Keywords
Neurogranin, Ischemic Stroke, Thrombotic, Embolic, Cardioembolism.
This article is included in the Fallujah Multidisciplinary Science and Innovation gateway.
Ischemic stroke (IS) is a severe neurological disorder that can lead to disability and mortality in adults. The management of acute ischemic stroke (AIS) requires the ability to predict functional outcomes. Blood biomarkers are valuable prognostic tools because of their rapid assessment, cost-effectiveness, and clinical accessibility. Given that Neurogranin (Ng) is a small postsynaptic neural protein, and when the blood-brain barrier (BBB) is damaged, Ng levels are altered in the bloodstream.
This study aimed to assess the concentration of Ng in patients with acute ischemic stroke. We investigated forty-six thrombotic patients, forty-five embolic patients, and forty-five healthy individuals. Serum concentrations of Ng, D-dimer, random blood sugar (RBS), lipid profile, renal function tests, liver function tests, complete blood count (CBC), and electrolyte tests were performed for all participants.
The results revealed that the embolic group had the highest serum level of Ng compared to the thrombotic and control groups (0.600±0.339 ng/ml, 0.464±0.121 ng/ml, and 0.304±0.065 ng/ml, respectively). Moreover, correlation analysis revealed that serum Ng was positively correlated with some biomarker parameters, specifically platelets, total cholesterol (TC), and low-density lipoprotein (LDL), in the embolic group. Meanwhile, Receiver Operating Characteristic (ROC) Curve analysis yielded area under the curve (AUC) values of 0.879 vs. 0.897 for the thrombotic and embolic groups, respectively.
These results led us to conclude that the assessment of serum Ng levels suggests that it can be used as a successful diagnostic parameter for ischemic stroke patients during the early stages of the disease.
Neurogranin, Ischemic Stroke, Thrombotic, Embolic, Cardioembolism.
Ischemic stroke (IS) is a cerebrovascular disease (CVD) and the most common subtype of stroke, accounting for approximately 70% of cases associated with substantial disability and mortality in adults worldwide. It occurs when a blood clot blocks or narrows an artery, depriving the brain of oxygen and glucose.1,2 The IS classification is based on the mechanism and includes two main groups: thrombotic stroke, which mechanism occurs when blood vessels are obstructed by an in-situ thrombosis, leading to a brain infarction in the area supplied by those ischemic strokes. An embolic stroke occurs when a blood clot forms in another part of the body, travels through the bloodstream to the brain, causing acute blockage of blood flow, resulting in infarction in the affected brain territory.3,4
Thrombotic events can precipitate strokes through multiple pathways. When blood clots obstruct major cerebral vessels, they result in either large-artery occlusion or small-artery occlusion (lacunar stroke). Thrombotic processes may also affect the cerebral veins and venous sinuses, thereby complicating clinical presentation. Conversely, embolic stroke occurs when cerebral arteries become occluded by thromboemboli originating from cardiac sources, and is termed cardioembolism. Moreover, approximately 50% of ischemic stroke cases are linked to cardiac embolism, 25% to large vascular occlusion, and 10% to small vascular occlusion.5–7
There is a critical need to novel and reliable biomarkers that can be utilized in stroke prognostic and severity assessments.8,9 When measured in blood samples, elevated levels of these biomarkers serve as indicators of cerebral damage.10,11 These biomarkers are proteins associated with the Central Nervous System (CNS). During stroke events, disorders of the Blood-Brain Barrier (BBB) lead to elevation of these biomarkers in the peripheral circulation.12,13 Neurogranin (Ng) is a calmodulin-binding protein predominantly found in the brain, especially in dendritic spines. It is a significant postsynaptic protein that is part of the signaling pathway for protein kinase C (PKC), regulating the availability of calmodulin by binding to it when calcium is absent.14 This protein demonstrates extensive distribution through multiple of the brain regions, showing up localization in cortical layers II-IV and notably appearing in the neocortex, amygdala, caudate nucleus, putamen, and hippocampus. Furthermore, its calcium-dependent interaction with calmodulin, this protein regulates numerous essential cellular processes, including cellular growth, transcriptional activity, cell movement, protein modification through phosphorylation and dephosphorylation cascades, ionic transport mechanisms, and modulation of neurotransmitter and hormonal signaling pathways. Consequently, the broad anatomical distribution and diverse functional involvement highlight the fundamental importance of this protein.15
This study aimed to assess the ability of Ng to be used as a potential biomarker for ischemic stroke patients and to determine the serum concentration of Ng in Iraqi individuals who have been diagnosed with ischemic stroke across various groups. Furthermore, it was designed to determine the correlation between Ng and the additional anthropometric and biochemical parameters.
