Keywords
canal size, isthmus, safety, sonic, periapical extrusion, EDDY irrigation
This study aimed to evaluate the effect of root canal preparation size on isthmus cleaning efficiency and periapical extrusion using high-frequency sonic agitation with the EDDY irrigation system.
Thirty custom-made epoxy split models were used to simulate a root canal system with two curved canals merging at the apical 1 mm and an isthmus extending along their length. A periapical lesion was simulated to assess the extrusion tendency. Canals were prepared using the WaveOne Gold system in small, primary, and medium sizes. The isthmuses were filled with bovine dentin debris, while dyed gelatin was used in the periapical lesion to quantify extruded irrigant volume. EDDY irrigation efficiency was evaluated based on the total cleared surface area (mm2) using ImageJ software. Statistical analysis was performed using one-way ANOVA and post hoc multiple comparisons, with a significance level of P ≤ 0.05.
Isthmus cleaning efficiency remained consistent across all canal sizes, with no significant differences among groups (P > 0.05). However, periapical extrusion increased significantly with larger canal preparation sizes, with medium-sized preparations showing significantly higher extrusion than small (P = 0.001) and primary (P = 0.026) sizes.
EDDY irrigation was effective in achieving isthmus cleanliness regardless of canal preparation size. However, larger instrumentation increased the risk of apical extrusion, underscoring the need for careful irrigation management in clinical applications.
canal size, isthmus, safety, sonic, periapical extrusion, EDDY irrigation
Successful endodontic treatment relies on the effective elimination of bacteria from the contaminated pulp-dentin complex (Tungsawat et al., 2021). However, residual bacteria may persist in anatomical structures such as dentinal tubules, deltas, isthmuses, and lateral canals, where standard chemo-mechanical preparation primarily targets the main canal (Kumar et al., 2023). These residual bacteria can lead to treatment failure.
One of the root canal instrumentation goals is to create sufficient space for irrigation and intracanal medication, preventing infection and promoting periapical healing while preserving healthy dentin for long-term function (Bolourchi & Pourmousavi, 2018). Ideally, instrumentation should incorporate the entire original root canal space, effectively removing infected pulp and dentin forming proper space to facilitate irrigant delivery and disinfection (Arias & Peters, 2022). However, due to the complexity of root canal anatomy, comprehensive mechanical preparation is rarely achievable. This highlights the critical role of irrigation in complementing mechanical instrumentation, as it enhances debris removal, ensures deeper penetration of antimicrobial agents, and improves overall root canal disinfection.
Nickel-titanium (Ni-Ti) files, while highly efficient, may fail to engage all canal walls due to the geometrical dissymmetry between the two (Moawad, 2017). Regardless of the fact that instrumentation files can almost entirely remove root canal content, mechanical instrumentation might struggle with organic matter or debris in isolated regions like fins and isthmuses. The smear layer, composed of inorganic dentin fragments and organic necrotic pulp tissue, can harbor microorganisms, viruses and yeasts rendering the chemical disinfection process and preventing complete cleaning of the root canal system (Robberecht et al., 2023). To address this issue, various instrumentation and irrigation techniques have been proposed (Sung et al., 2021).
Rotary Ni-Ti files significantly reduce preparation time while maintaining the original canal path integrity compared to manual instrumentation (Stavileci et al., 2015). Though its less time consuming, shorter instrumentation time limits irrigant contact, which may reduce disinfection efficacy. In addition, proper instrumentation to an optimal size is necessary for effective cleaning, particularly in the apical third, where sodium hypochlorite is essential for tissue dissolution (Metzger et al., 2013). Irrigation plays a crucial role in chemo-mechanical preparation by reducing instrument friction, enhancing cutting efficiency, dissolving tissue, and providing antimicrobial effects (Haapasalo et al., 2014). It remains the only means of accessing uninstrumented canal walls.
Traditional syringe irrigation relies on a flushing mechanism that is often insufficient for clearing debris from narrow irregularities such as isthmuses. Several advanced activation methods, including shaping files, ultrasonic and sonic devices, and lasers, have been introduced to improve irrigation efficiency (Dioguardi et al., 2018).
