Keywords
Temporomandibular Joint Disorders; Artificial Intelligence; Machine Learning; Deep Learning; Diagnostic Imaging; Osteoarthritis, Temporomandibular Joint; Juvenile Idiopathic Arthritis; Medical Image Processing.
This article is included in the AI in Medicine and Healthcare collection.
With the rapid advancement in artificial intelligence (AI), its integration into medical fields has gained momentum. AI is increasingly applied for diagnosing and managing temporomandibular joint disorders (TMD), affecting one of the most complex joints in the body. This review aims to highlight the AI-driven approaches in diagnosing and treating TMD.
A comprehensive literature search conducted using PubMed, Web of Science, and Google Scholar, focusing on English-language studies published since 2007. References from relevant articles were also examined.
A total of 21 studies met the inclusion criteria, covering diverse AI applications in TMD. These studies included AI-driven medical image segmentation (3), juvenile idiopathic arthritis (3), temporomandibular joint osteoarthritis (TMJOA) detection (4), Temporomandibular joint (TMJ) articular disc displacements and deformities (5), decision support systems (5), and AI-based sound analysis for TMJ assessment (1). The findings suggest that AI, particularly deep learning (DL) and machine learning (ML) techniques, has demonstrated promising accuracy in detecting and classifying TMD and its related pathologies. AI automates segmentation, improves diagnostic consistency, and assists clinicians through predictive modeling.
Despite advancements, challenges remain, as many studies rely on small datasets, limiting generalizability. The absence of standardized evaluation metrics and external validation hinders clinical adoption. Ethical concerns, data privacy issues, and workflow integration pose additional barriers. Future research should focus on larger datasets, model interpretability, and rigorous clinical validation.
AI is emerging as a valuable tool in TMD diagnosis and management, promising improved accuracy and efficiency, but further development is essential for real-world implementation.
Temporomandibular Joint Disorders; Artificial Intelligence; Machine Learning; Deep Learning; Diagnostic Imaging; Osteoarthritis, Temporomandibular Joint; Juvenile Idiopathic Arthritis; Medical Image Processing.
Artificial intelligence (AI) is transforming medical diagnostics by enabling systems to simulate human intelligence for tasks such as pattern recognition, prediction, and decision-making. In medical care, AI subfields, particularly machine learning (ML) and deep learning (DL), are increasingly utilized to process complex datasets and extract clinically relevant information with high speed and precision.1 These advancements have shown great promise in fields such as radiology, oncology, and pathology, and are now being explored in dental and maxillofacial medicine.
Temporomandibular disorders (TMD) are a group of musculoskeletal and neuromuscular conditions affecting the temporomandibular joint (TMJ), masticatory muscles, and associated structures. TMDs commonly lead to chronic orofacial pain, joint dysfunction, and reduced quality of life.2 Among the most prevalent orofacial conditions, such as bruxism, trigeminal neuralgia, Bell’s palsy, burning mouth syndrome, and orofacial clefts, TMD stands out as particularly challenging to diagnose and manage. Traditionally, the diagnosis of TMD involves a comprehensive approach that includes detailed medical history, physical examination, imaging methods, and subjective symptoms reported by the patient. Traditional diagnostic methods, including patient history, physical examination, and imaging, are time consuming and subject to inter-clinician variability. These limitations contribute to delayed or inconsistent diagnoses and may impact treatment outcomes.
AI offers significant potential to address these challenges by enhancing diagnostic accuracy and standardization. ML and DL models have been applied in various TMD-related tasks, including segmentation of TMJ structures, detection of osseous degeneration, classification of TMD subtypes, and analysis of joint sounds. These applications can improve early detection, reduce examiner bias, and support clinical decision-making by identifying patterns not easily perceived through conventional methods.1,3
Despite these advances, several barriers limit the widespread adoption of AI in TMD diagnostics. These include the scarcity of large, annotated datasets; lack of standardized performance metrics; concerns about data privacy and model transparency; and limited clinical validation. Moreover, the current literature is fragmented, with many studies focusing on narrow applications without addressing clinical integration.
This review aims to provide insight into AI-driven approaches for the diagnosis and management of TMD, with particular emphasis on diagnostic accuracy, specificity, and precision. It highlights the measurable impact of various AI tools, including convolutional neural networks (CNNs), decision support systems, ROC curve analyses, and a few others, on enhancing diagnostic performance. Additionally, it outlines existing challenges, including data limitations and clinical integration, while discussing future research directions. By synthesizing the available evidence, this review seeks to clarify the potential of AI to improve diagnostic consistency and support more effective clinical practice in TMD management, ultimately contributing to better patient outcomes.
The authors used the following keywords to search the NCBI database for published literature on TMJ and AI: “Artificial intelligence”, “Temporomandibular Joint,” “Osteoarthritis,” AND “Juvenile Idiopathic Arthritis.” Articles concerning TMJD and AI that were published in English after 2007 were included. The following criteria were used to exclude articles from this review; studies published before 2007, publications not available in the English language, studies that addressed (TMD) in general without incorporating any aspect of artificial intelligence (AI), and articles that lacked a clear focus on the integration or application of AI technologies in the diagnosis, assessment, or management of TMD. These criteria were used to ensure that the collected literature was directly relevant to the scope and objectives of this review, which focus on the role of AI in improving knowledge and clinical management of TMJ-related diseases. The authors first scanned the titles and abstracts for thirty-four publications. After reviewing the full text papers, twenty-one were shortlisted for this review.
