Keywords
Oral Health Research, Eastern Mediterranean Region, Dental Public Health, Scoping Review
Oral health is fundamental to general health and well-being, yet it remains a neglected priority in health systems, particularly within the World Health Organization (WHO) Eastern Mediterranean Region (EMR). Oral diseases affect an estimated 330 million people (46.5%) across the region, driven by unaddressed risk factors, inequalities, and weak integration into the Non-Communicable Diseases (NCD) and Universal Health Coverage (UHC) agendas. Despite this high burden, oral health initiatives to address this burden and prioritize the oral research agenda remain limited. This scoping review represents the first comprehensive effort to systematically map and identify the status of Oral Health Research (OHR), including scope, output, and collaboration within the WHO EMR (hereafter, EMR) between 2014 and 2024.
A comprehensive search strategy was developed in Ovid MEDLINE and translated into Embase, Cochrane Library, and Epistemonikos, and will be applied to global and regional databases. Searching covers January 2014 to December 2024 without language restrictions. Eligible studies will include primary and secondary research covering clinical, public health, and health systems research. Excluded will be in vitro, in vivo, case reports, protocols, editorials, and commentaries. Screening and data extraction will be managed using DistillerSR software. Extracted data will be analyzed in RStudio, using descriptive statistics, collaboration mapping, and interactive visualization.
This scoping review will provide the first comprehensive mapping of OHR across the EMR. The review will highlight oral health research strengths and gaps at regional and country-specific levels by examining research topics, study designs, funding sources, and collaboration patterns. These findings will guide policymakers, researchers, and academic schools and institutions to prioritize the OHR agenda and inform the development and enactment of oral health policies. Results will be disseminated through peer-reviewed publications, conference abstracts, and regional interest-holders engagement.
The protocol is registered with the Open Science Framework (OSF): https://doi.org/10.17605/OSF.IO/MXWKC.
Oral Health Research, Eastern Mediterranean Region, Dental Public Health, Scoping Review