Sonic devices operate at 1–8 kHz, generating lower energy compared to ultrasonic devices (25–40 kHz). Their plastic oscillating tips allow safe use in curved canals without damaging canal walls (Plotino et al., 2019). The EDDY system, operating at 6,000 Hz, has demonstrated superior power compared to other sonic systems. Studies indicate that EDDY enhances tissue dissolution efficiency and outperforms other activation techniques (Conde et al., 2017; Urban et al., 2017).
Recent research has examined the safety and efficacy of different irrigation activation techniques concerning periapical extrusion (Al-Jadaa et al., 2023). It was suggested that while EDDY achieves isthmus cleanliness comparable to passive ultrasonic irrigation, it carries a higher risk of periapical irrigant extrusion. Given its plastic tip design and high-frequency oscillation, further investigation into factors affecting its safe use is warranted.
This study aimed to evaluate the effect of canal preparation size on isthmus clearance and periapical extrusion risk using the high-frequency irrigant agitation (EDDY) irrigation system in a standardized in vitro simulation model.
In this study, an epoxy split model was created to simulate a two-canal system connected by an isthmus and extending to a periapical lesion. The designed root canal system consisted of two curved canals that merged at the apical 1 mm terminus. The total canal length was 16 mm, with a simulated pulp chamber measuring 5 mm in height, 6 mm in width, and 4 mm in depth. The isthmus extended from the pulp chamber floor to the apical joining point, measuring 0.1 mm in depth, 14.5 mm in height, and 3 mm in width. To replicate a periapical lesion, a space measuring 6 mm in width, 19 mm in height, and 1.5 mm in depth was created at the root canal terminus. The model featured a split design, enabling the two sections to be screwed together with an indexed guide to facilitate the placement of debris. The sample size was determined based on a previous study (Al-Jadaa et al., 2023). A total of 30 custom-built simulated models were fabricated and divided into three groups according to the WaveOne Gold file system canal sizes; Small, Primary and Medium (n = 10 per group).
The models were constructed following the methodology described in Al-Jadaa et al. (2023), using a multi-step molding technique.
A milling machine was used to mill two canals and an isthmus in plexiglass blocks that were cut to 4 × 3 × 1 cm. The blocks were then positioned in a high-precision CNC machine, which milled the desired root canal anatomy. The initial canal size simulated ISO 15, with the isthmus and half of the simulated pulp chamber were milled into one block, while the counterpart block contained only the remaining pulp chamber part ( Figure 1A). These blocks were molded using rubber duplication material (Ormaduplo, Major Prodotti Dentari, Italy) ( Figure 1B). The molds were filled with self-cured acrylic material, producing three duplicates of the milled blocks ( Figure 1C). The corresponding parts were assembled using screws, and the canals were prepared to a working length of 21 mm using WaveOne Reciprocating Files (Dentsply Sirona, USA) in small, primary, and medium sizes (one model per size). The acrylic models were then secured in a molding setup with positioning pins to ensure precise repositioning ( Figure 1D). These reference models were subsequently duplicated with rubber molding material to create the final test samples ( Figure 1E).
Due to the brittle nature of epoxy resin, an acrylic support frame was required to reinforce the model. A 3 mm-thick acrylic skeleton (Vertex-Dental, Netherlands) was first created using the final duplication mold. The acrylic pieces were trimmed into a U-shape ( Figure 2A) and fixed in a molding setup with positioning pins ( Figure 2B). Rubber molds were made using impression materials ( Figure 2C), and the single-step impression technique involved the application of Hydrorise light body (Zhermack, Italy) followed by Hydrorise Maxi heavy body (Zhermack, Italy). Metal pins were inserted into the designated holes, and self-cured acrylic was used to cast the frames ( Figure 2D). The frames were trimmed and polished to ensure a smooth and uniform surface.
The final test models were cast using clear epoxy resin (Art Epoxy Resin, EPOKE, India). Metal pins coated with Vaseline were inserted into the designated holes within the rubber molds to ensure precise alignment. Acrylic supporting frames were positioned inside the casting molds using the positioning pins ( Figure 3A). A 1 mm space was left between the mold floor and the acrylic frame to allow a thin layer of epoxy resin to ensure a flat and even joining surface. The epoxy resin components (base and catalyst) were mixed according to the manufacturer’s instructions (2:1 ratio by weight) and stirred for 10 minutes before being poured into the casting molds. The epoxy mixture had a self-bubble-releasing property, allowing for air-free curing. The molds were left undisturbed for 24 hours to ensure complete polymerization. Once set, the models were carefully removed from the molds, the pins were taken out, and the assembled components were secured using M3 screws and nuts, forming the final test sample ( Figure 3B).