TMD diagnosis
Diagnosing TMD is challenging because its symptoms overlap with other conditions. Accurate diagnosis requires a combination of patient history, physical examination, and imaging. History should address the chief complaint, prior trauma, infections, degenerative or arthritic diseases, systemic factors, and lifestyle aspects such as stress, sleep, and recreational activities, which can influence symptom severity.4 During the COVID-19 pandemic, 51.4% of patients reported worsened facial pain and significantly higher bruxism rates (p < 0.001), highlighting how psychological stress can exacerbate TMD beyond purely physical causes.5
TMJ examination involves dental evaluation, occlusal analysis, and palpation of the mandibular condyle with approximately 1 kg of pressure to assess tenderness. Mandibular function should be recorded, including mouth opening and closing, range of motion (protrusion and lateral excursions), and joint sounds, with brief sounds indicating clicking and prolonged sounds indicating crepitus. Bilateral palpation of the masticatory muscles is performed to identify trigger points and muscle tenderness.6 Imaging is indicated when clinical findings are inconclusive, using panoramic or cone-beam computed tomography for hard tissues and magnetic resonance imaging for soft tissue assessment.4
TMD is classified using several systems. The Helkimo clinical index assesses severity through three sub-indices: anamnesis for symptoms, dysfunction for mandibular mobility and muscle tenderness, and occlusion for dental assessment.7 The Wilkes classification, refined by Dimitroulis in 2012 into five stages, guides treatment based on clinical, radiological, and surgical findings.8,9
The RDC/TMD, introduced in 1992, established a dual-axis system: Axis I categorized patients into muscular disorders, disc displacement, and joint disorders based on clinical and radiological findings, while Axis II assessed psychosocial factors and pain-related disability.10 The criteria were updated in 2014 as DC/TMD, retaining the dual-axis framework but refining assessment algorithms and expanding tools to evaluate pain behavior, psychological status, and psychosocial functioning.2,9
In 2020, an international collaboration including OFHP SIG, INfORM, AAOP, and IHS released the first edition of the the International Classification of Orofacial Pain (ICOP), classifying all orofacial pain, including TMD-related pain, while excluding non-painful conditions. Painful TMDs are categorized as myofascial orofacial pain and TMJ pain, divided into primary and secondary disorders, with primary pain further classified as acute or chronic using a three-month threshold. Both types may be subclassified based on pain referral during palpation, following IASP definitions for primary and secondary pain.11
TMD can progress to advanced stages requiring treatment that depends on the diagnosis.12 Early-stage TMD is typically managed conservatively with a soft diet, drug therapy, physiotherapy, and occlusal guards tailored to each patient. Patients with anterior disc displacement who do not respond to conservative therapy may require minimally invasive procedures such as arthroscopy or arthrocentesis.13,14 Both techniques reduce joint friction by releasing adhesions, removing inflammatory mediators, and increasing joint space.15 Arthrocentesis involves single- or double-needle lavage with physiological saline or Ringer’s solution, while arthroscopy additionally allows direct visualization of intra-articular structures, fibrocartilage degeneration, and pathologies not detectable by MRI.16
Advanced TMD may require synovectomy, discectomy, disc plication, eminoplasty, eminectomy, or open joint surgery, with total joint replacement as a last resort.12 In 2025 systematic review of 684 videos and 550 social media discussions found that non-professional content, while more engaging, was generally low in quality (44.1% poor, 11.9% good), whereas professional content was more reliable but less engaging. Most content focused on treatment, with fewer than 6% addressing complications or prevention, highlighting the risks of misinformation and the need for evidence-based content in collaboration with healthcare professionals and influencers.17
AI is a branch of computer science focused on simulating intelligent behavior in machines, enabling them to learn from data, adapt to new environments, recognize patterns, make predictions, and understand complex relationships.1
ML is a type of artificial intelligence that uses data to improve performance without deliberate programming and Samuel defined it in 1959 as a field of study that enables computers to learn from experience without being explicitly programmed.18 The aim is to develop automated systems that recognize patterns, predict outcomes, and make data-driven decisions.ML can be categorized into three categories: supervised, unsupervised, and reinforcement learning. The model is trained on annotated or labeled data, with every input linked to the associated output. Unsupervised learning examines unlabeled data to uncover patterns and relationships among them. Reinforcement learning comprises directing an agent to interact with the environment and gathering information via feedback, such as punishments or rewards.
Traditional ML algorithms, which predate deep learning, use statistical approaches rather than deep neural networks. Although traditional ML methods have been successful but have not performed well with image and audio data. The success of Krizhevsky and colleagues in the 2012 ImageNet Large Scale Visual Recognition Challenge (ILSVRC) sparked interest in the emerging field of deep learning algorithms. Consequently, researchers have commonly used DL methods in their studies.1
