihydroxyindole to indolequinone.3,6 Thus, tyrosinase is the primary target for determining skin-lightening agents for cosmetic applications or skin lightening.5
While melanin shields the skin from UV radiation, excessive production can result in dermatological hyperpigmentation of the skin in clinical conditions such as dark spots, freckles, melasma, solar lentigo, linea nigra, and post-inflammatory hyperpigmentation (PIH), which can affect the appearance of the skin.7 Melasma is a common dermatological condition characterized by hyperpigmentation (light brown or dark brown), flaky or reticular patches, and macules that appear on the facial skin, and much less often on the neck and forearms. This condition primarily affects adult females, especially those with darker skin phototypes (Fitzpatrick skin phototypes III-VI).8,9 Although common to females, men are also affected; the incidence of melasma is estimated at 1% worldwide; however, it varies between 8.8-50% of at-risk populations.10,11 The pathogenesis of melasma is complex, as it is linked to melanocytes, keratin-forming cells, endothelial cells, fibroblasts, and alterations in the basement membrane.12,13 Visible light and UV exposure, hormonal changes, and genetic predisposition have all been linked to the onset of melasma.12–14 Melasma can also be caused by various factors such as the use of photosensitizing medication, thyroid diseases, ovarian tumors, hepatopathies, parasitic infestation, certain foods, and stress.9 Thyroid dysfunction, menstrual cycle irregularities, and insulin resistance which may also be caused by hormonal imbalance, have been linked to the diagnosis of melasma.9,14
Although not life-threatening, being a facial disorder, melasma is disfiguring, and as such, impacts patients’ lives and psychological well-being, potentially leading to anxiety, depression, and other disorders.12–15 Owing to this complex pathogenesis, melasma is difficult to treat. Currently, there is no cure or standardized treatment for melasma. Hydroquinone is a popular skin-whitening agent that inhibits tyrosinase. However, it is not suitable for all skin types, and prolonged and unsupervised use can cause undesirable side effects such as dermatitis, edema, allergic reactions, and ochronosis.15,16 Common treatments include sun protection, topical creams, such as niacinamide, vitamins A and C, oral medications, chemical peels, laser and light therapy, tranexamic acid, and microneedling. However, these treatments are prone to chemical irritation, inflammatory reactions, and hyperpigmentation. Other disadvantages include the possibility of recurrence and unpredictable efficacy.12–15
Patients often prefer the use of complementary and alternative medicines to supplement or replace traditional treatments, licorice extract, arbutin, and kojic acid are among the many tyrosinase inhibitors used in skin-lightening treatments.6,15 Additionally, tyrosinase inhibitors from phyto-molecules are equally valuable for scavenging ROS, which cause skin damage from excessive sun exposure.5 Since ancient times, medicinal plants have been used as therapeutic agents, dating back to 4000-5000 BC.17 The biological activities of plants are unique to specific species or groups, which supports the idea that a plant’s secondary metabolites are taxonomically distinct.18 Screening of such secondary metabolites as active compounds from plants has led to the invention of a drug discovery process in pharmaceutical science for isolating new natural drugs with efficient protection and treatment roles against various diseases.18
Studies have explored natural products that inhibit UV-induced ROS, suppress enzymes, and reduce melanin formation as potential alternatives to current treatments have been conducted. This strategic shift is due to the adverse effects of synthetic agents.19 Phytocompounds such as aloesin, arbutin, licorice, hesperidin, gentisic acid, flavonoids, niacinamide, polyphenols, and yeast derivatives have demonstrated great potential to inhibit melanogenesis without harming melanocytes.4,18 Topical botanicals are becoming increasingly popular for skin care because of their perceived safety, low side effects, formulation stability, efficacy, cost-effectiveness, and quick metabolism when applied to the skin compared with conventional treatments.12,17,20 According to ethnobotanical literature, topical application is the most commonly used mode of application because it guarantees direct and immediate interaction of specific botanical compounds with the site of action.21–23
Recently, three Cassipourea species (Cassipourea flanaganii, Cassipourea malosana, and Cassipourea gummiflua Tul. Verticillata) have been reported to be used for skin lightening by women in rural areas in the Eastern Cape and Kwa Zulu-Natal provinces of South Africa.20 Although common names of the species are often used interchangeably by the rural community, the phytochemical comparison showed that each species is distinct, but they share skin-lightening characteristics. All three plants have been demonstrated to be effective and safe for use as topical skin lighteners, with no side effects.17,22–24
In this context, the biological activities of the three Cassipourea species were systematically analyzed for their potential molecular mechanisms in the treatment of melasma using network pharmacology. Network pharmacology is an emerging science that examines the “compound-protein/gene-disease” system, thus, it is an effective measure for describing the intricacy of biological systems, drugs, and diseases from a network-based context.19,25 However, to gain further insight into the binding interaction and stability of the metabolites and hub targets, molecular docking and dynamics simulations were conducted.
Active compounds from the three Cassipourea species screened through Chromatography-Mass Spectrometry (LC-MS/MS) analysis in the negative mode were used to generate a library of compounds for Cassipourea. The LCMS/MS analysis detected twenty-four compounds from various chemical classes, including fatty acids, steroids, di- and triterpenoids, flavonoids, and phenolic acids (https://doi.org/10.6084/m9.figshare.26418361.v1) (accessed on 01 August 2024). Eighteen compounds were tentatively identified.20 The compounds were identified based on their structure and molecular mass, which shared similarities with known substances (https://doi.org/10.6084/m9.figshare.26418361.v1) (accessed on 01 August 2024). This was validated further with previous reports, characteristic fragmentation patterns, and data from a large bank and the SciFinder database.
The metabolites of the Cassipourea species were evaluated using Lipinski’s rule of five (Ro5) for drug-likeness properties of the metabolites.26 The SwissADME server (http://www.swissadme.ch/; accessed April 01, 2024) was used to predict the absorption, distribution, metabolism, and excretion properties of metabolites.27
The metabolite target genes were mined from two independent databases. Genes related to Cassipourea metabolites, whose SMILES were available on PubChem (Afzelechin, azelaic acid, cassipourol, chlorogenic acid, chrysin 8-C glucoside, decahydroretinol, emodin 6,8 dimethyl ether, hexose, isorhamnetin-3-O-rhamnoside, lupeol, lyoniside, methyl linoleate, sitosterol-glycoside and tricin) were identified from the Swiss Target Prediction (STP) database (http://www.swisstargetprediction.ch/) (accessed April 01, 2024) using the Simplified Molecular Input Line Entry System (SMILES) retrieved from PubChem (https://pubchem.ncbi.nlm.nih.gov/) (accessed April 01, 2024). Genes related to melasma were acquired from the Online Mendelian Inheritance in Man (OMIM) database (https://www.omim.org/) (accessed on April 01, 2024) and the GeneCards database (https://www.genecards.org/) (accessed April 01, 2024). The Venny 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/) tool was used to identify and characterize intersecting target genes28 between Cassipourea metabolites and melasma.29
The Search Tool for the Retrieval of Interacting Genes (STRING) database (https://string-db.org/) (accessed April 01, 2024) was used to construct a PPI network.30 The parameters for the analysis were set to Homo sapiens with a confidence level of < 0.4, followed by the input of common target genes between metabolites and melasma. The PPI network was then classified using Cytoscape v3.8.2.28 A degree algorithm was used to identify key genes in the network (Equation 1).31
To illustrate the roles of the identified common targets in biological processes (BP), cellular components (CC), and molecular function (MF), gene ontology was conducted using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) tool (https://david.ncifcrf.gov/tools.jsp),32 with search parameters fixed to Homo sapiens. The GO and KEGG enrichment pathway analysis plots were generated using SRPlot (http://www.bioinformatics.com.cn/en), and from the KEGG pathway enrichment, the most significant pathway was selected based on the lowest false discovery rate (FDR).33,34
The PCT comprising melasma-related signaling pathways, their interacting genes, and metabolites were constructed using the Cytomerger plugin in Cytoscape software v3.9.1.35 Thereafter, network topology analysis was conducted with edges depicting node interactions and their degrees of significance.35
Crystal X-ray structures of the target genes ESR2 [PDB: 2GIU] and ESR1 [PDB: 1QKU] were downloaded from the RCSB Protein Data Bank (PDB) (https://www.rcsb.org/) (accessed on April 01, 2024) and optimized by the elimination of all non-standard residues, co-crystallized ligands, and water molecules. Thereafter, ESR2 and ESR1 interacting metabolites and reference standards for melasma and the gene agonists tranexamic acid and tamoxifen, respectively, were developed. 3D conformers (ligands) were obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) (accessed April 01, 2024). Ligands were optimized using the Open Babel program plug-in on Python Prescription v 0.9.5 (PyRx)36 through the addition of Gasteiger charges. Binding at the gene active site was confirmed by the identification and selection of ESR2 and ESR1 active site amino acid residues. Docking studies were conducted on PyRx, from which the grid box coordinates, in correlation with the x-y-z coordinates, were ascertained using the BIOVIA Discovery Studio v21.1.0.37 Following molecular docking, the top five ligands with the highest negative docking score relative to their reference standards were visualized using Discovery Studio for their docking interactions before the simulation process. To avoid pseudo-positive binding conformations, the docking protocol was validated by measuring the root mean square deviation (RMSD) of docked ligands from the reference pocket containing native ligands (estradiol) in the experimental co-crystal Where an RMSD of 0.5 Å was obtained for both docking validations (Figure 1).38
MD simulations were conducted at 150 ns using the graphical processing unit (GPU) version of the AMBER 18 package (force field with FF18SB variant) of the Center for High Performance Computing (CHPC), Cape Town, South Africa.39 Ligand atomic partial charges were harnessed through ANTECHAMBER using a general amber force field (GAFF) and restrained electrostatic potential (RESP). The systems were neutralized using hydrogen atoms and Na+ and Cl− counter ions from the Leap module. The amino acid residues of ESR2 and ESR1 were subsequently numbered and encased in an orthorhombic box of TIP3P water molecules, such that all atoms were at most 8 Å away from any box edge. The SHAKE algorithm was used to constrain hydrogen atom bonds within simulated systems, with each simulation comprising a 2 fs step-size, concurrent with the isobaric-isothermal ensemble (NPT) randomized seeding, a Langevin thermostat with a collision frequency of 1.0 ps, 1 bar, 2 ps pressure-coupling constant, and 300 K temperature. Thereafter, along with the binding free energy, the post-dynamic parameters, namely the root mean square deviation (RMSD), root mean square fluctuation (RMSF), radius of gyration (RoG), and solvent-accessible surface area of each protein-ligand system were assessed.40
A total number of 18 common metabolites were identified from the previously characterized Cassipourea species. They are comprised of different chemical classes, including fatty acids, steroids, di- and tri-terpenoids, flavonoids, and phenolic acids (Table 1).