To simulate dentin debris, bovine teeth roots were dry-ground using a carbide bur, producing dentin powder. The powder was mixed with water at a 2:1 volume ratio before application. To prevent leakage and ensure a proper seal, a thin layer of Vaseline was applied to the model’s exterior. The debris sludge was placed in the isthmus region of one model half ( Figure 4A), after which the counterpart was positioned and secured with two 35-mm-long M3 screws ( Figure 4B). The models were stored in water until the experiment commenced.
To mimic granulation tissue, 10% gelatin (Merck, Germany) was dissolved in deionized water, stained with 1% red food dye (2 mL), and refrigerated at 5°C until set. Before use, the gelatin was minced to match the consistency of granulation tissue and was then injected into the simulated periapical area of the epoxy model using a 23-gauge syringe needle ( Figure 4C).
A custom-made acrylic holder was used to secure and transilluminate the model using an LED light (CHINLY, China) ( Figure 4C). A metal millimetric scale was fixed on the side of the acrylic holder for calibration purposes. The model was positioned in the holder with it’s canals aligned with the millimetric scale. A Nikon D7200 camera equipped with a VR Macro-Nikkor 105 mm lens was mounted on a tripod, with a continuous ring light placed in front for standardized imaging before and after irrigation.
Each sample underwent three irrigation cycles. In each cycle, 1 mL of 1.3% NaOCl was delivered into each canal using a 30-gauge endo side-vented needle. The EDDY activation instrument (VDW, Germany) was then used to agitate each canal for 20 seconds per cycle. Pre- and post-irrigation images were captured for analysis ( Figure 5A–D).
Since the isthmus thickness remained constant throughout it’s entire length, the pre- and post-irrigation images were analyzed using ImageJ software (ImageJ, Java, USA). The millimetric scale served as a reference for measurement calibration, and the surface area (mm2) of the cleared debris was calculated to compare irrigation efficiency across different canal sizes.
To assess data distribution, the Shapiro-Wilk test was conducted using a significance threshold of p > 0.05 to determine normality. The results indicated that debris removal within the isthmus across all three canal sizes and the periapical extrusion test followed a normal distribution.
ANOVA analysis was performed to determine the mean values and standard deviations for isthmus clearance and periapical extrusion among different canal sizes ( Table 1). The findings suggested that while isthmus clearance remained relatively consistent across canal sizes, periapical extrusion exhibited a notable increase with larger apical preparation sizes.
Capital letters indicate significant difference read vertical.
| Canal Size | Isthmus Clearance mm2 (mean ± SD) | Periapical Extrusion mm2 (mean ± SD) |
|---|---|---|
| Small | 5.33 ± 1.20 | 8.25 ± 2.82 A |
| Primary | 5.29 ± 1.44 | 9.96 ± 3.40 B |
| Medium | 5.00 ± 1.23 | 13.84 ± 3.15 AB |
A multiple comparisons test (Post Hoc Test) with a significance level of P ≤ 0.05 was conducted to determine differences between canal sizes. The analysis revealed no statistically significant difference in isthmus clearance among the different canal preparation sizes. However, periapical extrusion demonstrated significant differences between medium-sized preparations compared to both small and primary canal sizes (P = 0.001 and 0.026, respectively) ( Table 1). This finding indicates that apical preparation size significantly influences irrigant extrusion volume.
A graphical comparison of the mean cleared surface area among different sizes in both the isthmus and periapical areas ( Figure 6) suggests that isthmus clearance is relatively unaffected by canal preparation size, as values remained consistent across groups. However, a trend of increasing periapical extrusion with larger apical preparation sizes suggests a strong association between greater instrumentation and increased irrigant extrusion.
