Keywords
Allergy, infantile eczema, steroids, calcineurin inhibitors, phosphodiesterase inhibitors, predictive biomarkers, precision medicine.
Atopic dermatitis (AD) is a skin disease, but its clinical impact is systemic with more than 30% of patients suffering from other allergic diseases and mental health disorders. Early disease control could prevent the development of comorbidities or reduce the severity of the condition. Topical treatments, such as skin moisturizers, steroids, calcineurin inhibitors, and phosphodiesterase inhibitors, have proven to be effective in achieving clinical control in AD; However, sustained improvement is observed in approximately 50–70% of patients, depending on disease severity, treatment adherence, and individual response. Early detection of patients with a low probability of response to topical treatment would help start with other therapies and improve the quality of life of patients.
Several clinical and laboratory biomarkers have been proposed to predict the prognosis of AD. In this study, we present a protocol for developing a model to predict the clinical response of AD patients to topical treatment. To achieve this goal, a cohort study will be performed for the prognostic model using the following steps:
Selection of predictive variables for the model
Evaluation of the quality of the collected data and control of lost data
Data statistical management
Strategies to select the variables to include at the end of the model
Evaluation of the performance of different possible models (predictive accuracy)
selection of the best model.
Validation
The performance and internal validation of the model will be assessed, and if high diagnostic performance is confirmed, external validation will be justified to assess its clinical impact ( Figure 1).
Allergy, infantile eczema, steroids, calcineurin inhibitors, phosphodiesterase inhibitors, predictive biomarkers, precision medicine.
Atopic dermatitis (AD) is a skin disease that can significantly impact quality of life and cause frequent allergic and non-allergic comorbidities such as asthma, rhinitis, food allergy, depression, and anxiety.1–3 Topical treatment is the first-line for AD and is based on skin moisturizers, steroids, calcineurin inhibitors, or phosphodiesterase inhibitors; however, clinical control with this treatment is achieved in only 50%-70% of patients. While they are particularly effective in mild to moderate cases, their efficacy may be limited in severe or chronic forms of atopic dermatitis, which often require adjunctive systemic therapy.4–6
Early identification of topical treatment responses in AD is crucial for optimizing the clinical management approach and rationalizing the use of resources. This anticipation would enable more efficient management of financial resources and patient time, as well as reduce the risk or severity of some comorbidities.7,8 Although clinical and molecular biomarkers have been identified as potential predictors of response for some therapies in AD.9–17 So far, none of the biomarkers has proven to be sufficiently accurate on their own. Predictive models allow the addition of the contributions of different variables and achieve greater predictive accuracy than variables separately.18–21
In this article, we present a protocol for the development and internal validation of a predictive model to identify the response of AD patients to topical therapy. The creation of this predictive model could help identify patients who will respond poorly to topical therapy and define the early initiation of other therapies ( Figure 1).