The EMR office is one of the WHO’s six regional offices worldwide and oversees “… 21 Member States and occupied Palestinian territory (including East Jerusalem.)”1 Specifically, the EMR countries include Afghanistan, Bahrain, Djibouti, Egypt, Islamic Republic of Iran, Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Pakistan, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syrian Arab Republic, Tunisia, United Arab Emirates, and Yemen ( Figure 1).1,2 The region has a population of approximately 745 million people with vast cultural, economic, and societal differences.1
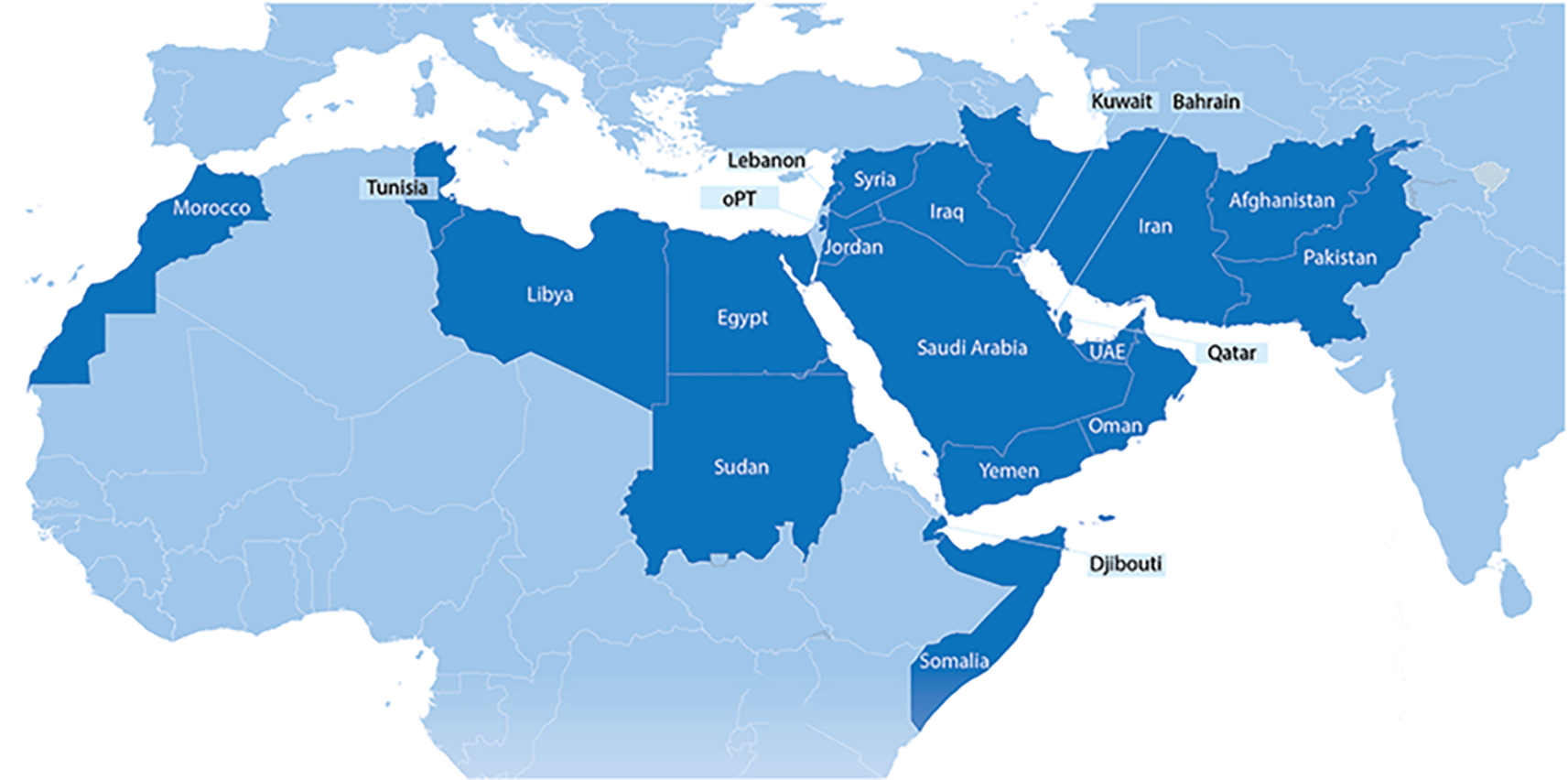
OPT: Occupied Palestine Territory, UAE: United Arab Emirates.
The burden of oral diseases is growing globally due to population growth (in low and lower-middle-income countries), inequalities, unaddressed risk factors, and social, commercial, and economic determinants of health.3 The burden of oral disease in the EMR affects around 330 million (46.5%) of the population.4 These oral diseases can be prevented and if present, effectively treated. However, the knowledge gap of OHR’s current status to prioritize initiatives and the lack of integration of oral health into the NCDs and UHC agendas weaken the oral health of the EMR population.3,5 Despite oral health’s integral role in overall health, oral health has been underprioritized within healthcare systems and policies at global, regional, and national levels.
The last coordinated regional initiative aiming to advance oral health, including research and policies, took place in Iran in 2012,6 where experts met to finalize a regional strategy for oral health promotion. Building on those early efforts, significant gaps remain in aligning the oral health research agenda and priorities with the evolving needs of the EMR. This information gap limits the capacity of EMR countries to address the increasing burden of oral diseases. Therefore, this scoping review will identify the status of OHR in the EMR from 2014 through 2024.
“Oral health is multi-faceted and includes the ability to speak, smile, smell, taste, touch, chew, swallow and convey a range of emotions through facial expressions with confidence and without pain, discomfort and disease of the craniofacial complex (head, face, and oral cavity).”7
“Oral health is the state of the mouth, teeth and orofacial structures that enables individuals to perform essential functions, such as eating, breathing, and speaking, and encompasses psychosocial dimensions, such as self-confidence, well-being and the ability to socialize and work without pain, discomfort and embarrassment. Oral health varies over the life course from early life to old age, is integral to general health and supports individuals in participating in society and achieving their potential.”8
What is the status of oral health research, including scope, output, and collaboration within the EMR from 2014 to 2024?