DL is a subset of ML that uses artificial neural networks with multiple hidden layers, earning the name ‘deep’ due to their layered structure. This framework, a branch of artificial intelligence trains algorithms to recognize patterns and representations in data. Its capacity to learn complicated features from raw data has rendered it popularity and made it a useful tool. DL uses Artificial Neural Networks (ANNs) to replicate neural connections similar to those found in the brain. These networks consist of interconnected neurons or nodes that process and transfer data to the next layer. The term “deep” refers to networks that have multiple layers and can learn abstract and complex representations of input data. Deep learning has shown remarkable results in a variety of applications, including image processing, voice recognition, and natural language processing. Its ability to handle complex patterns and massive amounts of data has resulted in widespread usage across a variety of industries.1
Individuals with TMD may experience a range of symptoms such as intense headaches, jaw pain, limited mouth opening, and tinnitus. The most common joint abnormalities in TMD are articular disc displacements and deformations. In the following section, we will present a meticulous review of studies that used artificial intelligence in diagnosing TMD. A study done in 2021 identified anterior disc displacement (ADD) utilizing 9009 sagittal TMJ MRI images. The accuracy and Area Under the Curve (AUC) were the comparative parameters to evaluate the optionality of severe complications after treatments. Deep learning models were created utilizing a Residual Networks (ResNet) based convolutional neural network (CNN) and a variety of approaches, including 5-fold cross-validation, data augmentation and oversampling. The maximum open mouth position model demonstrated high accuracy of 0.970 (±0.007) and AUC values of 0.990 (±0.005). In closed mouth position models, the diagnostic Criteria one accuracy was 0.863 (±0.008) and AUC was 0.922 (±0.009). The heat map showed the correct ADD and non-ADD. The ADD group’s heat map focused on the joint disc, condylar head, and posterior disc tissue, whereas the N-ADD group’s heat map focused on the condyle area in the open mouth position. The CNN model detects ADD with high accuracy and AUC, making it beneficial to clinicians to identify ADD before orthodontic therapy and improve treatment outcomes.19
Orhan et al. evaluated the efficacy of a ML algorithm for detecting TMJ abnormalities in MRI images. The study included 214 TMJs from 107 patients with signs and symptoms of TMD. The researchers used a radiomic system to detect imaging features linked with TMJ abnormalities, modifications in the condylar bone and disc displacements. The radiomic features were then determined using a variety of ML algorithms, including Logistic Regression (LR). DT, Random Forest, k-NN, SVM, and XGBoost classifiers were used to predict TMD pathology. Specificity, sensitivity, and Receiver Operating Characteristic (ROC) curves were used to evaluate the efficacy of various techniques. Experimental results revealed that k-nearest neighbors and random forest classifiers are the most effective machine-learning methods to assess ADD and condylar bone.20
A study done in Brazil by Diniz et al.,21 aimed to evaluate the performance of three ML techniques (radiomic, semantic, and radiomic-semantic) for detecting TMD in Infrared Thermography (IT) images. Radiomics in medical imaging entails extracting an enormous number of quantitative elements from medical images via data-characterization algorithms. Semantic features refer to high-level, human-readable features that are frequently manually annotated by specialists, such as size, location, and other clinically significant characteristics. Radiomic-semantic is basically the combination of both approaches to leverage the strengths of both high-dimensional quantitative data and high-level interpretative data. The study included 78 patients who met the Fonseca questionnaire and RDC/TMD criteria, 37 individuals were control individuals and 41 TMD patients. The approach involved obtaining each patient’s IT lateral projections and selecting the masseter and temporal muscles for the attributed extraction. The data was analysed using the classification algorithms support vector machine (SVM), Kernel nearest neighbor (KNN), and multilayer perceptron (MLP)Hopkins’ statistic, as well as the Tukey tests, Shapiro-Wilk and ANOVA. Semantic and radiomic-semantic association accuracy, precision, and sensitivity were significantly different from radiomic features (p = 0.008, p = 0.016, and p = 0.013, respectively). The radiomic-semantic association showed a statistically significant difference in testing accuracy value (p = 0.003). MLP stands out from the other classifiers in terms of radiomic-semantic association (p = 0.004). Accordingly, this study suggested detecting TMD via employing semantic and radiomic-semantic-associated ML feature extraction algorithms, as well as an MLP classifier, along with IT images and pain scale data.21
Kim et al.,22 published a study that aimed to establish a deep learning-based approach for predicting TMJ disc perforation using MRI data, and to confirm its performance by comparing it to previously published results. Data was collected by analyzing medical records at the Department of Oral and Maxillofacial Surgery, Gangnam Severance Hospital, Korea between January 2005 to June 2018. It included 299 joints from 289 patients and was divided into a perforated group and non-perforated group based on disc perforation detected during surgery. Experts evaluated the TMJ MRI scans to detect distinguishing features. Data containing these attributes were used to create and verify prediction models using random forest and MLP techniques, were MLP technique being based on the Keras framework, a new deep learning technology. The models’ performances were compared using the AUC curve. MLP scored the highest AUC (0.940), being followed by random forest (0.918) and disc shape alone (0.791). The MLP and random forest also previously published results utilizing MRI (AUC 0.808) and MRI-based nomogram (AUC 0.889) Figure 1. When predicting TMJ disc perforation, deep learning outperformed conventional techniques and previous findings.22
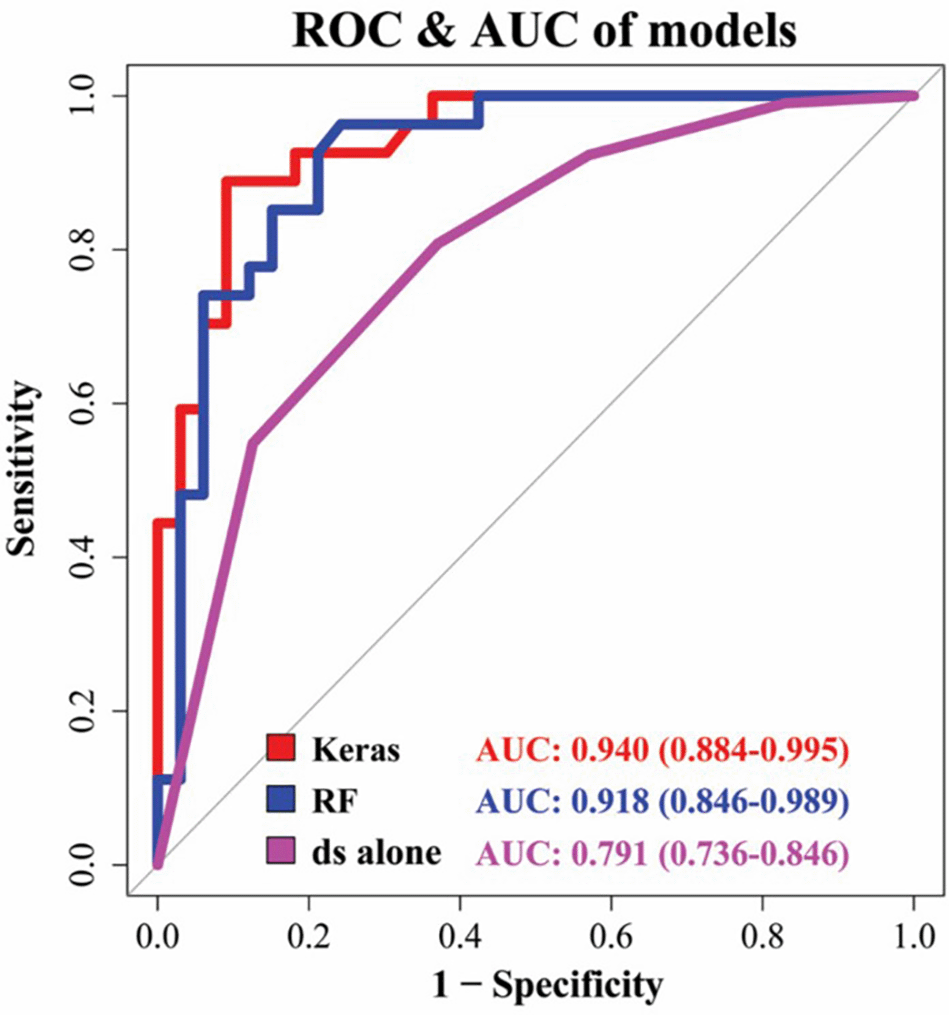
Multilayer perceptron had the highest AUC (0.940), being followed by random forest (RF) (0.918) and disc shape (ds) alone (AUC 0.630). Image adapted from Kim, J. Y. et al.22