Of the 14 common metabolites of Cassipourea species whose SMILES were available in PubChem, only lyoniside had three violations of Lipinski’s Ro5 (molecular weight ≤ 500 g/mol, hydrogen bond donor and acceptor ≤ 5 and 10, respectively, bioavailability score ≤ 0.55, and lipophilicity (MLOGP) ≤ 4.5), as it had a molecular weight of 552.57 g/mol, hydrogen bond acceptor and donor of 12 and 6, respectively; other metabolites had ≤ 2 violations (Table 2). Pharmacokinetic analysis revealed that, except for sitosterol-glycosides, the metabolites were soluble in water to varying degrees. The metabolites also possess relatively high gastrointestinal absorption, are mostly non-substrate for glycoproteins, and are impermeable to the blood-brain barrier (BBB). However, azelaic acid, decahydroretinol, and emodin 6,8 dimethyl showed BBB permeability (Table 2). The metabolites demonstrated significant non-inhibition of the cytochrome P (cytochrome P450) isoenzymes (Table 2).
In total, 644 and 545 Cassipourea metabolite genes were collected from the STP and SEA databases, respectively. A total of 202 genes were common in both databases. Of the 34 melasma genes acquired from the GeneCards database and 24282 genes acquired from OMIM, only 31 genes were common to both. A further probe of the gene interactions identified four genes directly linked to Cassipourea and melasma (Figure 2).
The PPI network constructed from common genes from melasma and Cassipourea metabolites comprised 4 nodes and 12 edges, an average node degree of 2, and an enrichment p-value of 0.0107 (Figure 3). The identified estrogen receptors 1 and 2 (ESR1 and 2), prostaglandin-endoperoxide synthase 2 (PTGS2), and tyrosinase (TYR) genes interact with each other.
Gene ontology analysis indicated the potential effect of Cassipourea metabolites on BP, CC, and MF relative to melasma. Amongst the BP, the intracellular steroid hormone receptor signalling pathway (2.0 × 10−3) was identified as the most enriched, followed by response to vitamin D (2.9 × 10−3) and cellular response to estrogen stimulus (3.4 × 10−3). Of the CCs, only the macromolecular complex (9.8 × 10−2) was marginally enriched. Estrogen receptor activity (6.3 × 10−4) was both the most enriched MF within the GO, followed closely by enzyme binding (1.2 × 10−3) and estrogen response element binding (1.7 × 10−3) (Figure 4).
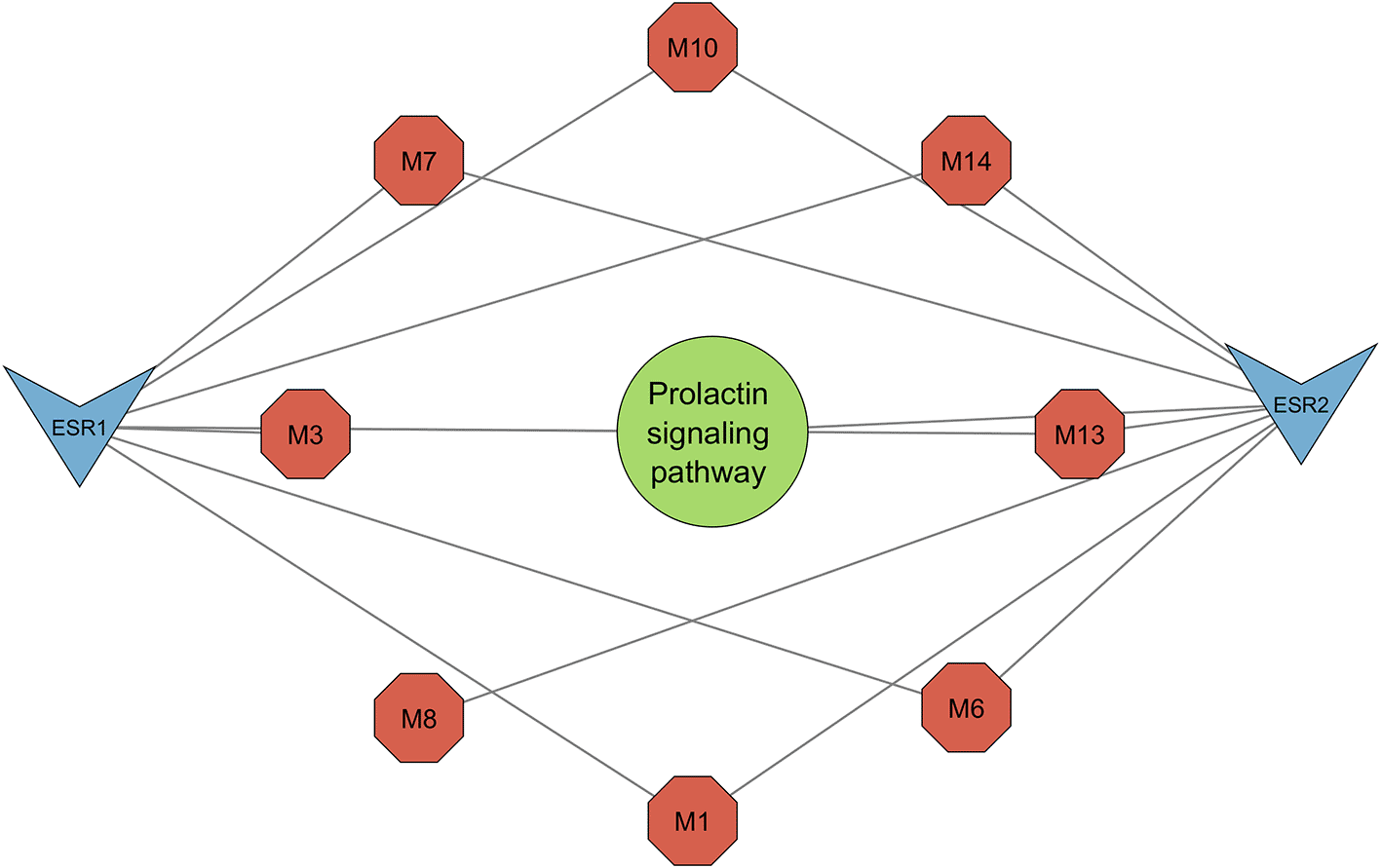