The present research collects ninety-one patients with acute ischemic stroke (AIS) who were admitted to three hospitals in Iraq: Dr. Saad AL-Witry Neuroscience Hospital (Baghdad), Neurosurgery Hospital (Baghdad), and AL-Hakeem Teaching Hospital (Maysan) between March 31 and September 1, 2025. All patients were initially evaluated in the emergency department (ED) and subsequently transferred to the neurology department for further management. Based on stroke etiology determined by neuroimaging, patients were classified into two groups: thrombotic stroke group (n = 46): 25 men and 21 women, age range–40-85 years, and embolic stroke group (n = 45): 26 men and 19 women, age range–45-88 years. Additionally, a control group (n = 45) of age- and sex-matched healthy volunteers (26 men and 19 women, age range–40-75 years) was included. All AIS diagnoses were confirmed by neurologists based on neuroimaging results, including computed tomography (CT) and magnetic resonance imaging (MRI) and clinical presentation.16 Electrocardiography (ECG) was performed to identify the potential cardiac sources of embolism.
The study excluded patients with a cancer history, acute inflammation, and chronic conditions diseases such as liver diseases, renal failure, hemorrhagic stroke, and transient ischemic stroke attacks (TIAs).
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Research Ethics Committee of the College of Science, University of Baghdad, Baghdad, Iraq (Approval Number: Ref. CSEC/0325/0053 on March 27, 2025) (as in Extended data).17 Appropriate permits were obtained from relevant hospital authorities. Written informed consent was obtained from all participants or their legally authorized representatives prior to enrollment (as in Extended data).17 For patients unable to provide consent due to their medical condition, consent was obtained from their next of kin in accordance with local regulations. All patient data were de-identified and coded to protect participant confidentiality. Demographic and medical history data were collected using a standardized questionnaire (as in Extended data).17
2.4.1 Sample collection
Five-milliliter blood samples were drowning from each participant and aseptically transferred to tightly sealed gel and EDTA tubes. An EDTA tube containing 2 ml of whole blood was used to assess the Complete Blood Count (CBC), while a gel tube containing 3 ml of blood was subsequently centrifuged at 1000 x g for 20 min until serum was separated. All samples were stored at -20 °C until biochemical analysis was conducted.
2.4.2 Neurobiochemical analysis
Serum levels of Ng protein were estimated using Enzme-linked immunosorbent assay (ELISA), with commercially available kits from MyBioSource (Catalog No: MBS8806622), USA, which are designed in accordance with established protocols for this biomarker.
2.4.3 Biochemical parameters analysis
Blood samples were analyzed using various automated device. Renal function tests, lipid profile, Random blood sugar (RBS), and calcium levels were measured using a spectrophotometer (APEL, Japan). The electrolyte levels (potassium, sodium, and chloride) were determined using an Electrolyte Analyzer EL-120 (Erma, Japan). Liver enzyme activities (ALT and AST) levels were analyzed using the analyzer cobas c 111 (Roche, Switzerland), and D-dimer concentrations were assessed by immunofluorescence assay using the i-CHROMA-II system. A Mindray BC-10 hematology analyzer (Mindray, Germany) was used to perform complete blood counts.
IBM SPSS software version 27.0 and GraphPad prism version 10 were used for statistical analysis. The numerical data were expressed as mean and standard deviation (±SD), while the categorical data were displayed as numbers and percentages. Group comparisons were performed using one-way ANOVA followed by Tukey’s post hoc test. Pearson correlation analysis was employed to examine relationships between serum Ng levels and other measured biochemical parameters. Statistical significance was set at p<0.05, with p 0.01 considered highly significant. Receiver operating characteristic (ROC) curve analysis was applied to distinguish ischemic stroke patients from healthy controls and to determine the diagnostic accuracy of neurogranin as a biomarker.
The results of the present study included one hundred and thirty-six participants who were divided into three groups: n=46 in the thrombotic group, n=45 in the embolic group, and n=45 in the control group. Table 1 summarizes the baseline demographic characteristics, including age ranges, numbers and percentages for sex, previous stroke, and relevant risk factors.