In Korea, Lee et al.,23 utilized AI to investigate whether psychosocial and biological factors influence the onset of TMD. The study investigated factors like stress, socioeconomic status, and working conditions as potential causes of TMDs. This study included data from 4744 participants, who filed information on their TMD status, and 37 independent factors, including socioeconomic position, demographic status, working conditions, stress, and health determinants. Researchers employed six AI methods to identify variables associated with TMJ disorders, including random forest, decision trees, naïve Bayes, logistic regression, SVMs, and ANN. Following that, predictive performance and the models’ accuracy were assessed. The highest average accuracy identified using methods such as logistic regression, artificial neural networks, random forest and support vector machines, for both reported TMD (r-TMD) and diagnosed TMD by a doctor (d-TMD), the highest AUC was observed using an artificial neural network and logistic regression for r-TMD and d-TMD, respectively.23
JIA is the most prevalent rheumatic condition in children. The International League of Associations for Rheumatology (ILAR) defines JIA as “arthritis of unknown etiology persisting for more than 6 weeks with an onset at 16 years of age or younger, after excluding other causes of joint inflammation”.24 Juvenile idiopathic arthritis can produce abnormal facial growth, pain, and impaired jaw function.25 To diagnose JIA, a medical history, physical examination, and laboratory investigations are conducted to rule out other possible causes and confirm the inflammation in the joint. Early diagnosis and treatment are critical for reducing joint injury and improving long-term results.25
Erika Van Nieuwenhove and colleagues investigated the use of machine learning (ML) to identify immunological signatures in juvenile idiopathic arthritis (JIA) patients. They conducted comprehensive flow cytometry profiling of the adaptive immune system in 85 JIA patients and 43 age-matched healthy controls. The results revealed distinct immunological differences between JIA patients and healthy individuals, with consistent immune alterations observed across all JIA subtypes. These alterations were especially pronounced in patients with systemic JIA and those with active disease, indicating a shared immunological mechanism underlying the various clinical forms of JIA.
To analyze and classify the immunological data, the researchers employed three ML models: random forests, artificial neural networks (ANNs), and potential support vector machines (pSVMs). The models were evaluated using tenfold cross-validation to ensure robust generalization. Random forests were implemented in R using the ‘randomForest’ package and included 10,001 trees with various hyperparameter and sampling scheme configurations to address class imbalance. ANNs were developed in Python using the Keras library with a TensorFlow backend, testing 672 hyperparameter combinations that varied in architecture, learning rate, dropout, momentum, class weighting, and regularization. The networks used ReLU activations in hidden layers and a sigmoid activation in the output layer, optimized using stochastic gradient descent and binary cross-entropy loss. The pSVMs, selected for their performance on imbalanced data, were tested in dyadic mode with different cost and shrinkage parameters, both with and without balancing. All models were assessed primarily based on the area under the ROC curve (AUC), and they achieved over 90% accuracy in distinguishing JIA patients from healthy controls. These findings highlight the potential of machine learning to enhance the diagnosis and understanding of immune mechanisms in JIA.26
Another study investigated the design and implementation of an AI-driven system to improve patient education for families struggling with JIA. This was accomplished by utilizing an AI-based argumentation theory to generate interactive educational conversations via using the Toulmin model of argument, a framework for constructing arguments, to transform Patient Education Materials (PEM) into a dialogue system. This paradigm assists in managing the content and structure of educational conversations, making them more dynamic and user-friendly. Educational information is organized as arguments with evidence-based suggestions. The dialogue system enables users to interact with information in a contextually appropriate and timely manner, resulting in improved comprehension and management of the condition. The dialogue system’s effectiveness was assessed using intellectual walkthroughs and semi-structured conversations with JIA topic experts. These studies yielded encouraging results, demonstrating that the system can effectively provide quality information to families when needed.27
The pathogenesis of TMJOA is defined by gradual cartilage deterioration, subchondral bone remodelling, and chronic synovial tissue inflammation. An increasing number of research has lately concentrated on subchondral bone remodelling and inflammation in the initial stages of TMJOA, which could offer light on the likely mechanism of initiation and development of TMJOA. The treatment approach for TMJOA focuses on decreasing discomfort and pain, halt subchondral bone and cartilage degradation, and improve the function of the joints. The most common treatments for TMJOA are nonsteroidal anti-inflammatory medications, physical therapy, splints and minor surgical intervention that includes low-energy laser and arthrocentesis. While these interventions are generally effective at treating symptoms and signs, their long-term therapeutic effects on degenerative articular structures are insufficient. As of now, there is no treatment for TMJOA damage that can reverse it. Therapies that stop the progression of subchondral bone damage and cartilage degeneration must be investigated, and TMJ regeneration may be the best long-term solution.28