To determine the status of oral health research, including breadth and scholarly productivity within the EMR from 2014 to 2024.
1. To identify and describe the output of oral health research conducted in or pertaining to the EMR from 2014 to 2024 through scientific publications.
2. To determine and assess the research topics and types, study designs, and funding sources used in oral health research in the EMR from 2014 to 2024.
3. To estimate the extent of national and international collaboration in oral health research in the EMR from 2014 to 2024.
This protocol follows the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P)9,10 and the findings will be reported using the PRISMA-ScR extension for Scoping Reviews.11
Primary and secondary studies addressing OHR topics according to the WHO and the FDI World Dental Federation oral health definitions will be included.7,8,12 The scope of the eligible study should include clinical, public health, or health system-related research, which means studies that can directly inform clinical practice, public health decision-making, and policy-making.
Primary study designs include randomized and non-randomized controlled trials, cross-sectional studies, cohort studies, ecological studies, case-control studies, interrupted time series, before and after studies, quasi-experimental studies (e.g., difference in differences, instrumental variables analysis), qualitative research studies (e.g., grounded theory, ethnography, phenomenology, case study, biography), mixed methods, economic analyses (e.g., cost-effectiveness, cost-benefit, cost-utility), measurement property studies (e.g., reliability, validity, responsiveness, cross-cultural translation of instruments) recruiting human participants where individuals, groups, or populations are the unit of analysis.
Secondary study designs and documents include systematic reviews with or without meta-analysis, health technology assessments, scoping reviews, rapid reviews, integrative reviews, realist reviews, policy briefs, clinical and public health guidelines, overview of reviews (i.e., umbrella reviews), and evidence mapping.
In vitro and in vivo experimental studies, case-reports studies (cases series or clinical cases), narrative reviews, editorials, protocols of primary and secondary studies, letters to the editor, commentaries, and news will be excluded.
To identify eligible articles for this scoping review, an informationist built a systematic search strategy in Ovid MEDLINE13 using keywords and subject headings. This strategy was translated into the following additional databases: Embase,14 Cochrane Library,15 Epistemonikos.16 A date limit of January 1st, 2014 - December 31st, 2024 was applied. A humans-only filter was extracted from the Cochrane Highly Sensitive Search Strategies for identifying randomized trials, and applied to this search. It was modified to also exclude in vitro and in vivo studies. No language restrictions were applied. In addition, the search will be translated into global and Eastern Mediterranean-specific electronic journal databases (e.g., African Journals Online,17 Eastern Mediterranean Region Index Medicus,18 and KAUST Repository19), the WHO International Clinical Trials Registry Platform,20 and ProQuest Dissertations & Theses Global.21 All results will be exported to EndNote. The EndNote library will be imported into DistillerSR,22 where the DistillerSR duplicate detection tool will be used to remove duplicates, leaving unique citations. The search will continue to be updated as needed. The search strategy can be found in the extended data section.23
Study selection will be done through two screening stages. First, titles and abstracts will be screened against eligibility criteria. Second, full text will be examined to determine final eligibility. In both stages, pairs of reviewers will independently assess article eligibility. Retrieved research articles published in Arabic, French, Urdu, Pashto, Parisian, Somali, and Dari will be designated to research team members who comprehend one of these languages at a native level. In case of disagreement, a third reviewer will be consulted. The corresponding author of a potentially eligible study will be contacted if there is any missing information limiting the determination of study eligibility. DistillerSR software will be used to manage the screening process and resolve disagreements.22 To support this process, a collaborative network that includes global and regional interest-holders, researchers, and experts has been established.