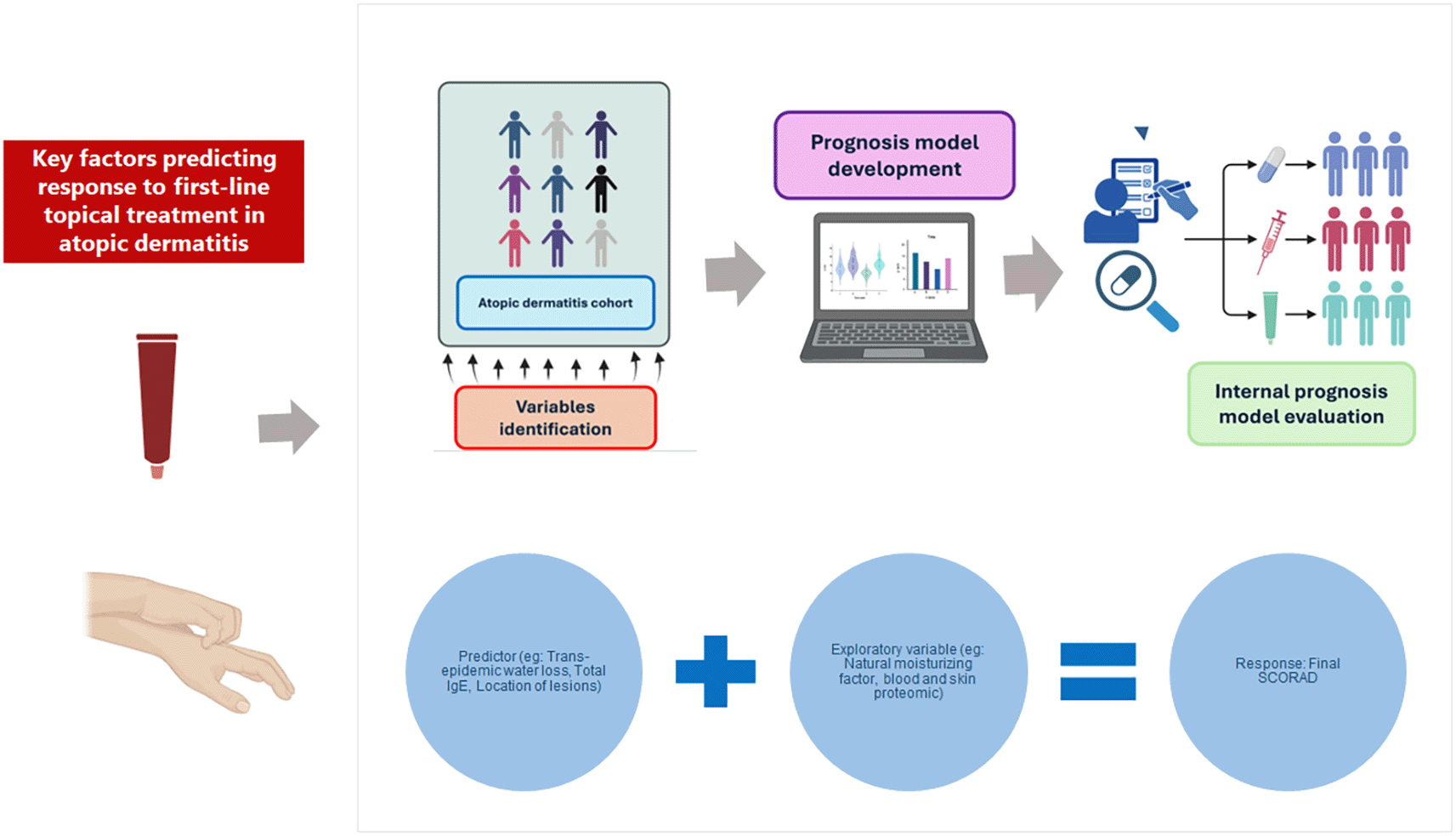
Summarizing key predictors of response to first-line topical treatment in atopic dermatitis. Variables such as trans-epidermal water loss, total IgE, and lesion location, combined with exploratory biomarkers including natural moisturizing factor and proteomic profiles from blood and skin, contribute to predicting treatment outcomes measured by final scoring Atopic Dermatitis (SCORAD).
Seeking the best research performance in clinical fields and in favor of collaborative work in the scientific community, this protocol is openly available through this online publication.
This is a registered protocol, with an ethics committee approved by the Hospital “Alma Mater de Antioquia” (Code IN62-2024) and CES university (265).
An analytical prospective cohort observational study with a three-month follow-up will be conducted to identify predictive factors for response to first-line topical treatment in patients with atopic dermatitis. The study will follow the existing guidelines for model development and internal validation22,23 and be reported following the “Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis” (TRIPOD) statement.24–27
Patients who meet the selection criteria for this project will be included and evaluation variables (predictive and outcome variables) will be recollected. All new patients with AD who attend one of the participating centers and meet the inclusion criteria will be invited to participate.
The reference population will include patients with AD located in Colombia attending dermatology, allergology, and/or pediatric services, and who voluntarily agree to participate in the study. The centers participating in the study will include hospitals and outpatient services willing to collaborate in the cities of Medellín and Bogotá, thus allowing the inclusion of patients with varying levels of severity.