In the University of Michigan, Bianchi et al.29 discussed the potential of early diagnosis of TMJOA using biomarkers and machine learning, via understanding the interactions between disease and health-related biomarkers. Biomarkers such as C-reactive protein (CRP), interleukins, and matrix metalloproteinases (MMPs) show potential in indicating early stages of OA and they are available in saliva, blood and synovial fluid. Advanced data science was employed to evaluate fifty-two clinical, biochemical, and high-resolution images CBCT (radiomics) parameters comprising TMJOA patients and control groups. The diagnostic effectiveness was assessed using four ML models XGBoost, logistic regression, lightGBM, and random forest. The XGBoost and the LightGBM model achieved (0.823) accuracy, F1-score (0.823) and AUC (0.870) in diagnosing TMJ OA Figure 2.29
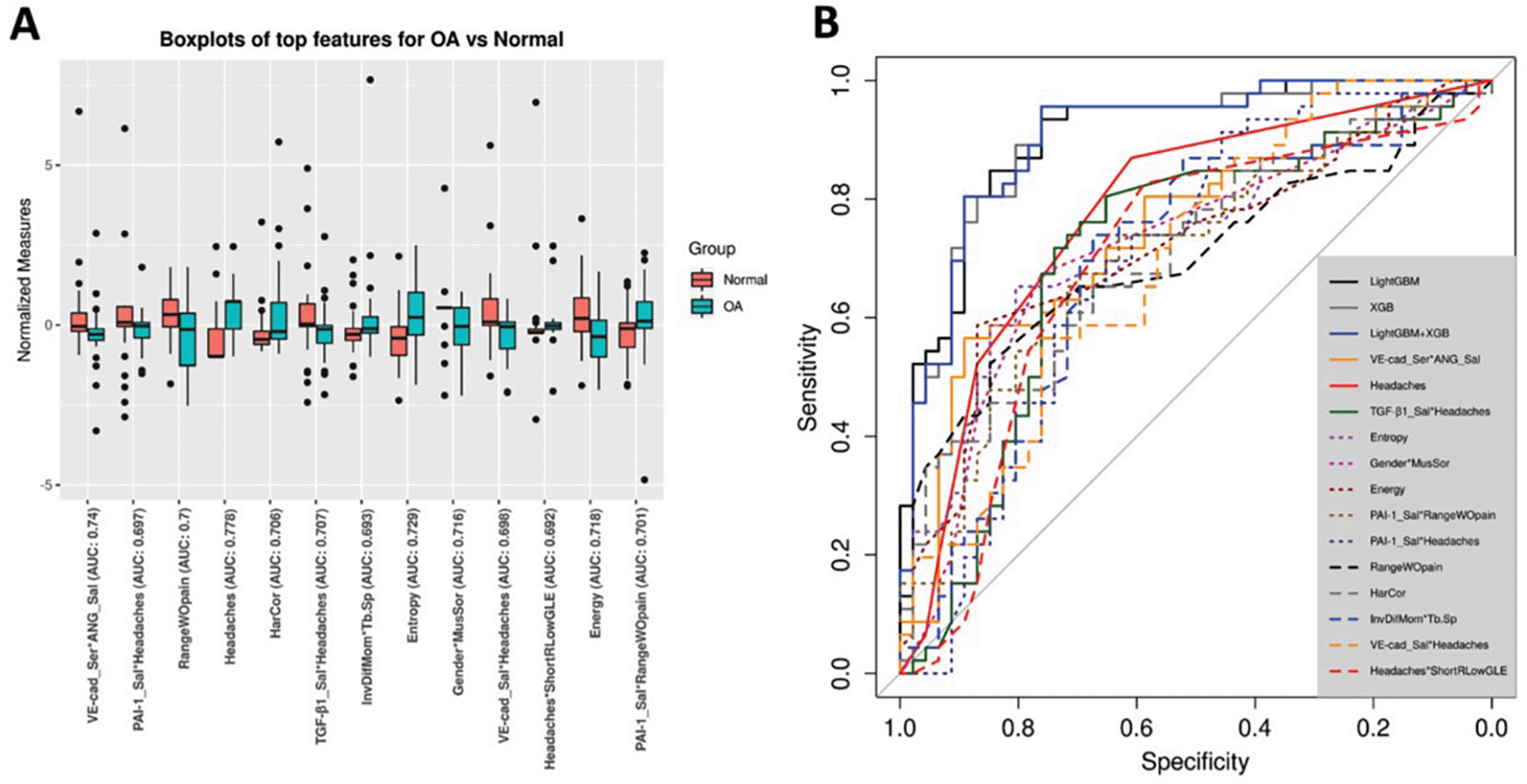
Image adapted from Bianchi, J et al.29
In 2020, Lee et al employed artificial intelligence to detect TMJOA in CBCT images from patients with TMJ dysfunction. Single-shot detection, an object detection approach, was optimized on 3514 sagittal CBCTs. TMJ radiographs revealed osseous variations in the condylar area. The relevant area (Condylar head) was characterized and divided into two categories, indeterminate for TMJOA and TMJOA based on the radiographic image analysis criteria for TMD diagnosis. The model was evaluated with two sets of 300 images in total. The average precision, accuracy, F1 scores, and recall for the two test groups were 0.85,0.86, 0.84, and 0.84, respectively. A deep neural network model can automatically identify TMJOA from sagittal CBCT images, which can greatly support the clinical diagnosis.30 Employing the same concept, in 2023, another study was done to use AI to classify TMJOA using (CBCT) sagittal images. The efficacy of the YOLOv5 architecture, a model of artificial intelligence, in segmentation TMJ and classifying osteoarthritis was assessed using 2000 sagittal sections (500 healthy, 500 erosion, 500 osteophytes, and 500 flattening images) from 290 patients’ CBCT DICOM images. The classification model predicts healthy joints with an 88% accuracy, 70% flattened joints, 95% erosion, and 86% osteophytes. The YOLOv5 model for TMJ segmentation has an F1 score of 0.99976, sensitivity of one, and precision of 0.9953. The model for TMJ segmentation has an AUC of 0.9723. Additionally, 0.9953 model’s accuracy in TMJ segmentation was found. The AI model utilized in this research can assist clinicians in saving time and providing convenience in disease diagnosis, with excellent results in TMJ segmentation and osteoarthritis classification.31
In the United Arab Emirates in 2023, Talaat et al.,32 published a study that aimed to create a DL-based model to accurately and efficiently identify TMJOA from 2737 CBCT images of 943 patients. They utilized a single convolutional network as a model base and a regression model for object detection. Two experienced evaluators conducted a DC/TMD assessment to generate a model-testing collection of 350 images, with the final diagnosis considered as the gold standard. The model’s diagnostic capabilities were compared to those of an experienced oral radiologist. The AI diagnosis revealed a substantially higher agreement with the golden reference. Cohen’s kappa revealed statistically significant differences in the AI and radiologist agreement with the gold standard for diagnosing subcortical cysts (P=0.0214) as well as all the remaining signs (P=0.0079), Table 1. In comparison to the human expert, the AI model utilized in this study performed equally well or better in the diagnostic process for TMJ osteoarthritis. They concluded that the use of AI in radiographic diagnosis of osteoarthritis is anticipated to speed up the diagnostic process and minimize the subjectivity involved in human interpretation.32
| Percentage of agreement (95% confdence limits) | Cohen’s kappa | P value | |
|---|---|---|---|
| Condylar fattening | 98.57% (97.33–99.81%) | 0.95 | <0.0001** |
| Subcortical cyst | 97.43% (95.77–99.09%) | 0.95 | <0.0001** |
| Surface erosion | 99.71% (99.16–100.00%) | 0.96 | <0.0001** |
| Osteophyte | 99.71% (99.16–100.00%) | 0.96 | <0.0001** |
| All signs | 98.86% (98.30–99.41%) | 0.96 | <0.0001** |
** Statistically.32
A research paper published in 2021 described a unique decision support system for detecting TMD that used deep learning and neural network techniques. A non-invasive apparatus was developed to record TMJ sounds. An interface was created that allowed the dentist to work on the captured audio data. The collected data comprised patient’s left and right TMJ sounds, ambient noise sounds, clinical data, and comments from the dentist about the patient’s diagnosis and therapy. The readings were subsequently categorized using artificial neural networks, signal processing, and DL algorithms, which resulted in the development of a system that determined the patient’s condition. Classification success was compared across frequency, statistical, and deep learning methods. The results revealed that the deep learning-based classification approach consistently outperforms the previous two methods, with a classification success rate of over 94.5 %. The proposed system can provide physicians with information regarding the efficacy of treatment approaches used on patients to address joint sounds, which are one of the most common symptoms of TMD.33
A cross-sectional study done by Waked et al. aimed to design a model with predictive skills that uses a statistical analysis classification tree to forecast the presence of TMD by categorizing participants as high or low risk of having the condition. A total of 776 persons requested dental or medical care in Brazil, Recife’s Family Health Units. The sample has been submitted to anamnesis using RDC/TMD. The data was imported to Statistical Package for the Social Sciences 20.0 software and processed for bivariate analysis using the Pearson Chi-square test and the multivariate analysis for the categorical tree method. Orofacial pain, aging, and depression may all be indicators of TMD. Individuals with orofacial pain, those aged 25 to 59, and those suffering from depression made up the high-risk group. Individuals who did not have orofacial pain made up the low-risk group. The authors concluded that orofacial discomfort was the best predictor of TMD and that the classification tree’s prediction model might be used as a tool to facilitate decision-making for TMD patients.34