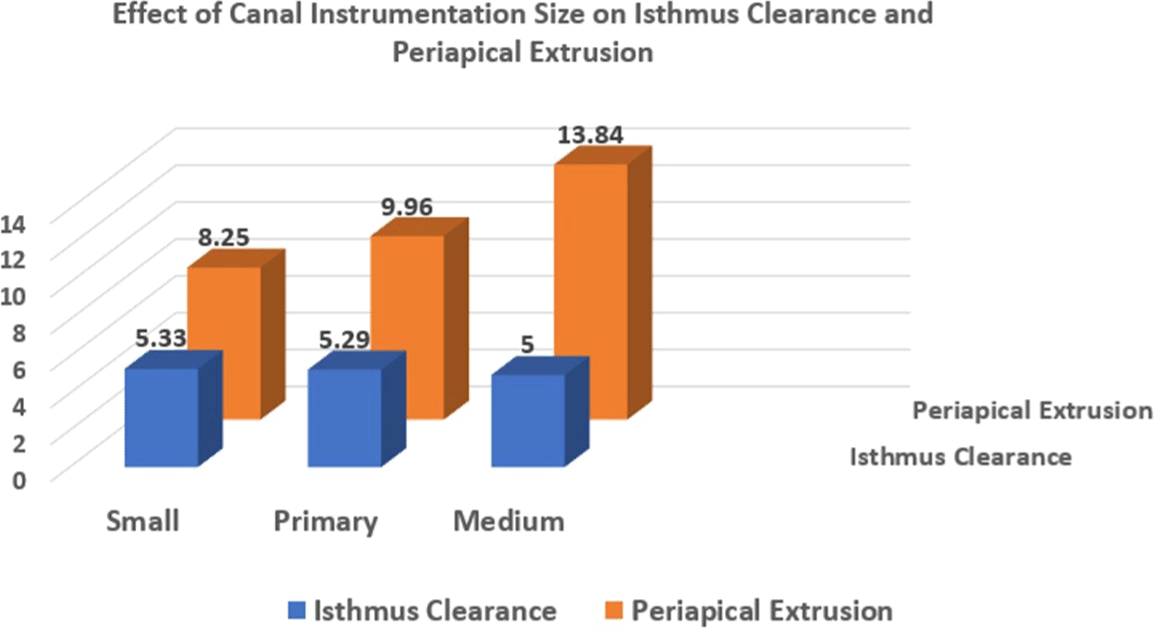
These findings emphasize the importance of carefully selecting the apical preparation size to optimize irrigation outcomes while minimizing the risk of excessive irrigant extrusion.
This study aimed to investigate the impact of varying canal preparation sizes on isthmus clearance and periapical extrusion using high-frequency sonic agitation (EDDY). The findings provide crucial insights into the complexities of root canal irrigation dynamics and their implications as recommendations for clinical application.
The study revealed consistent isthmus clearance across all tested canal sizes, indicating that the mechanical action of the EDDY system effectively removed debris regardless of canal dimensions. This outcome suggests that irrigation techniques may consistently achieve efficacy across varying canal sizes in clearing isthmuses. However, significant differences were observed in periapical extrusion levels among canal size groups, with larger preparations demonstrating a trend towards increased extrusion risks.
Sodium hypochlorite (NaOCl) was chosen as the irrigant in the current study as it is the most acceptable root canal irrigant in endodontic field. The study employed a standardized irrigation protocol across all groups to ensure consistency and minimize biases associated with variable irrigation techniques or solutions. This protocol involved three cycles of 20-second agitation intervals with 1 ml of 1.3% NaOCl refreshment per cycle, adhering to recommended practices in root canal treatment (Iqbal, 2012).
It is widely acknowledged that canal size significantly affects the conventional needle irrigation in root canals. Larger canals facilitate better irrigant replacement, increases shear stress, and pressure at the apical foramen, as suggested by Boutsioukis (2010). Conversely, the design of irrigation needles limits the depth of irrigant penetration, which is suggested to be more effective in smaller canals (Al-Sabbagh and Al-Huwaizi, 2013). Interestingly, the size of the canal found not to impact the penetration of endodontic irrigants into dentinal tubules (Zakaria, 2006). In conventional irrigation, to mitigate the risk of apical extrusion, recommendations include using larger canula sizes and side-vented needles to reduce apical pressure leading to higher extrusion risk (Chang et al., 2015).