Patients of any age who had AD who had not previously received topical treatment at the maximum potency allowed for the age, extent, and severity.28 AD will be defined according to the criteria of Hanifin’s and Rajka’s,29 and evaluated by a physician specializing in allergology, dermatology, or pediatrics, who have been previously trained in the study protocol.
Patients who suffer from a comorbidity that causes pruritus and could confuse the diagnosis will be excluded (for example, chronic itching, scabies, peripheral neuropathy, peripheral neuropathy, etc.). Patients taking any medication that may affect the evaluation of the clinical impact of topical treatment will not be included. Patients should not have any medical contraindications for initiating or maintaining topical therapies (for example, allergy to the active substance or its excipients, intolerable adverse effects).
Patients will be treated with topical medications, which may include corticosteroids, calcineurin inhibitors, or phosphodiesterase inhibitors at the maximum permitted dose and potency, as determined by the specialist based on the affected area. Calcineurin or phosphodiesterase inhibitors may also be used, with dosage adjustments for both active treatment and maintenance.
The outcome will be evaluated based on the response to topical anti-inflammatory treatment after a trial period of at least three months. A 75% reduction in the Score for Atopic Dermatitis (SCORAD75), with the maximum appropriate potency, according to age, extent, and affected area, will be considered as a control response.28,30
For this study, we will evaluate two groups of variables: the predictor variables will be evaluated for inclusion in the model, while the exploratory variables are focused on the search for potential variables that could be used to update the model in the future, which is in accordance with phase 2 of the TRIPOD proposal (prediction model for individual prognosis or diagnosis).
Table 1 describes the study variables that will be collected and the characteristics assigned to them (predictive, exploratory, outcome).
The predictor variables were selected based on biological plausibility and an exhaustive literature review. We also considered previous systematic reviews.9–11,13–17,31
Non-random, non-probabilistic convenience sampling from the population attending the participant centers will be carried out. Although it is not a census or probabilistic sampling, the realization of a generalizable predictive model will imply, as an a posteriori strategy, an external validation in the population with atopic dermatitis to determine its clinical applicability in other scenarios. Validation studies provide estimates of the capacity of a model to discriminate between patients with different outcomes and the agreement between the predicted and observed risks.32
The sample size was calculated according to Peduzzi’s recommendations for predictive indices,33,34 following the “events per variable” criterion, which requires obtaining at least ten outcomes for each possible predictive variable. Ten variables will be included in this study. Furthermore, considering that it is estimated that 30– 60% of patients will achieve control with the first line of management,27,28,35 which is a topical treatment for both adults and children, the following formula was applied:
“N = 10*k/I”, where “k” is the number of predictive variables and “i” is the incidence of the outcome of interest: “N = 10*11/0.5” so N = 220.
In the processing of the variables and data analysis, the analysis of the data will be as follows:
Descriptive analysis of variables: The descriptive analysis will be conducted on the entire cohort, considering the univariate analysis with absolute and relative frequencies for the qualitative variables. The distribution of the quantitative variables will be considered by applying the Shapiro-Wilk test (considering the cutoff point p ≤0.05; we will reject the null hypothesis that the distribution is normal). The mean and standard deviation for the quantitative variables with a normal distribution will be used. The variables will be described using the median and interquartile range if a non-normal distribution is evident.
Bivariate analysis: Bivariate analysis will be performed to select variables related to the response to topical treatment. Categorical variables will be analyzed with the chi-square hypothesis test of independence, if for all cases the expected frequencies will be greater than 5; otherwise, the Fisher test will be used (for both cases, a p-value <0.05 will be considered significant). The quantitative variables will be evaluated based on their distribution. The unpaired t-test will be used for continuous variables if the data distribution is normal, or the Mann-Whitney U test if they did not meet this criterion.