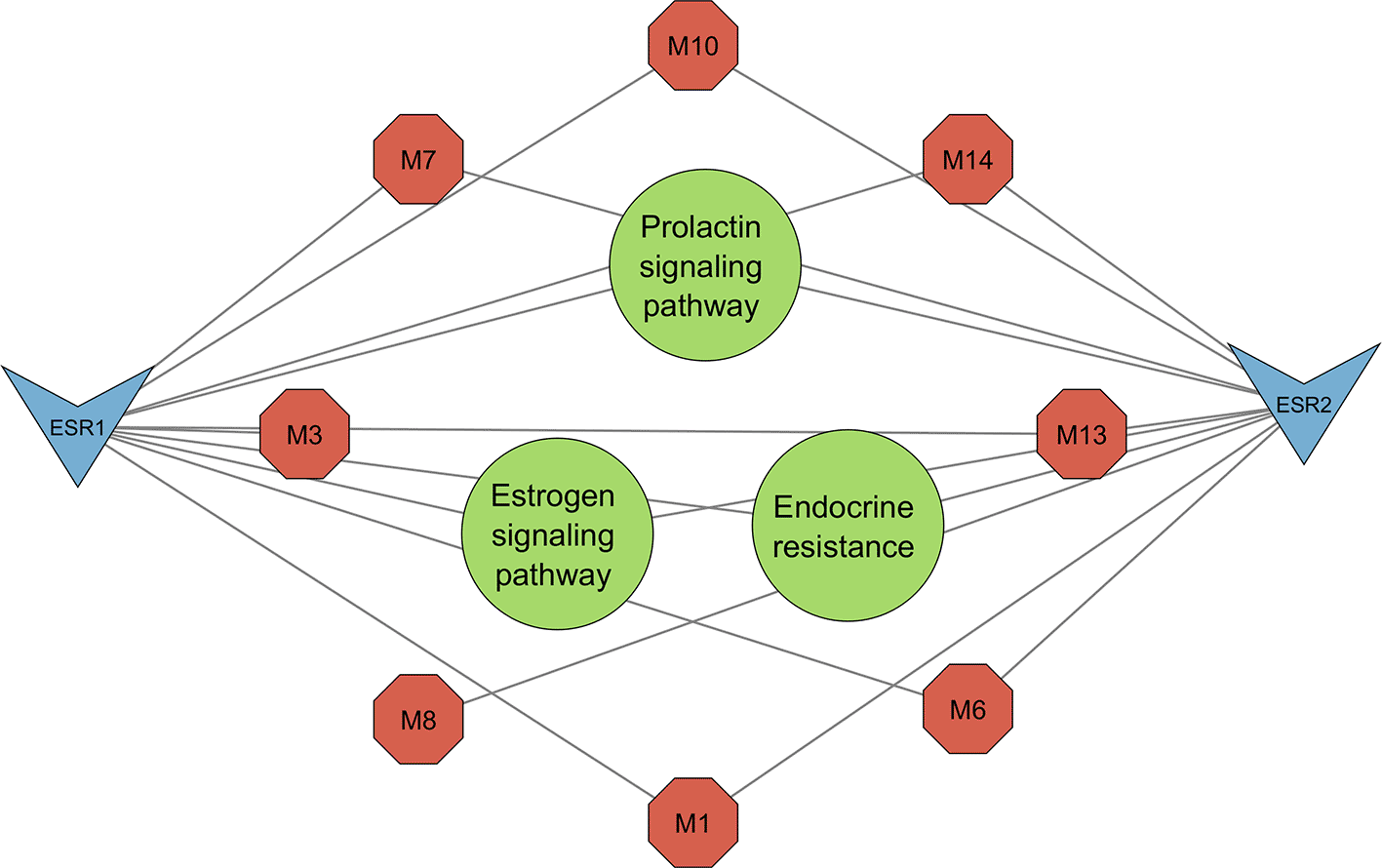
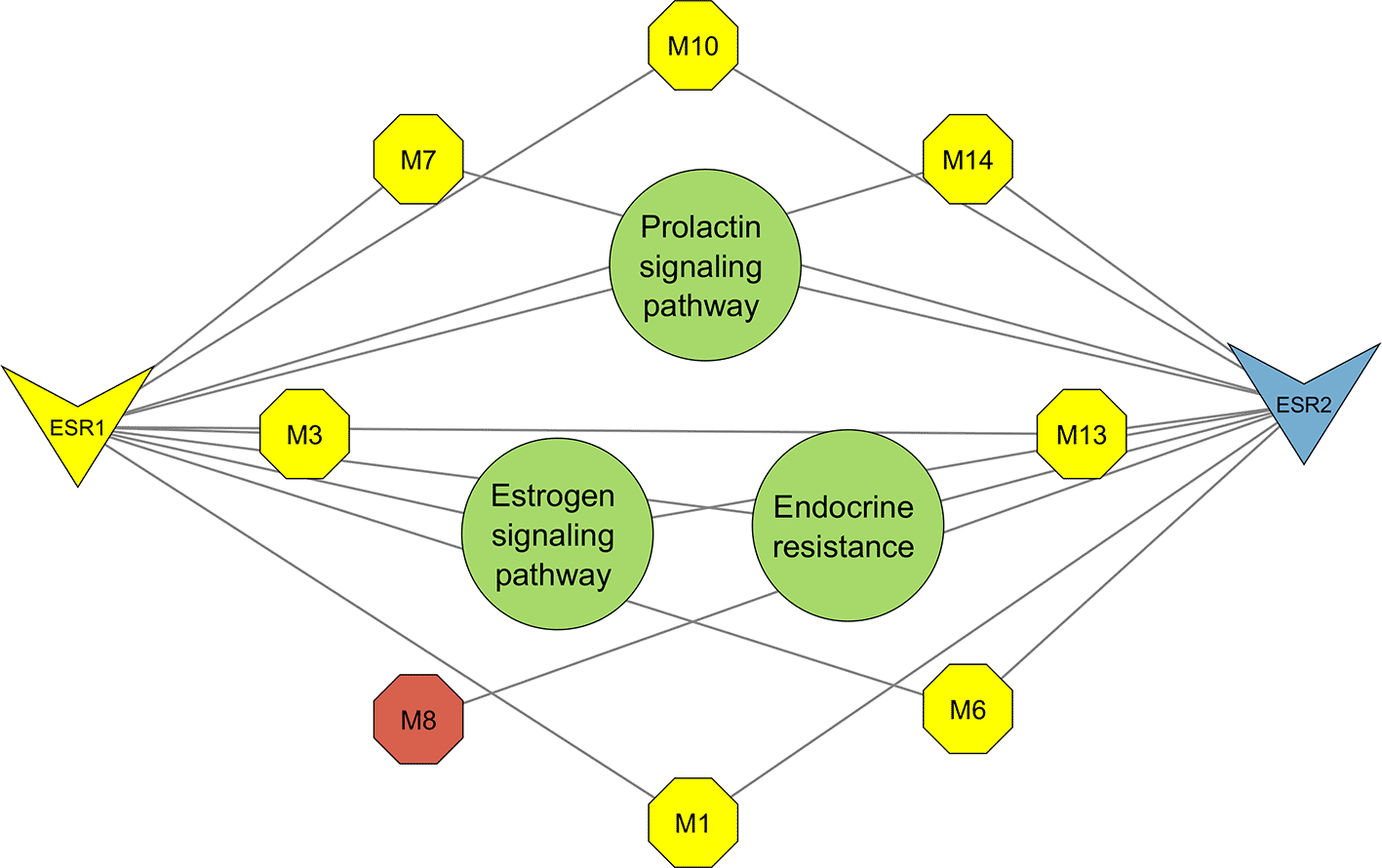
KEGG pathway enrichment analysis generated five pathways, three of which were related to melasma (Figure 5). All three pathways, namely the prolactin signaling pathway (0.0238), estrogen signaling pathway (0.0238), and endocrine resistance (0.0238), shared the same FDR. To account for this, pathway strength was used to screen for the most enriched pathways. The prolactin signaling pathway was subsequently identified as the most prominent pathway (Table 2).
The resultant PCT comprised the prolactin signaling pathway, estrogen signaling pathway, and endocrine resistance (Figure 7) encompassing 13 nodes, 20 edges, and an average node degree of 3.07. From the top three pathways identified, the prolactin signalling pathway was highlighted as the most significant for its greater pathway strength (Figure 6). Network topology analysis identified seven metabolites that interact with the significant genes ESR1 and ESR2 (Figures 8 and 9).
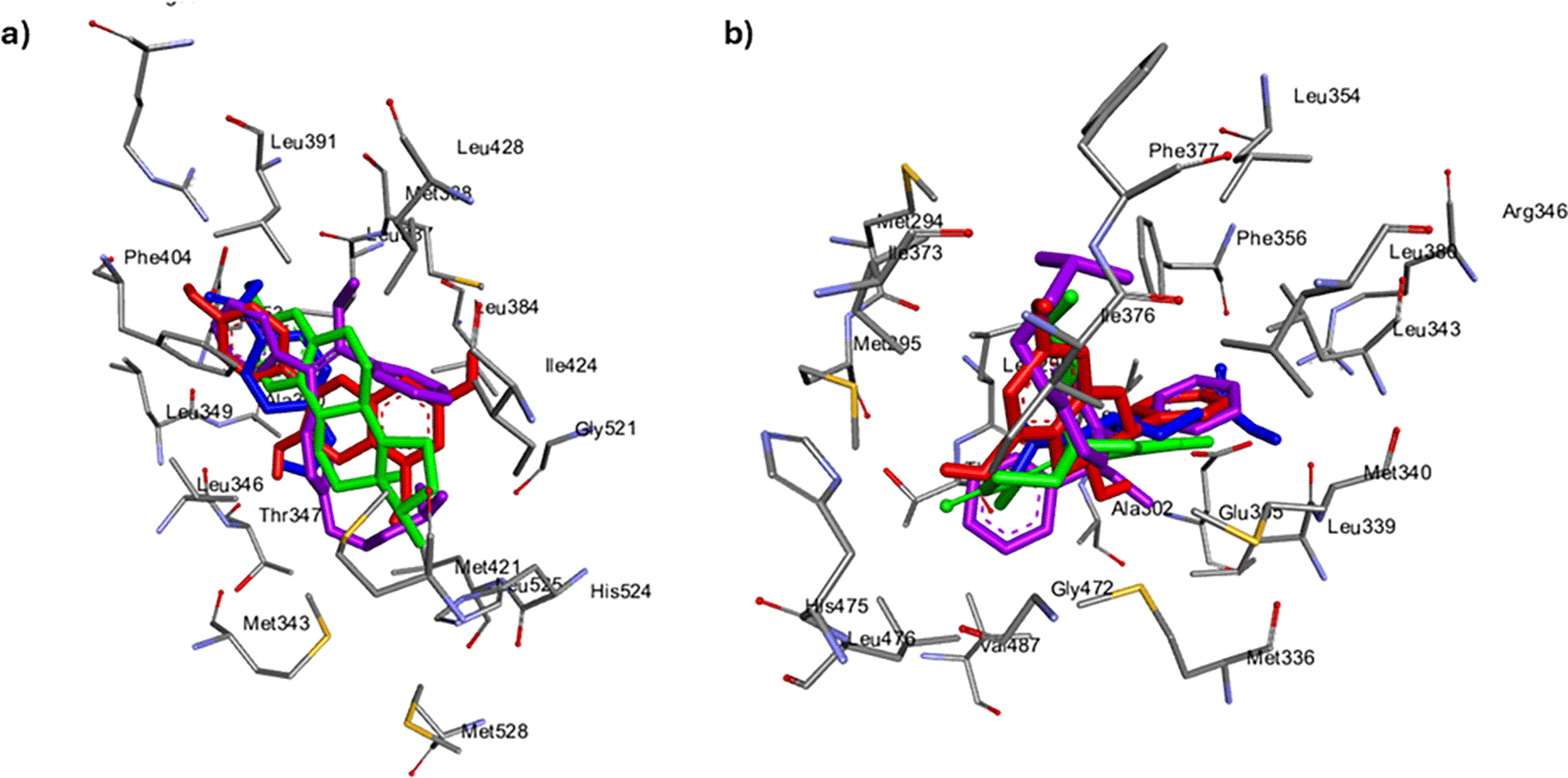
From the ESR2 bound Cassipourea metabolites, hexose (-5.4 kcal/mol), lupeol (-5.4 kcal/mol) and sitosterol-glycoside (-4.9 kcal/mol) had lower negative docking scores than tamoxifen (-6.2 kcal/mol) and tranexamic acid (-5.5 kcal/mol) (Table 3). Except for decahydroretinol (-6.0 kcal/mol), all ESR1-bound metabolites exhibited higher negative docking scores than both tamoxifen (-6.1 kcal/mol) and tranexamic acid (-5.9 kcal/mol) (Table 4). Observably, when bound to both ESR2 and ESR1, afzelechin exhibited the highest negative docking scores of -7.3 kcal/mol and -8.8 kcal/mol, respectively. Regarding ESR2, afzelechin formed 27 intermolecular interactions comprising two hydrogen bonds (Ile373, Arg346), 12 van der Waals interactions (Met340, Ile376, Leu362, Met294, Phe377, Leu380, Leu354, Val487, Thr299, Trp335, Leu301, Arg346), and nine other important interactions, viz. hydrophobic interactions (Glu305, Leu343, Phe356, Ala302, Leu339, Leu476, Met336, Met295, Leu298). Twenty intermolecular interactions and bonds were observed in ESR1 bound afzelechin namely, two hydrogen bonds (Phe404, Met517), 13 Van der Waals interactions (Arg394, Glu353, Thr347, Leu346, Met343, His524, Leu525, Met421, Gly521, Ser518, Ile424, Leu428, Leu387), and five other important interactions (Met388, Leu384, Leu391, Ala350, Leu349) (Tables 4 and 5).