Compared to other agitation systems, Sen and Kaya (2018) compared the safety of the EDDY root canal irrigation system with other techniques. Their findings indicated that EDDY and similar systems are safe for irrigating canals with intact apices, but caution is advised in over-instrumented canals due to increased potential for irrigant extrusion—a critical consideration for clinical practice, consistent with observations from the current investigation. In addition, comparative studies on irrigation systems have shown varied efficacy and safety profiles. While EDDY demonstrated effective debris removal comparable to ultrasonic systems, it also exhibited a higher risk of periapical extrusion (Al-Jadaa et al., 2023). Uğur Aydın et al. (2021) observed higher bacterial extrusion through the apex compared to other irrigation techniques. These findings emphasize the need for tailored irrigation strategies to minimize procedural risks and optimize treatment outcomes.
Literature exploring the influence of canal instrumentation size on the application and safety of the EDDY agitation system remains sparse. In the current study the observed trend towards increased periapical extrusion with larger canal sizes underscores the need for careful management and precise execution of irrigation procedures, particularly in cases involving larger canals, to minimize the risk of periapical complications during endodontic treatments. The suggested explanation may lay in available canal space for the irrigation tip to osculate within the canal.
The effect of anatomical variation on irrigation was demonstrated in previous studies. Short-wide isthmuses can be better cleaned regardless of the irrigation technique used. This is because lengthy intercanal distances present more obstacles, and wide isthmuses provide more surface area and space for irrigant flow. In contrast, narrow isthmuses provide little contact surface area and flow space. Since the cleaning effectiveness of long and short isthmuses with the same width does not differ significantly, the width of the isthmus is more important than its length (Robberecht et al., 2023).
The adoption of standardized 3D-milled root canal modPels in the current study effectively mitigated potential anatomical variables, thereby enabling further experimentation under diverse clinical scenarios, augmenting sample size, and streamlining the ethical approval process. Moreover, this approach offers a cost-effective, easily analyzable method suitable for both research and educational purposes.
Conversely, while Epoxy Resin blocks used in this research allow for replication of various root canal configurations and scenarios, they lack the ability to mimic dentinal tubules crucial for assessing the effectiveness of endodontic treatments. Furthermore, as an in vitro study, the direct application of its findings to clinical settings is limited. Nevertheless, these findings lay groundwork for future research that could inform clinical guidelines and practices.
In summary, this study advances our understanding of how canal size influences root canal irrigation dynamics, isthmus clearance, and periapical extrusion. The findings underscore the importance of tailored irrigation protocols and meticulous technique application in mitigating procedural risks and optimizing treatment outcomes. Further research incorporating diverse patient populations and real-world clinical scenarios will be pivotal to translate these findings into evidence-based practices in endodontics.
This study provides valuable insights into the impact of root canal preparation size on isthmus clearance and periapical extrusion when using high-frequency sonic agitation (EDDY) for root canal irrigation. The findings confirm that EDDY effectively clears isthmuses across different canal sizes, reinforcing its mechanical efficacy regardless of preparation dimensions. However, a significant increase in periapical extrusion was observed with larger canal preparations, emphasizing the need for careful irrigation management to minimize potential clinical risks.
These results highlight the critical balance between effective cleaning and procedural safety in endodontic practice. While EDDY has demonstrated strong efficacy in debris removal, the increased risk of irrigant extrusion in over-instrumented canals necessitates a cautious approach. Clinicians should tailor irrigation protocols based on individual root canal morphology, considering both canal size and patient-specific factors to enhance treatment safety and efficiency. Despite the inherent limitations of in vitro studies, including the use of epoxy resin models that do not fully replicate dentinal tubule characteristics, this research provides a foundation for further clinical investigations. Future studies should incorporate natural teeth and in vivo conditions to validate these findings and refine endodontic irrigation protocols. Additionally, exploring alternative activation methods and refining irrigation parameters could further optimize root canal disinfection while minimizing extrusion risks.
Overall, this study contributes to the growing body of evidence on endodontic irrigation dynamics, reinforcing the necessity of precise instrumentation and irrigation techniques to improve treatment outcomes and patient safety. These findings emphasize the importance of balancing canal preparation size with irrigation safety, ensuring effective root canal disinfection while minimizing the risk of periapical extrusion.
Figshare. MEASUREMENTS copy.docx. https://doi.org/10.6084/m9.figshare.30227821.v1 (Al-Jadaa A, Saidi F, Al-Musawi G, Musaad R, Alsenan J, 2024).
This project contains the following underlying data:
Study tables that contains the full data for analysis.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)