The general clinical data and biochemical parameters for all studied groups are shown in Table 2. The Statistical ANOVA and post hoc test showed that there was a highly significant difference in systolic blood pressure (SBP) between the thrombotic group and control (156.50 vs 138.37 mmHg, p<0.01), but no discernible difference existed between the embolic and healthy groups or between the patient groups. However, the WBCs showed a highly significant difference between the thrombotic group and embolic group compared to control group (8.17 and 8.47 vs 6.61 103/μL, p<0.01). In contrast, RBCs, Hb, and platelets showed that were not any significant differences between the three groups. In comparison to the embolic and healthy groups, the embolic group’s the mean of serum random blood sugar (RBS) was noticeably higher (11.00 vs 8.4 and 7.67 mmol/l, p<0.01). Moreover, the mean of serum total cholesterol showed a highly significant difference between the thrombotic group and control group (198.50 vs 150.60 mg/l, p<0.01) and a significant difference between the embolic group and control (177.27 vs 150.60 mg/l, p<0.05). The mean of serum TG level showed a highly significant difference in the thrombotic group and embolic group compared to control group (226.93 and 208.24 vs 163.60 mg/l, p<0.01).
| Parameters | Thrombotic n=46 | Embolic *Cardio embolism n=45 | Control n=45 | p-value |
|---|---|---|---|---|
| BMI (Kg/m2) | 30.85±7.55** | 31.38±7.20 | 28.97±3.57 | 0.175 |
| SBP mmHg | 156.50±24.64**a | 145.62±22.91 | 138.37±20.44 | <0.01 |
| DBP mmHg | 90.63±17.66 | 88.00±15.86 | 88.11±15.31 | 0.685 |
| WBCs 103/μL | 8.17±2.42**a | 8.47±2.68**b | 6.61±1.65 | <0.01 |
| RBCs 106/μL | 4.50±0.66 | 4.70±0.79 | 4.79±0.51 | 0.112 |
| Hb g/dl | 12.83±1.76 | 12.88±2.16 | 13.55±1.46 | 0.114 |
| Platelets 103/μL | 233.19±84.46 | 223.80±94.46 | 229.88±59.19 | 0.854 |
| RBS mmol/l | 11.00±4.29**a,c | 8.41±3.27**c | 7.67±2.89 | <0.01 |
| TC mg/dl | 198.50±48.06**a | 177.27±53.76*b | 150.60±32.03 | <0.01 |
| TG mg/dl | 226.93±63.84**a | 208.24±93.18**b | 163.13±30.70 | <0.01 |
| HDL mg/dl | 35.78±11.72**a,*c | 29.03±10.87**b,*c | 53.55±12.87 | <0.01 |
| vLDL mg/dl | 45.38±12.76**a | 41.64±18.63**b | 32.62±6.14 | <0.01 |
| LDL mg/dl | 117.33±43.81**a | 106.58±45.60**b | 63.58±29.95 | <0.01 |
| Ca mmol/l | 2.15±0.16 | 2.15±0.25 | 2.15±0.11 | 0.989 |
| Na+ mmol/l | 135.53±6.80**a | 136.22±5.05**b | 141.06±2.47 | <0.01 |
| Cl- mmol/l | 103.77±4.01 | 104.06±4.17 | 103.97±2.61 | 0.927 |
| K+ mmol/l | 3.94±0.50 | 3.92±0.44*b | 4.16±0.44 | 0.02 |
| ALT u/l | 15.97±5.67 | 16.08±6.63 | 14.18±5.33 | 0.233 |
| AST u/l | 19.46±8.17 | 23.02±15.79 | 18.17±4.57 | 0.083 |
| Urea mmol/l | 5.95±2.40 | 5.88±2.54 | 5.06±0.94 | 0.098 |
| Creatinine μmol/l | 70.23±21.50 | 75.88±26.10 | 68.60±12.90 | 0.226 |
| D.dimer ng/ml | 3905.45±1745.84**a,c | 6036.30±2941.49**b,c | 285.36±133.40 | <0.01 |
| Ng ng/ml | 0.464±0.121**a,c | 0.600±0.339**b,c | 0.304±0.065 | <0.01 |
** Statistically highly significant differences at p≤0.01, a denotes to significant differences between thrombotic group & control. b denotes significant differences between the embolic and control groups. c denotes significant differences between thrombotic and embolic groups. BMI = body mass index; SBP = Systolic Blood Pressure; DBP = Diastolic Blood Pressure; WBC = white blood cell; RBC = red blood cell; Hb = hemoglobin; RBS = Random blood sugar; TC = total cholesterol; TG = triglyceride; HDL = high-density lipoprotein; vLDL = very low-density lipoprotein; LDL = low-density lipoprotein; Ca = calcium; Na+ = sodium; Cl-- = chloride; K+ = potassium; AST = aspartate aminotransferase; ALT = alanine aminotransferase; Ng = neurogranin.