Regarding ESR2 bound systems, all Cassipourea metabolites displayed lower negative binding affinities (ranging from -18.71 kcal/mol to -40.50 kcal/mol) than tamoxifen (-47.68 kcal/mol) and higher negative binding affinities than tranexamic acid (17, 60 kcal/mol). From the top performing Cassipourea metabolites bound to ESR1, sitosterol-glycoside (-78.97 kcal/mol), decahydroretinol (-63,34 kcal/mol), lupeol (-61.23 kcal/mol), emodin 6,8 dimethyl ether (-56.45 kcal/mol), and tricin (-48.22 kcal/mol), had higher negative binding affinities than tamoxifen (-47.61 kcal/mol) and tranexamic acid (-16.68 kcal/mol), with emodin 6,8 dimethyl ether (-36.26 kcal/mol) exhibiting the lowest negative binding affinity (Table 6).
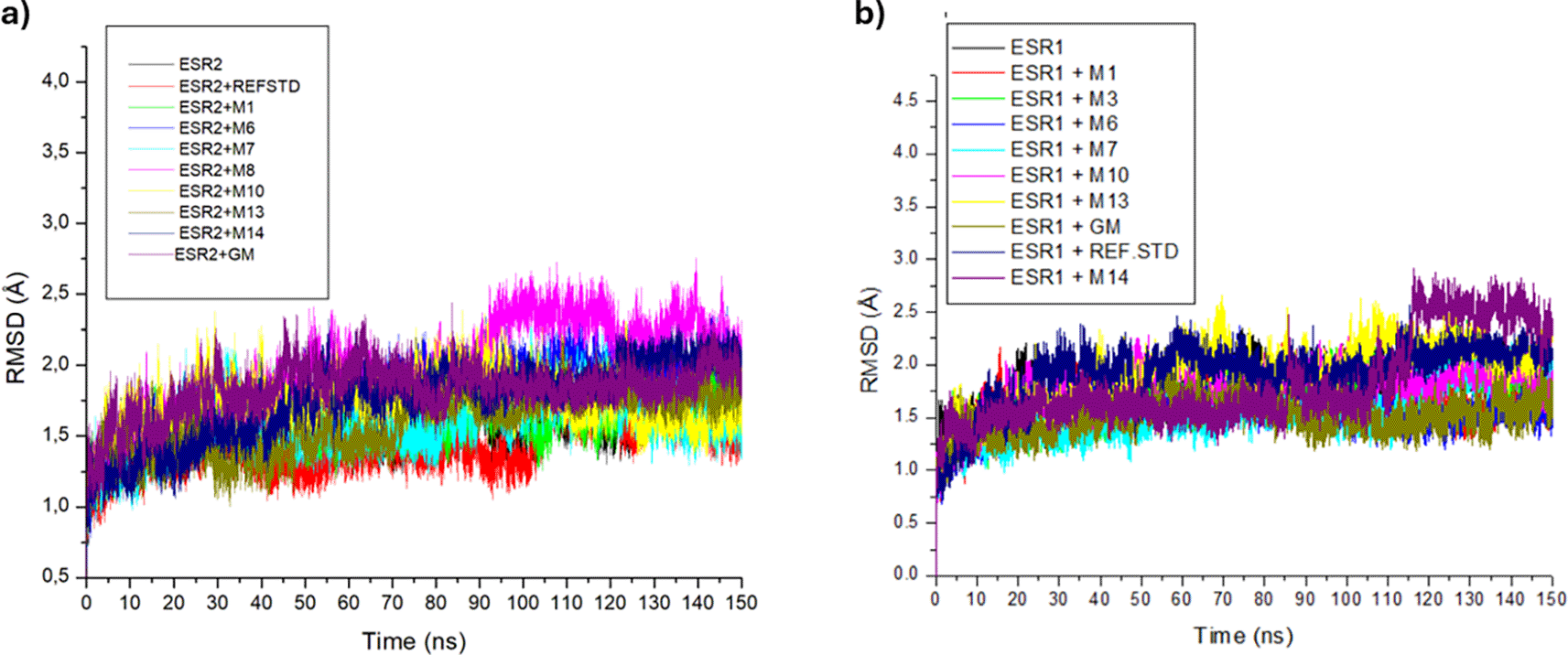
With the exception of ESR2-hexose and ESR1-tricin bound complexes at 90 ns and 120 ns, respectively, all trajectories of ESR2 and ESR1 bound and unbound systems were relatively stable and experienced minimal fluctuations (Figure 10a and 10b). Among ESR2-bound metabolites, tranexamic acid (1.44 Å) had the lowest RMSD value. Along with tranexamic acid, afzelechin (1.60 Å), emodin 6,8 dimethyl ether (1.58 Å) and sitosterol glycoside (1.59 Å) exhibited RMSD values comparable to or lower than apo-ESR2 (1.60 Å). Excluding hexose (1.98 Å), all ESR2-bound metabolites had lower RMSD values than tamoxifen (1.98 Å) (Table 7). In ESR1 bound systems, tamoxifen (1.51 Å) had the lowest RMSD value. Conversely, tranexamic acid (1.91 Å) and sitosterol-glycoside (1.91 Å) had the highest RMSD values among the ESR1-bound metabolites. Whereas tamoxifen (1.51 Å), afzelechin (1.61 Å), cassipourol (1.70 Å), decahydroretinol (1.55 Å), emodin 6,8 dimethyl ether (1.55 Å) and lupeol (1.78 Å) had similar or lower RMSD values than apo-ESR1 (1.78) (Table 7).
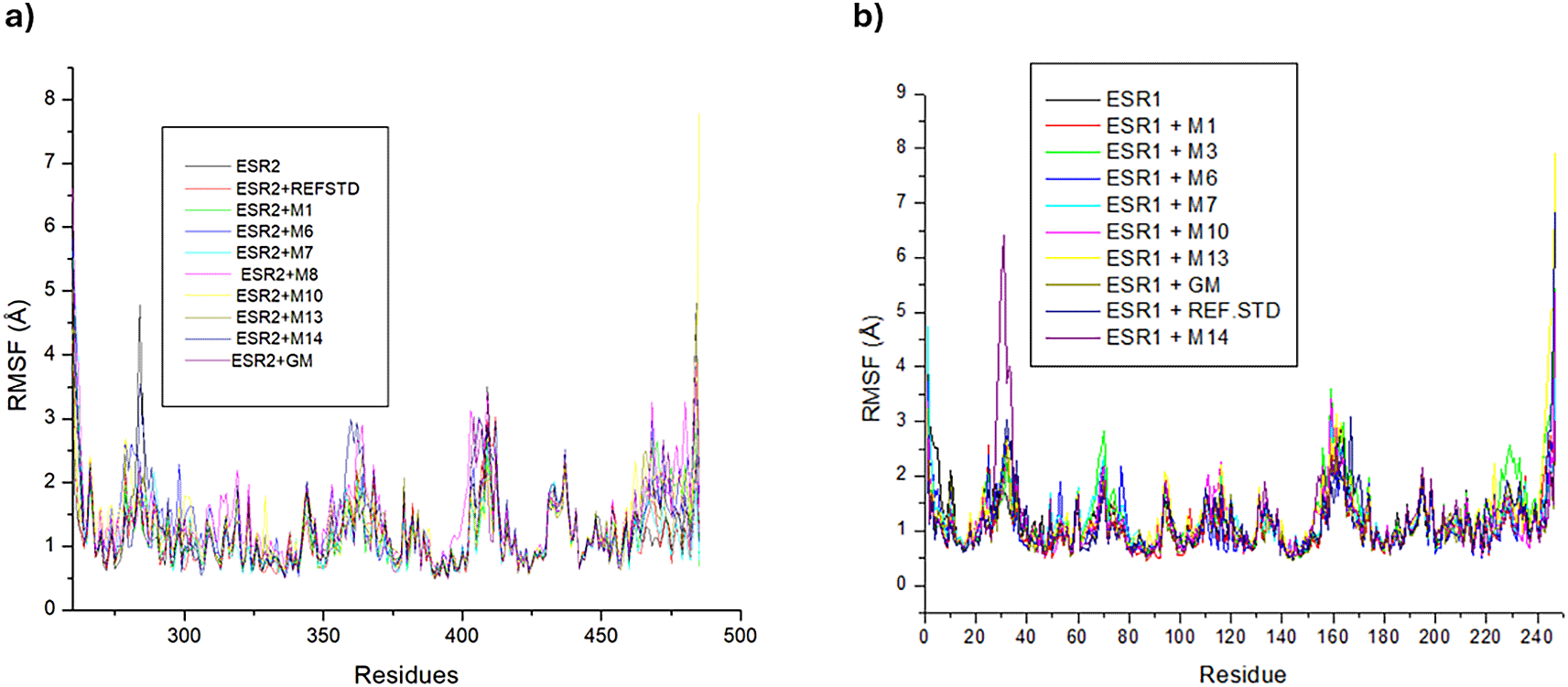
Trajectories of ESR2 bound systems experienced a multitude of high-rising fluctuations on amino acid residues 290, 215-218, and 402-412 and again from residues to 469-482 (Figure 11a). All ESR2 bound and unbound systems had comparable RMSF values, with hexose (1.50 Å) and tranexamic acid (1.15 Å) exhibiting the highest and lowest RMSF values, respectively. Against apo-ESR2 (1.20 Å), tranexamic acid, afzelechin (1.20 Å), and emodin 6,8 dimethyl ether (1.17 Å) had lower RMSF values (Table 7). In the ESR1 bound and unbound systems, fluctuations in residues 30, 70, 230, and 247, and from residues 155-165 were prominent (Figure 11b). Besides sitosterol-glycoside (1.30 Å), cassipourol (1.28 Å), and emodin 6,8 dimethyl ether (1.21 Å), all other ESR1 bound metabolites had comparable or lower RMSF values than apo-ESR1 (1.18 Å) and tranexamic acid (1.19 Å). From the ESR1-bound metabolites, only afzelechin (1.05 Å) had a lower RMSF value than apo-ESR1, tranexamic acid, and tamoxifen (1.07 Å) (Table 7).