Additional research concerning dentistry covering the clinical decision support systems includes Mago et al.35 Polášková et al.,36 Mendonça,37 and Machoy et al.38 Here is a summary of the articles mentioned in Table 2.
| Data set | Method | Outcome | Reference |
|---|---|---|---|
| 9009 sagittal TMJ MRIs for patients diagnosed with anterior disc displacement in one or both joints | Deep learning models based on the ResNet architecture and the Imagenet database were generated using a convolutional neural network (CNN), which included fivefold cross-validation, oversampling, and data augmentation. Then the accuracy under the carve (AUC) and accuracy were determined. | The maximum open mouth position model showed high accuracy values of 0.970 (±0.007) and AUC values of 0.990 (±0.005). The closed jaw position ranged between 0.863 (±0.008) and 0.922 (±0.009). The CNN model detects ADD with great accuracy and AUC. | 19 |
| A total of 107 patients with TMJ signs and symptoms were included (214 TMJs) | Machine learning algorithms, including Logistic Regression (LR). DT, k-NN, SVM, Random Forest, and XGBoost classifiers | k-nearest neighbours and random forest classifiers are the most effective in predicting temporomandibular displacements and condylar changes | 20 |
| 41 patients with TMD and 37 individuals as a control group | Lateral infrared thermography projections (temporal muscles and masseter) were chosen to assess radiomic, semantic, and radiomic-semantic correlations using KNN algorithms, MLP-Hopkins’ statistics, SVM, Shapiro-Wilk and Tukey tests ANOVA. | Semantic and radiomic association accuracy, precision, and sensitivity were significantly different from radiomic features (p = 0.008, p = 0.016, and p = 0.013, respectively). | 21 |
| 299 joints from 289 patients classified either perforated or non-perforated according to disc perforation during surgery and an MRI reading. | The deep learning method used multilayer perceptron (MLP) techniques and random forest, with the first utilizing the Keras framework. | MLP achieved the highest AUC (0.940), followed by random forest (0.918) and disc shape alone (0.791). The MLP and random forest exceeded previously published results with (AUC 0.808) for the MRI and an (AUC 0.889) for MRI-based nomogram | 21 |
| 4744 TMD patients | Logistic regression, Random forest, decision trees, naïve Bayes, SVMs, and ANN. | For both r-TMD and d-TMD, approaches based on support vector machines, random forest, logistic regression, and artificial neural networks produced the highest mean accuracy. | 22 |
| 85 JIA patients and 43 healthy controls | Machine learning, Random Forests, Artificial Neural Networks and Support Vector Machines | Machine learning models were able to distinguish JIA patients from healthy individuals with an accuracy of over 90%. | 23 |
| Patients’ families | AI-based argumentation theory using the Toulmin model of argument | The dialogue system enables users to interact with information in a contextually timely manner, resulting in improved comprehension and management of the condition | 26 |
| CBCT of 92 patients enrolled (46 TMJOA and aged 46 and sex-matched controls) | Machine learning models: Logistic Regression, Random Forest, LightGBM, XGBoost | The XGBoost + LightGBM model had an accuracy of 0.823, AUC of 0.870, and F1-score of 0.823 for diagnosing TMJ OA. | 29 |
| 413 TMJOA patients included with 3,514 sagittal CBCT images. | Artificial intelligence, Single-shot detection. | The average accuracy was 0.86, precision of 0.85, recall of 0.84, and F1 scores of 0.84. | 30 |
| 290 patients were enrolled, there were 2000 CBCT DICOM images of sagittal sections (500 healthy, 500 erosion, 500 osteophytes, and 500 flattening images) included. | YOLOv5 architecture | The YOLOv5 model for TMJ segmentation has a sensitivity of one, a precision of 0.9953, and an F1 score of 0.9976. | 31 |
| 2737 CBCT images of 943 patients | Single convolutional network and single regression model | Cohen’s kappa revealed significant variations in AI and radiologist agreement with the golden reference when diagnosing all signs collectively (P = 0.0079) and subcortical cysts (P = 0.0214). | 32 |
| 21 healthy and 27 TMJ patients. | Signal processing, artificial neural networks, and deep learning algorithms | Deep learning-based classification approach consistently outperforms the previous two methods, with a classification success rate of over 94.5 % | 33 |
| 776 participants | classification tree statistical analysis | The authors concluded that orofacial discomfort was the best predictor of TMD and that the classification tree’s prediction model might be used as a tool to facilitate decision-making for TMD patients | 34 |
| 100 samples | Mamdani inference algorithm | The Chi-square value of the test is 3.843565 which is less than the critical value which is 12.592. | 35 |
| Review | Decision-making supportive system | Th DSS is a valuable tool for improving decision-making in dental implantology. It aids practitioners by providing evidence-based recommendations and clinical guidance during the planning and execution of dental implant treatments | 36 |
| Review | Clinical decision-support systems (CDSSs) | CDSS have significant potential to improve the quality of dental care. By integrating patient data, clinical guidelines, and decision-making algorithms, CDSS can assist dental practitioners in making more accurate diagnoses, treatment plans, and care decision | 37 |
| Review | machine learning (ML) | ML has a growing and valuable role in modern dentistry, helping to automate complex tasks, improve diagnostic accuracy, and optimize treatment plans based on large datasets. | 38 |