In addition, the mean of serum HDL level showed a highly significant difference in the thrombotic group and embolic group compared to the control group (35.78 and 29.03 vs 53.55 mg/ml, p<0.01), and a significant difference between the thrombotic and embolic groups (35.78 vs 29.03, p<0.05). Similarly, by comparing the means of very low-density lipoprotein (vLDL) and low-density lipoprotein (LDL), there were a significantly increased in thrombotic and embolic stroke patients compared to healthy individuals (p<0.01).
Therefore, the Sodium (Na+) was significantly higher in the control group compared to the patients groups (p<0.01). In addition, potassium (K+) showed a significant difference increased in the control group compared to that in the embolic group (4.16 vs 3.92 mmol/l, p= 0.02). Furthermore, the mean of serum D-dimer level was showed a high significantly different between the thrombotic group and embolic group compared to that in the control group (3905.45 and 6036.30 vs 285.36 ng/ml, p<0.01). As well, Figure 1 used to show that the serum Ng level in the embolic group was higher than that in the thrombotic and healthy groups.
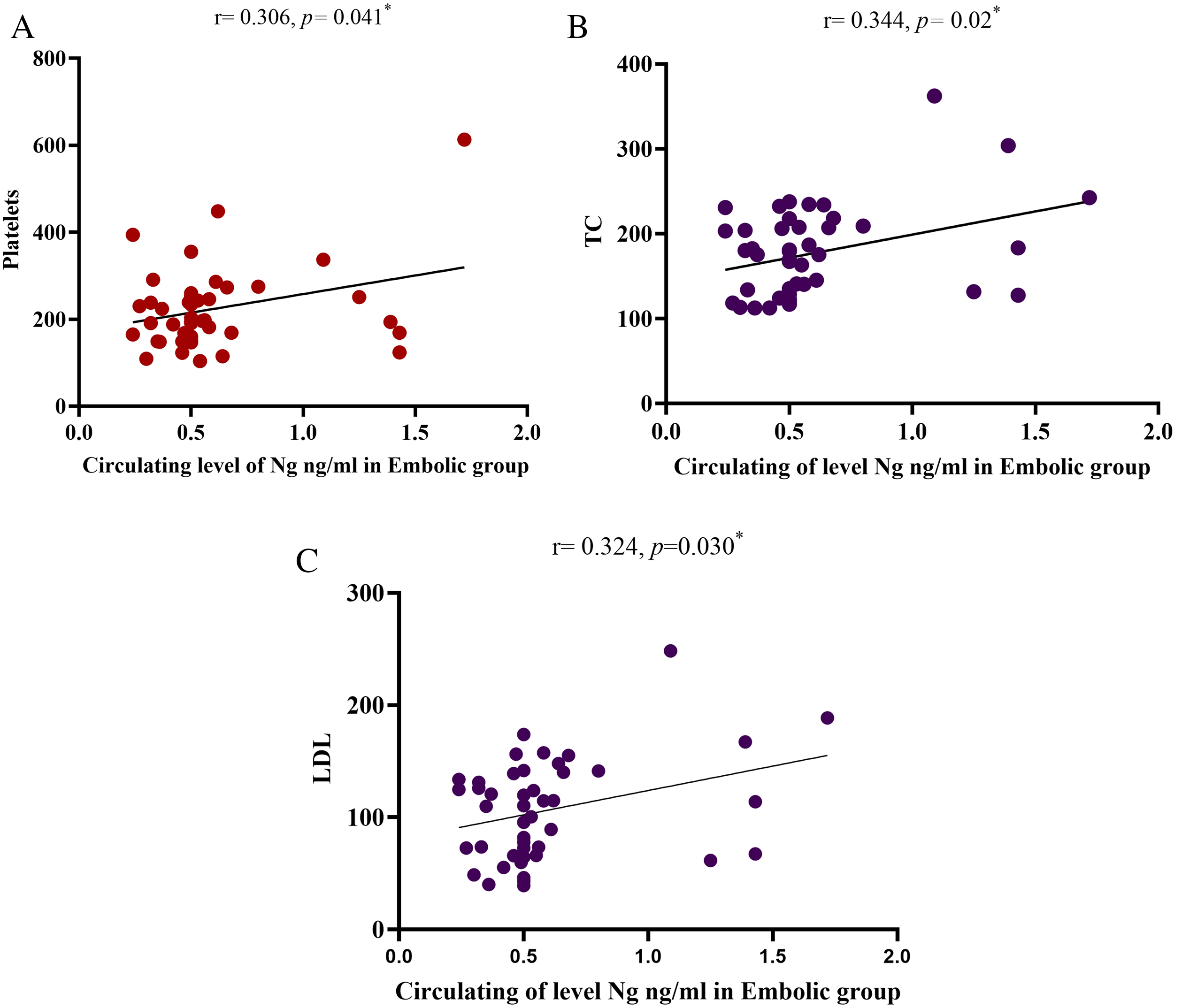
Table 3 shows the Pearson correlation coefficients of serum Ng with other biochemical parameters in the thrombotic and embolic groups. Based on these results, the serum Ng level in the embolic group (cardioembolism) had a positive correlation with platelets (p=0.041), total cholesterol (TC) (p=0.021), and LDL (p=0.030). Similarly, as illustrated in Figure 2.
| Parameters | Circulating Levels of Ng Protein ng/ml | |||
|---|---|---|---|---|
| Thrombotic n=46 | Embolic*cardio embolism n=45 | |||
| r | p-value | r | p-value | |
| Demographic Data | ||||
| Age (year) | 0.147 | 0.329 | 0.055 | 0.720 |
| BMI (kg/m2) | -0.107 | 0.478 | -0.033 | 0.827 |
| SBP mmHg | 0.080 | 0.597 | 0.166 | 0.276 |
| DBP mmHg | 0.026 | 0.864 | 0.148 | 0.332 |
| Hematological Parameters | ||||