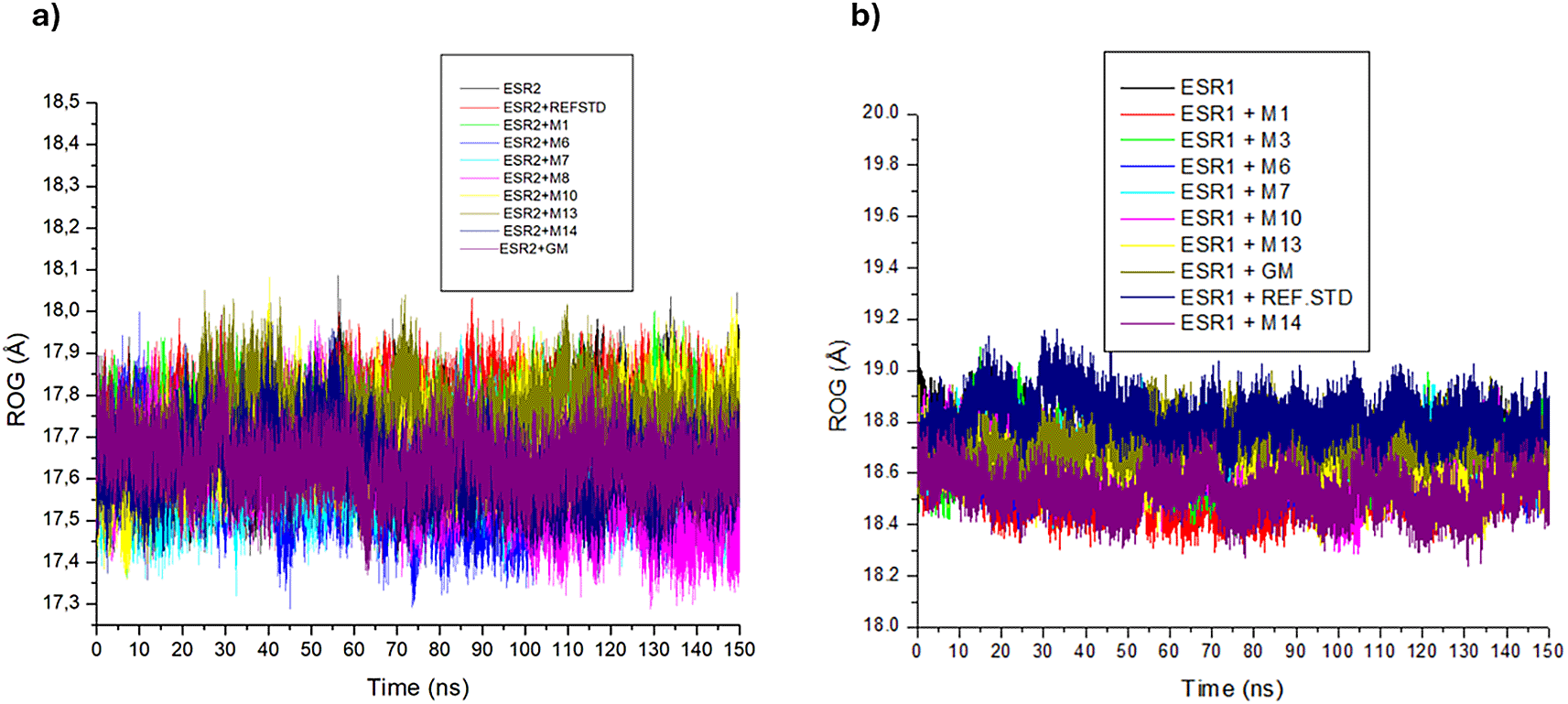
In the ESR2 and ESR1 bound and unbound systems, no complex fluctuations were observed (Figure 12a and 12b). The ESR2 bound systems exhibited non-significant differences in their RoG values, with decahydroretinol (17.59 Å), emodin 6,8 dimethyl ether (17.62 Å) and hexose (17.60 Å) displaying lower RoG values than tranexamic acid (17.76 Å), apo-ESR2 (17.70) and tamoxifen (17.64 Å) (Table 7). There was a marginal variance in ESR1 bound the RoG values. Excluding emodin 6,8 dimethyl ether (18.69 Å), all ESR1 bound metabolites complexes had lower RoG values than apo-ESR1 (18.67 Å), tranexamic acid (18.81 Å) and tamoxifen (18.71 Å), with tricin (18.52 Å) displaying the lowest RoG value (Table 7).
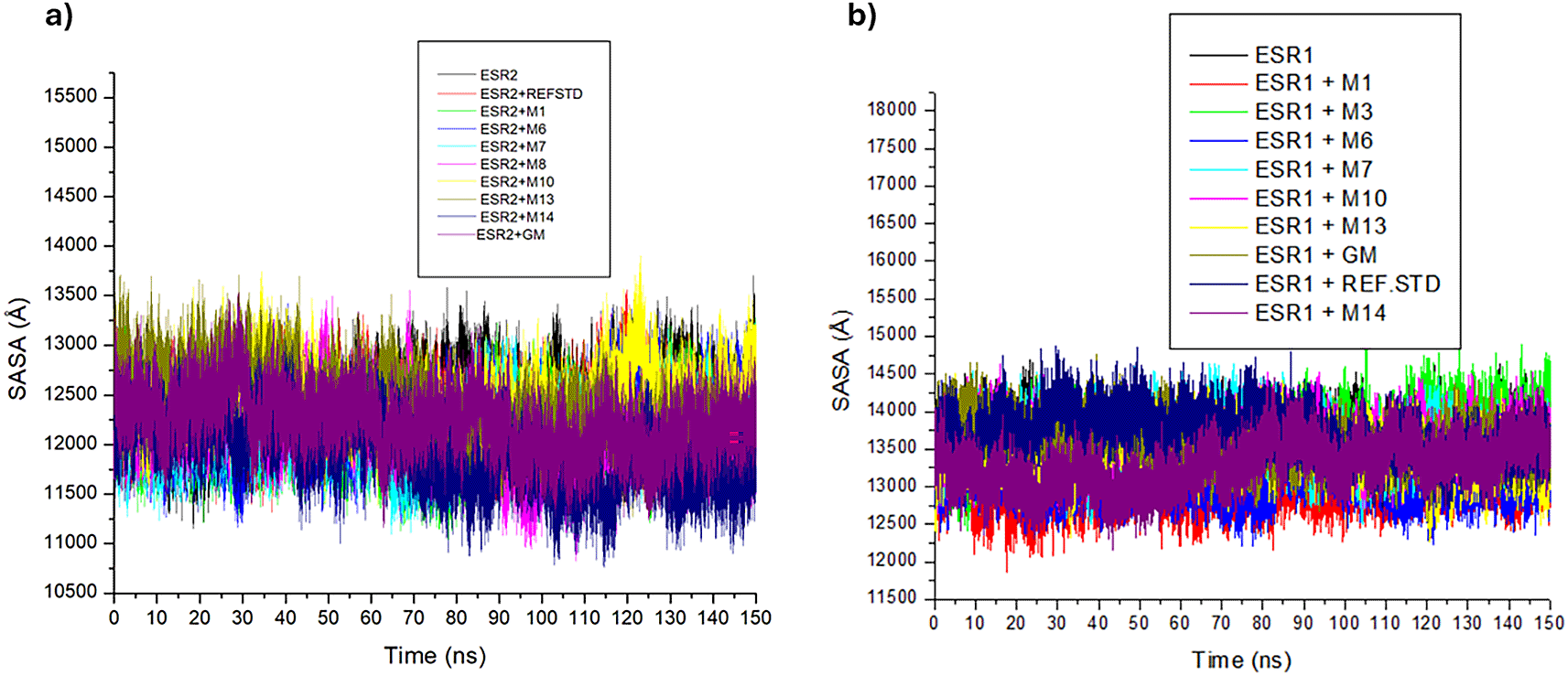
Excluding ESR2 bound lupeol and ESR1 bound tricin, the trajectories of ESR2 and ESR1 bound and unbound systems experienced minor disruptions (Figure 13a and 13b). Except for sitosterol-glycoside (12397.75 Å) and lupeol (12475.06 Å), ESR2-bound metabolites exhibit lower mean SASA values than tranexamic acid (12291.71 Å), tamoxifen (12227.35 Å) and apo-ESR2 (12411.51 Å), with tricin (11892.74 Å) exerting the most impact on the SASA (Table 7). In ESR1 bound and unbound systems, tranexamic acid (13706.67 Å) had the highest SASA value. ESR1 bound cassipourol (13672.21 Å), Emodin 6,8 dimethyl ether (13615.75 Å) and lupeol (13602.97 Å) exhibited higher SASA values than tamoxifen (13558.03 Å) and apo-ESR1 (13584.25 Å), with afzelechin (12995.41 Å) exhibiting the lowest SASA value (Table 7).