Pairs of reviewers will independently and in duplicate extract data from included studies using a data extraction form developed in DistillerSR software,22 including all variables listed in Table 1. In case of disagreement or discrepancy in any single piece of data extracted, a third reviewer will make the final decision. The extraction sheet will be updated iteratively throughout the process, and modifications will be reported in the methods of this scoping review.
| Variable | Sub-variable | Note |
|---|---|---|
| Reference ID | Assigned by the software (DistillerSR) | |
| PMID or alike | ||
| Publication Year | ||
| List of Authors |
| |
| Title | ||
| Abstract | ||
| Journal |
| |
| Country of origin of the study |
| Step 1. Identification of the primary location of the study (i.e., the location of the center recruiting participants). If more than one country is reported, all locations will be extracted. Step 2. If no description about the primary location of the study was provided based on step 1, the affiliation of the last and the corresponding author will be extracted. When these two authors have different affiliations, the location for the corresponding author will be the primary location of the study |
| Author |
| Partnerships are classified according to the type of collaboration: National: This category includes collaborations between two different entities within the same country EMR: This category includes collaborations between entities from different countries within the EMR Out of the EMR: This category includes collaborations beyond the EMR, involving global partners |
| Source of funding |
| |
| Study design |
| Primary studies: Include randomized and non-randomized controlled trials, cross-sectional studies, cohort studies, ecological studies, case-control studies, interrupted time series, before and after studies, quasi-experimental studies (e.g., difference in differences, instrumental variables analysis), qualitative research designs (e.g., grounded theory, ethnography, phenomenology, case study, biography), mixed methods, economic analyses (e.g., cost-effectiveness, cost-benefit, cost-utility), measurement property studies (e.g., reliability, validity, responsiveness, cross-cultural translation of instruments) recruiting human subjects where individuals, groups, or populations are the unit of analysis. Secondary studies: Include systematic reviews with or without meta-analysis, health technology assessments, scoping reviews, rapid reviews, integrative reviews, realist reviews, policy briefs, clinical practice guidelines, overview of reviews (i.e., umbrella reviews), and evidence mapping. |
| Primary studies discipline |
| List of the study disciplines: General dentistry, dental anesthesiology, dental public health, endodontics, oral and maxillofacial pathology, oral and maxillofacial radiology, oral and maxillofacial surgery, oral medicine, orofacial pain, orthodontics and dentofacial orthopedics, pediatric dentistry, periodontics, and prosthodontics.25 |
| Oral health topic/condition |
| List of topics/conditions: Dental caries, periodontal disease, oral infections, oral lesions, malocclusions, oral cancer, temporomandibular disorders, dental anomalies, endodontic conditions, dental trauma, salivary gland disorders, halitosis, edentulism, craniofacial anomalies, maxillofacial trauma, oral health needs, oral health systems, oral health education, oral health status, anthropometrics, and psychological factors and behavioral issues.25 |
| DPH subtopic |
| List of DPH sub-topics: Oral health situation, healthcare professionals' education, preventive education, oral health-related quality of life, community outreach and promotion, oral health services, oral health access, oral health need assessment, policymaking, oral health interventions, workforce studies, and burden of disease. |
| Research type |
| These categories defined in the WHO document titled “A systematic approach for undertaking a research priority-setting exercise”26 Problem - Research to measure the size of the health problem through epidemiology, estimating the burden of disease and other forms of data collection; Cause - Research to understand the causal agents, risk factors and determinants of the health issue (this research may include, for instance, study of infection cycles, vectors, role of socioeconomic factors, environment, diet and the interaction of multiple factors); Solution - Research to develop new interventions, including therapeutics, devices, and procedures and also policy interventions, public health campaigns etc; Implementation - Research to translate new interventions into policy and practice and understanding the barriers to delivering known interventions; Evaluation - Research to monitor and evaluate the effectiveness or health impact of an intervention or program. |
| Citations |
| Web of Science or alike will be used to extract citation numbers. |
R Studio will be used to summarize, analyze, and display data. A taxonomy will be developed or cited to classify and categorize the qualitative data from the identified studies. The data will be analyzed at the regional (i.e., EMR) and country-specific levels. The variables will be modified according to the level of analysis, which will depend on whether it is regional or country-level (i.e., data will be aggregated for the regional level and disaggregated for the country-specific level).