Development of the predictive model: There will be seven follow-up steps for the analysis and construction of the model ( Figure 2):
The selection of candidate prognostic variables has been explained previously ( Table 1). Selection of the prognostic variables in the final model will be based on the magnitude of its effect assessed according to the coefficients, and/or its biological plausibility; variables with a p < 0.20 in the initial comparisons (Hosmer-Lemeshow criterion) will be included.
A multiple imputation strategy will be applied to complete the missing data exclusively for variables that do not exceed 10% of the losses. Suppose the losses were greater than 10% or were not random (i.e., the data loss is independent of the observed and unobserved characteristics of the sample). In that case, the variable will not be included in the model.
In the multiple imputation method, each censored value will be replaced by a set of ‘m > 1’ simulated values (typically 5–10), resulting in ‘m’ complete datasets. Each of these datasets will be analyzed using standard analytical methods, and the results will be combined while accounting for the variability across imputations. The Monte Carlo Markov Chain (MCMC) method will be used, as it is suitable when the missing data pattern does not meet the monotonicity assumption (the missing data pattern is non-monotonic, meaning that data are missing in different combinations and do not follow a predictable sequence).
The steps to follow in the imputation are as follows:
The collinearity assumption will be analyzed in continuous variables using the correlation matrix (where a value of r > 0.7 is considered to indicate collinear variables) or by the Variance Inflation Factor (VIF > 5 indicating collinearity), and in categorical variables using the chi-square test of independence (relationship between categorical variables with p > 0.05 indicating collinearity).
The monotonic function relationship assumption will be graphically evaluated in the continuous variables using the Lowess function; those variables that do not meet this assumption by graphic criteria will be transformed or dichotomized.
For each variable remaining in the model, the degree of association with the dependent variable will be estimated (bivariate analysis) by calculating the Odds Ratio (with its respective 95% confidence interval), and a multivariate regression model will be adjusted to identify the independent variables associated with the outcome of interest.36 The final decision will be made based on the Wald statistic, considering a p-value < 0.05 to include a variable in the multivariate model, or biological plausibility along with the magnitude of the association.
To compare the predictive capacity of the models, we will evaluate the discrimination and calibration.36 Discrimination will be graphically evaluated in each model and using the c-statistic statistic and area under the Receiver Operating Characteristic (ROC) curve. Calibration tells us about the model’s goodness of fit; we will use the Hosmer–Lemeshow hypothesis test (a value of p > 0.050020indicates that the model has adequate calibration).
Several models will be built based on the inclusion and exclusion of variables to select the best model. To compare these prognostic models, the data obtained from a bootstrap analysis will be used as a comparison sample (internal validation). Among the models, we will evaluate the Discrimination Improvement Index (DII), Integrated Discrimination Improvement (IDI) which evaluates between the models, the change in estimated probability of outcome in a linear fashion. It will be calculated as the difference between the means of the estimated outcome probabilities of one model vs. those estimated in the other, minus the same difference between those that did not have the outcome, and Net Reclassification Improvement (NRI) which considers the changes between the different risk categories (success and non-success).37
The IDI linearly evaluates the change in the probability estimate of the outcome between the models. It will be calculated as the difference between the means of the estimated probabilities of the outcome of one model versus those estimated in the other, minus the same difference between those who did not have the outcome. In other words, IDI represents the average improvement of a model in terms of predicting patients who have the outcome, removing what is worsened by the prediction of the result in patients who ultimately do not have it.
Meanwhile, the NRI considers the changes between the risk categories (success and nonsuccess). The outcome will be used to estimate the categorical NRI, and two comparison tables will be constructed separately according to whether the event of interest has been recorded for the patients.
Similarly, the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) will also be evaluated (the lowest value obtained will define the best model) for selection.
The bootstrap resampling method will be used with 1000 simulations. Resampling methods are based on repeatedly extracting samples from a training dataset, which will be the development sample, adjusting the model of interest for each simulated sample. Using the bootstrap method, we can evaluate the accuracy of a model’s estimation by emulating the process of obtaining new samples using the available data.