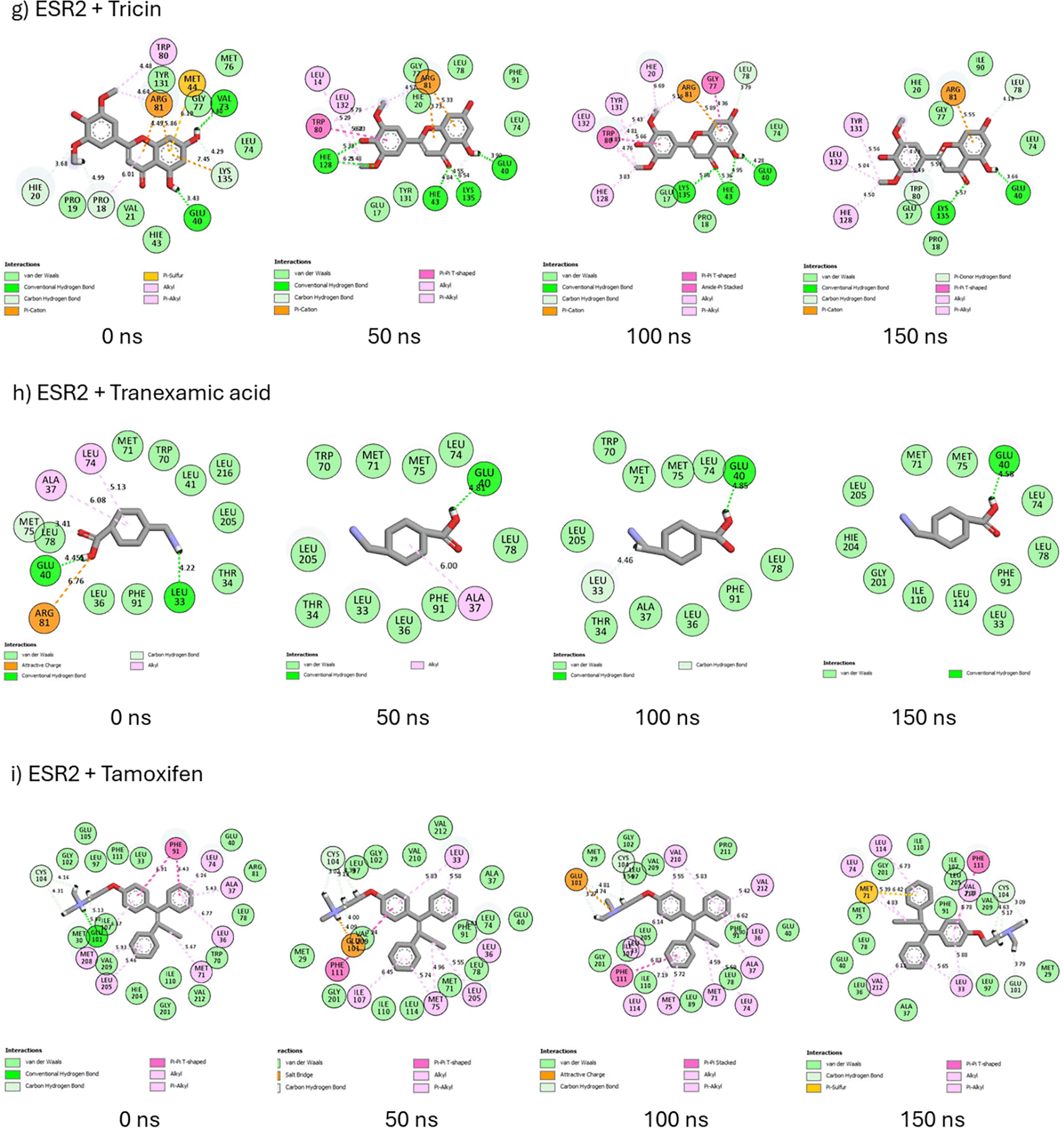
The 2D interaction plots of ESR2 bound systems displayed the formation of hydrogen bonds (carbon-hydrogen, pi-hydrogen donor, and conventional), hydrophobic interactions (pi-pi-T-shaped, alkyl, pi-alkyl, pi-sulfur, pi-cation, amide-pi-stacked, attractive charge, salt bridge, and unfavorable donor-donor bonds), and van der Waals interactions (Figure 14). From 0 ns to 150 ns, afzelechin is unable to maintain hydrogen bond formation (4 → 1) and forgoes its unfavorable donor-donor bond to form a π-sulfur bond, thus maintaining six important interactions and bonds (Figure 14a). Decahydroretinol retains six hydrophobic interactions at 0 ns and 150 ns but is unable to maintain its hydrogen bonds (2 → 1) I (Figure 14b). Emodin 6,8 dimethyl ether formed nine hydrophobic interactions and three hydrogen bonds at 0 ns, which were reduced to seven hydrophobic interactions and two hydrogen bonds at 150 ns (Figure 14c. However, at 0 and 150 ns, hexose formed four and seven hydrogen bonds, respectively, and experienced a decrease in the total intramolecular interactions from 15 to 13 (Figure 14d). At 150 ns, lupeol was unable to maintain hydrogen bond formation on Arg81 and retained only four hydrophobic interactions (Figure 14e). Sitosterol-glycoside retained four hydrogen bonds at 0 ns and 150 ns and formed a hydrophobic alkyl interaction on Met292 at 150 ns (Figure 14f). Regarding tricin, at 0 ns and 150, the number of hydrogen bonds decreased from five to four, and the number of hydrophobic interactions increased from three to four (Figure 14g). Based on its interactions at 0 ns, tranexamic acid was unable to retain its hydrogen bond (one), hydrophobic interactions, and intramolecular interactions (12) at 150 ns (Figure 14h). Although tamoxifen was able to maintain its hydrophobic interactions (seven) at 0 and 150 ns, it did not retain its hydrogen bond formation (one) or intramolecular interactions (22) (Figure 14i).


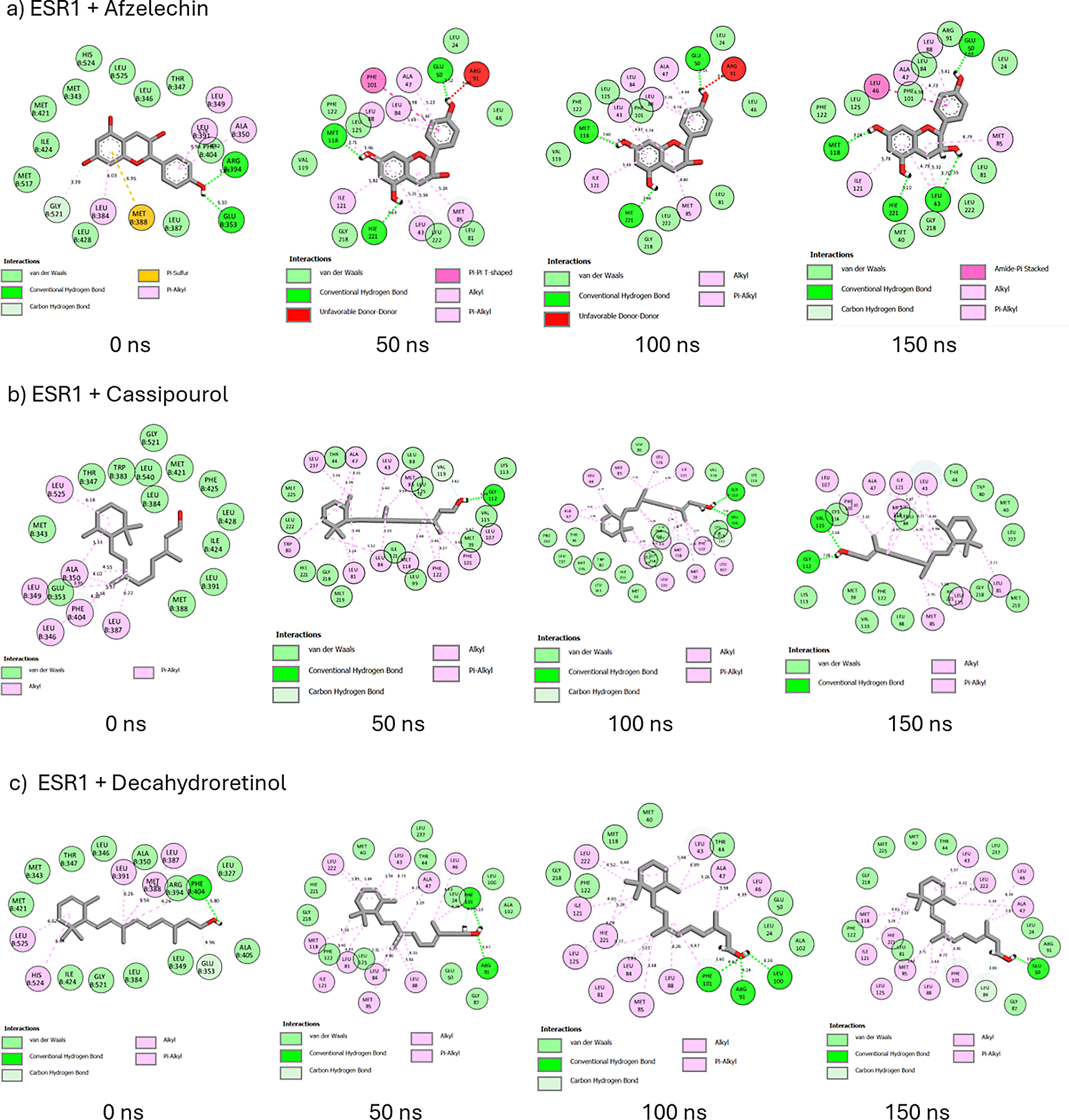
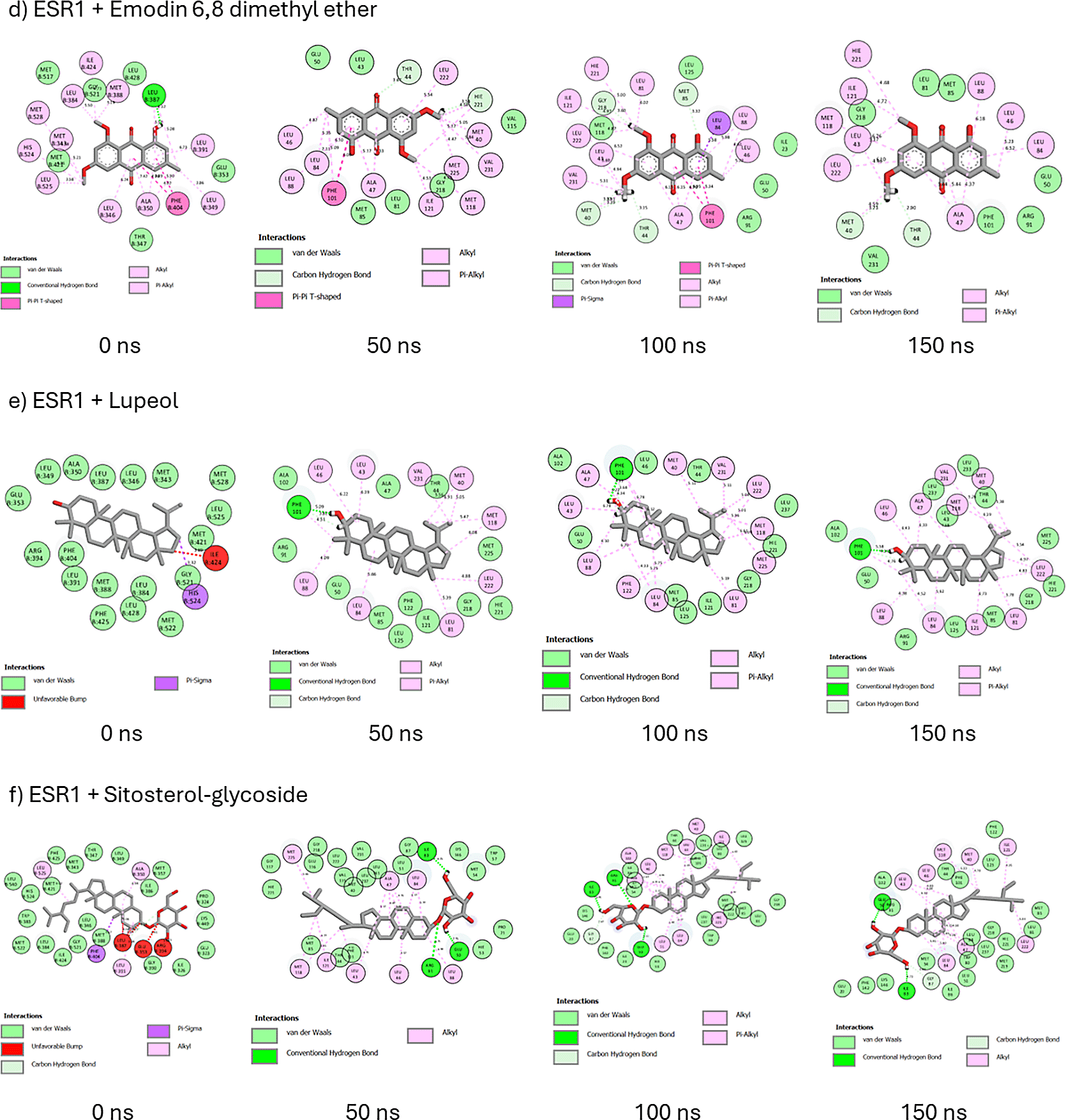
A probe into the binding interactions of ESR1 and its metabolites revealed the presence of hydrogen bonds, van der Waals interactions, and hydrophobic interactions such as pi-sulfur, pi-alkyl, alkyl, pi-pi-T-shaped, amide-pi-stacked, pi-sigma, and unfavorable donor-donor interactions (Figure 15). At 0 and 150 ns, afzelechin retained its five hydrophobic interactions and formed four hydrogen bonds at 150 ns (Figure 15a). From 0 to 150 ns, cassipourol forms nine hydrophobic interactions and two hydrogen bonds (Figure 15b). Decahydroretinol maintains one hydrogen bond and, in total, forms 11 hydrophobic interactions from 0 to 150 ns (Figure 15c). Against emodin 6,8 dimethyl ether’s intramolecular interactions at 0 ns, hydrogen bond formation increased to two, and hydrophobic and total intramolecular interactions decreased to nine and 18, respectively (Figue 15d). Lupeol exhibited hydrogen bond formation on Phe101 and an increase in hydrophobic (10) and intramolecular interactions (23) (Figure 15e). Sitosterol-glycoside forms three hydrogen bonds and replaces the three unfavorable bumps with eight hydrophobic alkyl interactions (Figure 16f). At 0ns and 150 ns, tricin forms two and five hydrogen bonds, respectively, and forgoes the unfavorable acceptor bond at 0ns for eight hydrophobic interactions (Figure 15g). In tranexamic acid, from 0 ns to 150 ns, hydrogen bond formation increased to three, intramolecular interactions were altered to 18, and hydrophobic interactions decreased to two (Figure 15h). Similarly, in tamoxifen, hydrogen bonds and intramolecular interactions are increased to two and 22, respectively, and hydrophobic interactions are reduced to six (Figure 15i).