TMDs are complex conditions that often require detailed clinical evaluation and imaging for accurate diagnosis. Traditionally, diagnosis has relied on physical examination, patient symptoms, and interpretation of imaging studies such as X-rays, CT scans, and MRI by oral and maxillofacial surgeon. However, recent advancements in AI are transforming TMJ diagnostics by enabling faster, more consistent, and potentially more accurate interpretations. Table 3, compares the differences between conventional diagnostic techniques and emerging AI-based approaches in TMJ assessment.1,39–41
The comparison delineates the superior capabilities of AI-assisted diagnostic approaches over conventional methods in the evaluation of TMD. AI techniques enhance diagnostic precision, expedite image interpretation, and significantly reduce inter-observer variability. Furthermore, through continuous learning and data integration, AI systems offer a more objective and standardized diagnostic process, thereby augmenting clinical decision-making and potentially improving patient outcome.
The integration of AI into clinical medicine, while holding substantial promise, is encumbered by a range of complex challenges. Chief among these are concerns related to the quality, security, and privacy of medical data. The effective training of AI algorithms necessitates access to extensive datasets; however, the fragmented nature of healthcare systems and stringent regulatory frameworks such as the Health Insurance Portability and Accountability Act (HIPAA) and the General Data Protection Regulation (GDPR), significantly limit data sharing and interoperability across institutions. Nonetheless, several barriers persist. Notable among these are persistent data privacy concerns, the risk of algorithmic bias, and a general lack of trust from clinicians, particularly due to the opaque nature of so-called “black box” AI models.42
AI has demonstrated remarkable utility across numerous medical specialties, notably in radiology and pathology, by enhancing diagnostic precision, enabling early disease detection through predictive analytics, and facilitating personalized treatment strategies through developments in genomics and pharmacology. These advancements have the potential to address critical healthcare challenges, including the growing burden of chronic diseases, shortages of healthcare professionals, and the escalating demand for cost-effective care.43,44
The ethical concerns of using AI in diagnosing TMD disorders revolve around key principles such as patient autonomy and evidence-based practice. AI introduces risks, including lack of transparency that undermines informed consent, potential misdiagnosis due to unvalidated or biased algorithms, and reduced clinician responsibility. On the other hand, AI can detect anomalies that were missed by the physician.AI systems require large amounts of patient data, raising concerns over data handling, storage, and sharing, which may lead to breaches of confidentiality or misuse of data compromising patient trust and violate ethical standards of privacy and professional responsibility. Furthermore, commercialization may lead to prioritizing profit over patient welfare, and data privacy concerns may erode trust.45–47
While AI shows potential in TMD diagnosis and treatment, its integration into clinical practice must consider ethical implications, such as patient safety, clinician oversight, and potential biases in AI models. Future research should focus on ensuring AI tools align with evidence-based TMD management principles and patient-centered care approaches.
DL architectures of AI, is gaining popularity in various industries. DL models have considerable usage in the medical field because of their capacity to generate extraordinary results due to their multi-layered design. Consequently, these models have a widespread application in medical data analysis. However, upon evaluating the literature, dentistry has seen relatively few research using AI compared to the other medical sectors. Diagnosing TMD, which are among the most frequent in the human body, is a challenging task. TMD diagnosed based on medical observations and radiographic interpretations of the physicians. It is vital to highlight that diagnostic processes are time and effort-consuming and because of that, developing an automated diagnosis system is essential. In a review published on 2025, the diagnostic accuracy of AI models was examined. The studies’ accuracy levels ranged from 0.5948 to 1.32,49 Farook and Dudley discovered a sensitivity of 0.63-0.95 using DL models.50
This article reviews computer-based technologies, specifically AI, for diagnosing and treating TMD. It covers segmentation, decision support systems, and speech data investigations, as well as disease-specific applications to ensure comprehensiveness. We used Google Scholar search engines, Web of Science and PubMed to find relevant material. Considering the rise in popularity of approaches to AI in medicine, the major goal of this review is to highlight the substantial contribution of AI in dentistry. Additionally, through providing a comprehensive summary of the most pertinent research, this review seeks to present significant insights that will influence the direction of future studies and improve the efficiency of the literature review process.
A thorough collection of 21 studies was identified, encompassing a wide range of topics. This comprises three segmentation studies, three on JIA, four on TMJOA, five on TMJ articular disc displacements and deformities, five on decision support systems, and one on sound analysis.
Because of the limits of classical ML methods, DL approaches were expected to become more common in segmentation investigations. The scientific literatures in JIA are noticeably weak, with only a few research, the majority of which use ML approaches. In the field of TMJOA research and TMD, DL algorithms have established a greater presence than traditional ML methods. The shift towards the adoption of DL-based methodologies over traditional models is well noticed in the new publications. In studies focused on voice analysis, signal processing techniques are commonly used. Nonetheless, the combined use of both imagery and audio for diagnostic purposes remains underrepresented in the current literature.