| WBCs 103/μl | 0.027 | 0.861 | 0.221 | 0.146 |
| RBCs 106/μl | -0.118 | 0.435 | 0.102 | 0.506 |
| Hb g/dl | -0.038 | 0.803 | 0.106 | 0.487 |
| Platelets 103/μl | -0.050 | 0.743 | 0.306 | 0.041* |
| Biochemical Parameters | ||||
| RBS mmol/l | -0.225 | 0.133 | -0.226 | 0.135 |
| TC mg/dl | -0.074 | 0.626 | 0.344 | 0.021* |
| TG mg/dl | -0.019 | 0.901 | 0.256 | 0.09 |
| HDL mg/dl | -0.036 | 0.815 | -0.097 | 0.527 |
| vLDL mg/dl | -0.019 | 0.901 | 0.256 | 0.09 |
| LDL mg/dl | -0.066 | 0.663 | 0.324 | 0.030* |
| Ca mmol/l | 0.010 | 0.948 | -0.097 | 0.527 |
| Na+ mmol/l | 0.184 | 0.220 | -0.020 | 0.897 |
| Cl- mmol/l | -0.108 | 0.476 | -0.078 | 0.609 |
| K+ mmol/l | -0.243 | 0.103 | -0.003 | 0.983 |
| D.dimer ng/ml | 0.062 | 0.681 | -0.024 | 0.899 |
| ALT u/l | 0.133 | 0.380 | -0.168 | 0.271 |
| AST u/l | 0.233 | 0.119 | -0.114 | 0.456 |
| Urea mmol/l | 0.076 | 0.616 | 0.056 | 0.713 |
| Creatinine μmol/l | -0.059 | 0.696 | 0.029 | 0.849 |
The Receiver Operating Characteristic (ROC) Curve of serum Ng level analysis was used to identify ischemic stroke groups (thrombotic and embolic) and to distinguish ischemic stroke patients from healthy controls. Table 4 shows the comparison between the thrombotic and embolic groups. For the thrombotic group, the AUC value for Ng was 0.879, with a cut-off of 0.39991 ng/ml (p<0.001) and sensitivity and specificity of 80.4% and 88.9%, respectively, with a positive predictive value (PPV) was 88.1%, a negative predictive value (NPV) was 81.6%, and an accuracy was 84.61%. For the embolic group, the AUC value for Ng was 0.897, with a cut-off of 0.34443 ng/ml (p<0.001) and sensitivity and specificity of 75.6% and 100%, respectively. PPV, NPV, and accuracy were 100%, 80.4%, and 87.7%, respectively. Furthermore, Figure 3 illustrates the differences in ROC curves between both patient groups and demonstrates the overall diagnostic performance accuracy of serum Ng levels in distinguishing the thrombotic and embolic groups.