Several studies have established the benefits of natural products, including plants, for various biological purposes.41 Metabolites identified in the Cassipourea species are reportedly implicated in skin pigmentation, treatment of skin diseases, and inhibition of tyrosinase activity.20,24 Specifically, azelaic acid, a dicarboxylic acid found in many grains, has been reported to possess anti-tyrosinase and anti-inflammatory effects,42 whereas lupeol and its derivatives are implicated in wound healing.43 These studies corroborate the therapeutic potential and identify putative leads of Cassipourea metabolites in melasma.
Interestingly, Cassipourea metabolites demonstrated remarkable drug-likeness and pharmacokinetic properties, indicating their use not only as potential topical agents but also as oral drug candidates for melasma treatment. This is evident by the finding that most metabolites are in conformity with Lipinski’s Ro5 for oral drug candidates and show appreciable bioavailability for therapeutic applications. In addition, the metabolites were relatively non-inhibitors of CYP450 enzymes; hence, they can be easily biotransformed into more polar and excretable by-products in biological systems. The GIA and Pgp-S profiles also potentiated Cassipourea for both applications. For example, sitosterol-glycoside and lupeol are hydrophobic compounds, and their low GIA and poor solubilities are suggestive of their biological effect being more applicable topically with potentially longer-lasting and better effects on the skin due to their hydrophobicity. To gain further insight into the mechanism of action of Cassipourea metabolites in melasma treatment, NP and MD simulations were conducted.
Although only four genes were common between Cassipourea metabolites and melasma, they have been reported to exert significant effects on skin pigmentation and melasma. Factors that reportedly affect the pathogenesis of melasma include genetics and hormonal activity.44 Estrogen is a female sex hormone that influences skin pigmentation by stimulating melanin synthesis in melanocytes, improving skin tonicity, thickness, healing, elasticity, and preventing age-related diseases when produced in adequate amounts.45 Estrogen levels differ with stages in women; for example, estrogen levels gradually rise from the pre-menopausal to childbearing stages, followed by a decline during post-menopause. This could be attributed to the reduction in melanin synthesis with increasing age.45
Estrogen exerts its effects through signalling pathways and genes that are important in skin physiology and pathology. Dysregulation of estrogen gene activity in the skin might result in abnormal skin pigmentation, including melasma. Furthermore, overexpression of ESR2 has been implicated in melasma.46 Melasma is more prominent during pregnancy, hormone replacement therapy, and oral contraceptive use.47 The observation that estrogen genes, receptors, and signalling pathways were some of the most enriched genes in the PPI, the most significant molecular functions in the GO analysis, and the second most significant signalling pathway KEGG, respectively, in this study lend credence to the vital role of Cassipourea metabolites in melasma treatment.
The PTGS2 gene (cyclooxygenase 2) stimulates the synthesis of prostanoids, such as prostaglandins, thromboxanes, and protacyclins, involved in inflammation caused by the 20-C atom arachidonic acid.48 Therefore, through the modulation of PTGS2, Cassipourea metabolites may regulate melasma-induced inflammatory responses. Furthermore, sitosterol has been reported to be an anti-inflammatory agent.49 The involvement of tyrosinase (TYR) in the PPI network corroborates the findings of Mpofana et al. 2023,20 who stated that Cassipourea metabolites possess an anti-tyrosinase effect. TYR regulates melanin synthesis; hence, its inhibition aids in replenishing normal skin pigmentation and decreasing the risk of melasma.