Prior to enrollment, written informed consent will be obtained from all participants in accordance with ethical research standards. In cases involving minors, informed assent will be secured in addition to the written consent provided by their legal guardians, ensuring compliance with applicable regulatory and ethical guidelines.
The research project will be conducted at each center once authorization has been obtained from the respective ethics committee. It is essential that each patient provides their informed consent, as well as informed assent in the case of minors, if applicable.
In this project, we will comply with the current national and international regulations on research, adhering to the recommendations of the Nuremberg code (1946), the UNESCO Universal Declaration on Bioethics and Human Rights (2005), and the Helsinki declaration (2024), followed by international ethical guidelines for health-related research in the aspects that correspond to this project.
In prospective cohort studies, selection biases are rare, since the selection and recruitment of the population occurs before the outcome of interest, so it can be assumed that the selection of participants is carried out independently of the event. However, a referral bias may occur because, as the centers participating in the study specialize in AD, there would be a potential risk of including more severe cases than mild ones. However, for the study, this does not negatively affect the prognostic model since it is specifically intended for use by AD specialists and pediatricians; therefore, the distribution found represents that of the model’s end users.
Another potential selection bias is that a census will not be conducted for the sample size, affecting the results’ generalizability. However, if the model performs well before being used in clinical practice, it requires external validation in a different cohort.
The subjects surveyed may not have understood and answered the study questions correctly. To control for this bias, the participants will be trained to ensure that the questionnaires are carried out clearly and adapted to the different ages of the patients. The data provided by the patients will also be verified using the medical records kept at the participating institutions.
AD is one of the most common skin diseases in childhood and significantly affects the quality of life of patients and their families on a personal and social level.38–40
Currently, management guidelines focus on recommending therapeutic steps based on the risk-benefit balance, but do not consider specific aspects of each patient.4,5,41–43 Precision medicine seeks a medical approach in which the information of each patient defines more appropriate management.
This approach has multiple advantages for patient spatiality.
▪ It could offer a more rapid clinical control.
▪ Allows the most appropriate management to be selected for each patient.
▪ Avoids selecting therapies with a low probability of success and reduces the therapeutic trial period.
▪ Allows faster treatment adjustments.
▪ Generates economic and time-saving benefits.
▪ It helps to profile phenotypes and endotypes.
Although several biomarkers seem to be associated with the clinical response to topical treatment in AD patients, none are sufficient to correctly predict symptom control.9–11,13–17,31 Some prognostic models have been developed for AD but have generally focused on predicting clinical remission or progression of the disease.
With this protocol, we present for the first time the development of a prognostic model that could be useful for the selection of therapies in AD patients. If the model has a high predictive performance, patients identified as unlikely to respond to topical therapy could be offered systemic treatment early and the therapeutic evaluation period with topical therapies could be reduced or avoided. On the other hand, if the patient has a high probability of response to topical treatment, aspects of adherence can be reinforced, and a longer therapeutic evaluation period with topical therapies can be offered.
Both the candidate and exploratory variables included in the protocol allow the study of different clinical and molecular aspects of atopic dermatitis. With the development of this model, it is possible to explore the characteristics of patients with AD that could be related to specific endotypes or phenotypes, allowing the construction of clinical clusters with different clinical evolution and medical needs.19
In this protocol, we have applied the methodological recommendations made by the PROGRESS strategy (Prognosis Research Strategy) and the TRIPOD statement (Transparent Reporting of a multivariate prediction model for Individual Prognosis or Diagnosis). Additionally, suppose the model presents a high prognosis performance in the internal validation. In that case, it is planned to subsequently carry out an external validation that allows evaluating aspects such as reproducibility and transportability.18,22,44
In conclusion, we propose a protocol for creating a multivariable prognostic model focused on predicting the response to topical treatment in patients with AD. From this model, new lines of research can emerge and contribute to both external validation of the model and a better understanding of the disease according to its clinical and molecular characteristics.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
No
Are sufficient details of the methods provided to allow replication by others?
No
Are the datasets clearly presented in a useable and accessible format?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Allergy, Immunology, Infectious Disease, Biologics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 02 Oct 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)