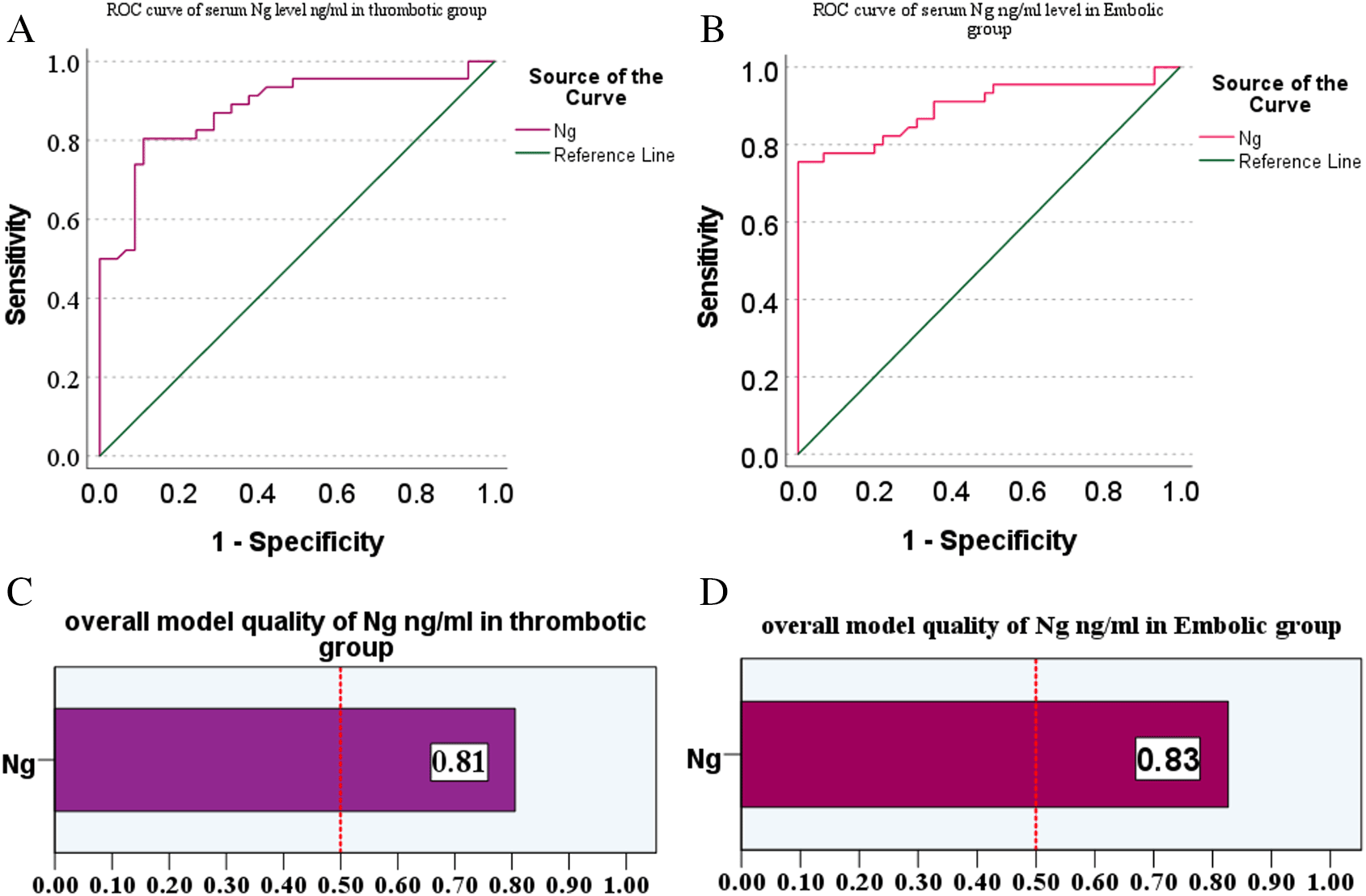
Overall model quality (C, D) illustrates model performance for serum Ng concentrations (ng/ml) in thrombotic and embolic groups, respectively, demonstrating that optimal diagnostic parameters consistently achieve AUC values above 0.5.