The finding that the intracellular steroid hormone receptor signalling pathway was the most enriched biological process in the GO analysis denotes the potential of Cassipourea metabolites in the treatment of melasma. Other biological processes, such as cellular response to estrogen and estradiol stimulus, estrogen receptor signalling pathway, and regulation of inflammatory response, have also been implicated in skin pigmentation. However, steroid hormones, such as estrogen, estradiol, and progesterone, have been implicated in the control of skin pigmentation via certain receptors.50 These hormones, as well as the overexpression of tyrosinase and melanocyte-stimulating hormone, are attributed to the excessive pigmentation observed during pregnancy.51 The interaction of these steroid hormones and signalling pathways is suggestive of the possible role of Cassipourea in preventing pregnancy-induced melasma. Interestingly, the signalling pathways and genes identified in this study are in line with the study by Yin et al.,25 who studied the mechanism of action of Croci stigma on melasma.
The hyperstimulation of melanocytes by UV irradiation also induces high melanin secretion and ROS generation, resulting in cellular damage.44 Vitamin D plays an important role in skin functions, including skin homeostasis and anti-inflammatory, immune, and cell proliferation responses that are essential for wound healing and restoration of skin pigmentation anomalies.52 The observation that response to vitamin D was one of the most enriched biological processes denotes the ability of Cassipourea metabolites to stimulate vitamin D production, which is necessary to prevent UV-induced melasma.
However, the macromolecular complex was the only enriched cellular component. This complex is vital to cellular processes. It is composed of assemblies of proteins and nucleic acids involved in transcriptional and translational processes that convert DNA to RNA and protein, respectively. In signaling pathways, these assemblies are transient,53 which might imply that melasma temporarily affects signaling pathways and that skin anomalies could be restored to normalcy upon appropriate treatment.
Prolactin, a hormone secreted during lactation and reproduction, is involved in metabolism, immunoregulation, protection, growth, and development. The binding of prolactin to the prolactin receptor initiates the prolactin signaling pathway.54 The observation that the prolactin signalling pathway was the most enriched in the KEGG analysis signifies that the functions of prolactin, its predominance in females who are largely affected by melasma, and the activation of its signalling pathway during the reproductive process, the regulation of this pathway could also serve as a target for the action of Cassipourea metabolites. Furthermore, the most significant genes (ESR2 and ESR1) in this pathway were analyzed for their binding affinity, stability, and interaction with top-ranked metabolites.
Molecular docking identifies the top therapeutic metabolites by assessing their binding affinity for the active site of selected receptors to further augment the understanding of their bioactive mechanisms of action.55 Higher negative docking scores are associated with better docking poses and fitness.56 Except for ESR2-bound hexose, lupeol, sitosterol-glycoside, and ESR1-bound decahydroretinol, the higher negative docking scores of receptor-bound Cassipourea metabolites, relative to tamoxifen and tranexamic acid, are suggestive of their better biological activity.57 Afzelechin acts as a dual modulator of both ESR2 and ESR1, with the highest negative docking scores within each receptor-bound system, further highlighting its potential therapeutic application. However, molecular docking merely assesses the crystallographic binding orientation of ligands, while molecular dynamic (MD) simulations help to elucidate binding stability.58
To better understand the biomolecular affinity and efficacy of Cassipourea metabolites as therapeutic candidates, their thermodynamic binding free energies were analyzed over 150 ns,59 with higher negative values indicative of greater binding affinity. The higher negative binding free energy of tamoxifen than that of ESR2-bound metabolites suggests that tamoxifen offers marginal superiority as an ESR2 modulator. The lower negative binding free energy of tranexamic acid relative to that of ESR2-bound metabolites indicates that Cassipourea metabolites are more thermodynamically stable. Comparatively, the higher negative binding free energies of ESR1 bound cassipourol, decahydroretinol, lupeol, tricin, and sitosterol-glycoside complexes with tamoxifen and tranexamic acid were presumed to be stable and had better modulatory effects on ESR1. Moreover, MD simulations identified sitosterol-glycoside as a dual modulator of ESR2 and ESR1 because of its high negative binding free energy and superior thermodynamic stability among all other Cassipourea metabolites, further augmenting its biological activity in melasma-based therapeutics.
Thereafter, the post-dynamic parameters RMSD, RMSF, RoG, and SASA of the ESR2 and ESR1 bound systems were assessed at 150 ns for better insight into their thermodynamic stability.60 RMSD measures the stability of protein-ligand systems by quantifying the change between their initial and final structural conformation, with lower deviations (values) denoting greater stability.61 According to Umar et al.,62 an RMSD value of ≤ 3 Å is an acceptable measurement of protein-ligand stability, validating the stability of ESR2 and ESR1 bound and unbound systems. The lower RMSD of the ESR2-bound tranexamic acid and ESR1-bound tamoxifen complexes is presumed to be due to their better stability as modulators of their respective genes. Conversely, the higher RMSD of ESR2-bound tamoxifen and ESR1-bound tranexamic acid systems compared to Cassipourea metabolites suggests their potential stability with the ESR2 and ESR1 genes. Comparatively, the lower RMSD of ESR2-bound emodin ethyl ether and sitosterol-glycoside and ESR1-bound afzelechin, cassipourol, decahydroretinol, emodin 6,8 dimethyl ether, and lupeol against their apo-genes indicated that these metabolites formed stable complexes with their relative genes.
RMSF analysis of ESR2 and ESR1 bound and unbound systems measures the flexibility of protein-ligand interactions. Higher RMSF values and fluctuations denote greater flexibility but less stability, whereas lower RMSF values and fluctuations indicate greater stability.63 Generally, an RMSF value <2 Å is an optimal measure of ligand flexibility.64 Thus, all metabolites bound to ESR2 and ESR1 exhibited suitable flexibility, with ESR2-bound emodin 6,8 dimethyl ether and ESR1-bound afzelechin, decahydroretinol, and lupeol exerting stabilizing effects on their relative apo-genes. However, among Cassipourea metabolites, tranexamic acid may potentiate greater stability as an ESR2 modulator, and tamoxifen may exhibit comparable flexibility as an alternate ESR1 modulator.
The RoG measures the time-dependent compactness of protein-ligand systems, with lower values indicating greater overall compactness and stability.65 The similar RoG values of the ESR2 and ESR1 bound systems relative to their apo-genes are suggestive of the marginal impact exerted by Cassipourea metabolites on ESR2 and ESR1 protein folding. The lower RoG values of ESR2-bound decahydroretinol, emodin 6.8 dimethyl ether, and hexose complexes and ESR1-bound afzelechin, cassipourol, decahydroretinol, sitosterol-glycoside, lupeol, and tricin complexes relative to tranexamic acid, tamoxifen, and their apo-genes are indicative of their greater compactness, stability, and suitability as ESR2 and ESR1 modulators.
The SASA plot was used to measure the rate of interaction between complexes and their surrounding hydrophobic environment, with higher values denoting greater environmental exposure and lower values depicting more stable systems.66 The lower SASA values of ESR2-bound metabolites (excluding lupeol) and ESR1-bound metabolites (excluding emodin 6,8 dimethyl ether, cassipourol, and lupeol) relative to their apo-genes suggest Cassipourea metabolites induce protein-ligand stability and compactness through protein folding. The minor fluctuations experienced by ESR2-bound lupeol at 125 ns and ESR1-bound tricin and cassipourol at 85 ns and 105 ns, respectively, suggest less interaction during that period. The lower SASA values of ESR2 bound afzelechin, hexose, emodin 6,8 dimethyl ether, decahydroretinol, and tricin complexes and ESR1 bound afzelechin, decahydroretinol, sitosterol-glycoside, and tricin complexes relative to tamoxifen and tranexamic acid are presumptive of their weaker interactions with the surrounding hydrophobic environment, resulting in better stability and modulation of ESR2 and ESR1.
The presence of hydrogen bonds significantly induces protein-ligand stability, binding specificity, and several pharmacokinetic properties such as metabolism and adsorption.67 The 2D interaction plots of ESR2 and ESR1 bound systems also formed van der Waals and hydrophobic interactions, both of which augment their stability and affinity. In both ESR2 and ESR1 systems, sitosterol-glycoside could retain and steadily increase its hydrogen bond formation, corroborating its highest negative binding free energy among Cassipourea metabolites in both systems. The higher negative binding free energy of ESR2-bound tamoxifen can be attributed to the formation of multiple hydrophobic interactions throughout the 150 ns simulation.
The results of this study provide pharmacological support for the development of an alternative treatment for the management of hypermelanosis disorders such as melasma. The constituents of Cassipourea have potential commercial value. The finding that several sex hormones implicated in melasma, transcription processes, and vitamin D are highly involved in this study is a strong indication of the multi-target pathways by which Cassipourea metabolites potentially exert effects on melasma. The potential regulation of estrogen synthesis by these metabolites, depicted by their binding interactions, stability, and compactness, is suggestive of melanogenesis control for normal skin pigmentation and the possible restoration of melasma skin. Further studies are recommended to validate and assess the safety of these metabolites.
The study was conducted following the approval of the University of KwaZulu-Natal Biomedical Research Ethics Committee (UKZN BREC) (protocol reference number: BREC/00002721/2021).
Conceptualization: NM; methodology: NM; CP and SS; software: SS.; formal analysis: SS, CP, and HYL; investigation: NM, CP, and SS.; resources: SS; writing: original draft preparation: NM, CP, and HYL; writing: review and editing, NM, CP, HYL, SS, and MUM.; visualization, SS; supervision: NCD, NG, and AH; funding acquisition: NM and NG. All authors have read, agreed to, and approved the final version of the manuscript.
FIGSHARE: Compounds elucidated from the three selected Cassipourea species as well as phytochemical comparison, https://doi.org/10.6084/m9.figshare.26418361.v1. 68
The project contains the following underlying data:
• Compounds elucidated from the three selected Cassipourea species
• Phytochemical comparison of the compounds
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: melasma, phototherapy, keloids, basic science research, AN, cosmetics, skin of color
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 22 Aug 24 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)