Methods of analysis will be as follows:
a. To identify and describe the output of OHR in the EMR from 2014 to 2024, a database of relevant studies and all extracted variables ( Table 1) will be created.
b. To determine and assess the research output, including (research topics and types, study discipline, study designs, and funding sources) in the EMR from 2014 to 2024, tables and figures will summarize the distribution of publications across each variable, stratified by the level of analysis (i.e., regional or country-level) ( Table 1).
c. To estimate the extent of national and international collaboration in OHR in the EMR from 2014 to 2024, authors’ names, and the institutional affiliations (university and department), city, and country for authors, and the source of funding will be identified. Collaboration mapping analysis will be created. A database that includes the relevant citation data (title, abstract, publication year), authors’ names, institution, city, and country of affiliation will be created. Application Programming Interface (API) from the US National Library of Medicine and Elsevier will be used to obtain this public data. Additionally, the Shiny package in RStudio will be used to create an interactive data visualization tool for the regional collaboration map. The public citation data will be available in a publicly accessible file. The collaboration map will be generated using authors’ country of affiliation from the public data, which will help identify existing partnerships and trends in research collaboration among researchers within and out of EMR countries. The funding source will be used to identify which country and institution supports OHR in the region and identify the level of OHR funding for each country (e.g., national, EMR, or out of the EMR).
To advance OHR and strengthen evidence-informed decision-making in the EMR, it is crucial to first map the scope, nature, and distribution of existing research. This scoping review will systematically identify and synthesize evidence on OHR, including study designs, research topics, funding sources, and patterns of collaboration in the EMR from 2014 through 2024. The findings will help highlight strengths, gaps, and opportunities for enhancing the regional and country-level OHR agenda. In addition, findings can inform the development of national and regional research priorities and national evidence-informed guidelines, foster stronger collaboration within and out of the EMR, and guide investment in research capacity. The interactive data visualization and collaboration mapping will provide researchers, policymakers, and academic schools and institutions with a practical tool to monitor trends, track partnerships, and facilitate knowledge exchange across the EMR.
Some limitations are expected. The broad scope of OHR may result in heterogeneity across study designs, disciplines, and terminologies, requiring intensive team training and careful synthesis of diverse evidence types. Regional literature may also be inconsistently indexed across databases, and access to the full text of some eligible articles could be restricted. Additional limitations may include language barriers, cultural differences, and region-specific factors such as political instability. A collaborative network that brings together representatives with global expertise and regional knowledge from within and out the EMR has been established to mitigate these pitfalls, ensuring access to regional databases, language capacity, contextual insights, and region-specific expertise.
The findings of this scoping review will be disseminated by presentation of abstracts at international conferences, publication in peer-reviewed journals, and circulation of findings with key regional interest-holders, researchers, and deans of dental schools or academic institutions in EMR countries. These country-level interest-holders will help reiterate the project’s findings with their constituencies and colleagues.
The search strategy has been built in Ovid MEDLINE and will be completed by November 2025. Data collection is expected to begin by February 2026, and data analysis by April 2026.
Ethical approval is not required for this project since it does not involve human subjects.
Figshare. PRISMA-P checklist. https://doi.org/10.6084/m9.figshare.30214912.v1.10
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC BY 4.0).
Figshare: Search strategy: Oral Health Research in the WHO Eastern Mediterranean Region between 2014 and 2024, https://doi.org/10.6084/m9.figshare.30154495.v1.23
This project contains the following underlying data:
• Search Strategy - Oral Health Research in the WHO Eastern Mediterranean Region between 2014 and 2024
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC BY 4.0).
OSF: Oral Health Research in the WHO Eastern Mediterranean Region between 2014 and 2024: a scoping review protocol, https://doi.org/10.17605/OSF.IO/MXWKC.24
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Dental research, oral health, oral microbiology.
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Partly
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Oral health and Mental health domain, particularly focusing on Bibliometrics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 16 Oct 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)