The number of published articles on TMD considered relatively minimal. However, as AI, particularly DL, gains prominence, the number of research in this topic is projected to rise in coming years. The main explanation for this low volume of research when compared to others, is the challenge of data availability. Neural networks rely substantially on a wide range of high-quality data effective learning and generalization. Typically, neural networks require large volumes of labeled data to operate properly. Limited accessibility to data can lead to overfitting, which is when the network fails to perform effectively with unseen data. Furthermore, neural networks’ generalization capability improves when the training data covers a wide range of scenarios and variations, emphasizing the importance of data diversity. Without diversity, the network’s ability to deal with unexpected conditions may be limited. As a result, creating a broad and representative training dataset is critical for generating dependable and accurate neural network models.
There are many challenges facing the adoption of AI in the diagnosis of TMJ. A fundamental challenge in this field is the difficulty of developing a comprehensive dataset, as accessibility to data is frequently restricted by security measures, which leads to a limited number of samples. To address these data challenges, researchers and practitioners employ a variety of approaches, including transfer learning, data augmentation, adversarial training and pre-trained models. Data augmentation is the technique of artificially extending the training dataset by applying different transformations to existing samples. including picture flipping, rotation, scaling, shearing, zooming, translation, and color jittering, which are common in computer vision. Additionally, the generation of synthetic data using Generative Adversarial Networks (GANs) is an advanced approach to data augmentation. This innovative technique leverages GANs to generate authentic and heterogeneous synthetic data, which could improve DL model training and addressing data scarcity in different applications such as computer vision, natural language processing and medical imaging. GANs are made up of two competitive networks: discriminator and generator, which are trained together to generate synthetic data. Nevertheless, employing GANs to augment data has its own set of challenges. Obtaining high-quality synthetic data necessitates a properly educated and well-trained GAN, and there is a risk of producing unrealistic or noisy outcomes. This strategy’s effectiveness depends on ensuring the reliability and variety of the generated data.51
Other challenges include the fact that AI networks are often considered “black boxes.” Traditional neural networks, particularly DL models, are characterized by their complex mathematical transformations and the use of high-dimensional data, which make their decision-making processes difficult to understand. Although these models perform exceptionally well in natural language processing, image recognition, and speech recognition, their increasing complexity makes the task hard to interpret their predictions. As these networks acquire information from input data and modify internal parameters (such as weights and biases), they form complex structures that are difficult to comprehend. This complexity makes it difficult to pinpoint features or patterns in the data that lead to certain predictions. The lack of interpretability is particularly concerning in vital industries like medical care, financial services, and auto-driving cars, where transparent and understandable decision-making is required.52
In order to solve this “black box” problem, researchers established a new concept termed as “Explainable AI” (xAI), a subset of AI research aimed at improving the interpretability and transparency of AI systems. The fundamental purpose of xAI is to present comprehensive justifications and explanations for AI model decisions and forecasts, allowing a better comprehension and understanding of the results for both individuals and stakeholders. This is particularly crucial in areas where AI decisions have a large impact on individuals and society. xAI frequently uses techniques like feature rule-based models, visualization, saliency maps, feature attribution, and contrastive explanations. These methods seek to illuminate on the internal workings of AI models and the factors that influence their forecasting abilities, allowing users to build trust and confidence in AI systems.52
Additionally, AI, specifically ML and DL-based algorithms, has a substantial impact on medical applications. The rapid development of new algorithms in this field has been extraordinary. The use of DL techniques on dental photos has the potential to automate inferences in the future. ResNet introduces layer-level connections that prevent the vanishing gradient issue in DL networks, enabling efficient and rapid learning of intricate patterns in complex datasets like MRI or CBCT scans. Its deep architecture captures both fine-grained and large-scale structural features, enhancing its capability to detect subtle abnormalities.53
However, a major challenge hindering its full potential is the scarcity of large, high quality datasets specific to TMD. Most available datasets are limited in size, diversity, and standardization, which increases the risk of overfitting where models perform well on training data but fail to generalize to new, unseen cases. This limitation compromises the reliability and clinical applicability of AI tools. Moreover, variability in imaging techniques, patient demographics, and diagnostic criteria further exacerbates data inconsistency, making it difficult to develop robust, widely applicable AI models for TMD.
While AI has shown high accuracy in image-based TMD diagnosis, it cannot yet replace the clinical expertise of oral and maxillofacial surgeons. Diagnosis often requires integrating patient history, physical exams, and complex data interpretation, areas where current AI systems remain limited and task specific. The comparison table in our manuscript highlights AI’s strengths in efficiency, consistency, and objectivity, suggesting its value as a supportive tool in clinical workflows. Looking forward, the use of Large Language Models (LLM) can help in organising the molecular pathway in a way that AI can easily mine the data and identify the relevant diagnostic biomarker. Another upcoming development is the research in implementing Artificial General Intelligence (AGI) which may eventually emulate human reasoning across broader tasks, potentially matching expert-level diagnostic capabilities, however, AGI remains theory theoretical at this stage. Therefore, AI should currently be regarded as a complementary aid, not a substitute for clinical judgment in TMD diagnosis.
Our present review focuses on TMD, with the aim of contributing to the current knowledge and assisting dentists in establishing the correct diagnosis. We anticipate that our findings will serve as the foundation for future research aimed at creating more precise and dependable models for TMD. These advances will surely enhance patient care and significantly contribute to the oral health field.
Rapid technological breakthroughs have altered several fields, including medicine, where computer-based research and AI-driven algorithms have grown in prominence. This review investigated the use of computer-based and AI approaches in the diagnosis and treatment of TMD. The evaluation revealed that there are insufficient studies in this field. The fundamental causes of this constraint were investigated, and some solutions were given, such as using xAI to improve the interpretability of the created AI and provide contrastive explanations. Another solution is to use GANs to generate a large dataset and train deep learning models more effectively. Fortunately, considering the current surge in published research and continuing efforts to solve these problems, it is expected that new studies will emerge in this area, resulting in significant expansion in both the quantity and quality of research.
This Figure 3 compares existing diagnostic models with developing AI-based techniques for diagnosing TMD.
The dataset supporting the results and analyses of this article is openly available in Zenodo at https://doi.org/10.5281/zenodo.17069908.54 The repository includes the extracted sample sizes, AI/ML methods, outcome measures, and variables (e.g., study references, conditions studied, diagnostic accuracy values) used in the review. The dataset file (“TMD_AI_Review_Data.xlsx”) is made available under a CC0 license to permit unrestricted reuse. All other data supporting the findings are available within the article and its referenced sources.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)