By comparing the results of this study, it was demonstrated that serum Ng concentrations were significantly elevated in patients with ischemic stroke of embolic etiology compared to those with thrombotic stroke and healthy control subjects, as shown in Figure 1. These findings provide evidence that increased Ng concentrations in serum positively correlate with the degree of acute cerebral infarction volume. Kuşdoğan et al. and De Vos et al.18,19 Additionally, L. Li et al. observed elevated protein levels in the lesion area as early as 24 hours post-middle cerebral artery occlusion, which peaked at day 7, and subsequently declined progressively.20 Pohlan et al. established a positive correlation between Ng concentration and infarct volume, which confirmed its diagnostic value in distinguishing ischemic from hemorrhagic stroke. The authors also reported that Ng phosphorylation through the PKC pathway decreased its calmodulin-binding affinity.21 Subsequently, Gerendasy et al. explained that unphosphorylated Ng maintains a high binding capacity to CaM, thereby decreasing intracellular calcium availability.22 These results agree with the findings of the current study, which demonstrated that elevated levels of unphosphorylated Ng corresponded to reduced intracellular calcium concentrations. To further characterize the relationship between Ng and other clinical parameters, we performed comprehensive biochemical analysis. The statistical tests showed that concentrations of Ng, urea, creatinine, ALT, AST, Hb, and platelets ( Table 2) have a highly significant difference in the patient groups compared to healthy individuals. These findings were consistent with Ozensoy et al.23 Additionally, Canturk et al. reported that serum Ng, WBC, and sodium levels showed highly significant differences in patient groups compared to controls.15 In the current study, however, while Ng and WBCs ( Table 2) findings were consistent with those of Canturk et al., serum sodium levels were paradoxically increased in control subjects compared to patient groups. Moreover, the Pearson correlation coefficient of serum Ng with other biochemicals showed a positive correlation with platelets, TC, and LDL in the embolic group ( Table 3), as shown in Figure 2. Consequently, this study provides the first evidence of a correlation between Ng and these biomarkers in patients with ischemic stroke, thereby addressing a previously unexplored gap in stroke biomarker research and establishing a foundation for future investigations. However, in ROC curve analysis, Faraggi et al. found that the AUC of any biomarker parameter can be classified as follows: 0.90-1.00, excellent; 0.80-0.90, good; 0.70-0.80, fair; 0.60-0.70, poor and 0.50-0.60 failing,24 in the current study, the AUC of serum Ng in the thrombotic and embolic groups was AUC (0.879 and 0.897, respectively). This means that Ng is an excellent biomarker parameter to be used for diagnosis and to distinguish patients with ischemic stroke, as illustrated in Figure 3. Additionally, the cut-off values in the thrombotic and embolic groups were (0.3991 and 0.4443 ng/ml, respectively) with a sensitivity and specificity of (80.4% and 88.9%, respectively) in the thrombotic group, while in the embolic (cardioembolism) group they were (75.6% and 100%, respectively), as illustrated in Table 4. These findings were consistent with those reported by Kuşdoğan et al.18
Overall, the results obtained from the conducted questionnaire showed that cardiac conditions, such as myocardial infarction, heart failure, and atrial fibrillation were among the most significant contributors to embolic ischemic stroke in our cohort ( Table 1). These results are consistent with established literature demonstrating a strong association between cardiac comorbidities and cerebrovascular events.25 In contrast, thrombotic stroke is primarily associated with metabolic syndrome and atherosclerotic vascular diseases. Metabolic syndrome, characterized by high blood pressure (HBP), insulin resistance, obesity, and dyslipidemia, contributes to cerebrovascular pathology through distinct inflammatory and vascular mechanisms. This syndrome promotes a chronic pro-inflammatory state characterized by elevated levels of tumor necrosis factor-alpha (TNF-α), which weakens endothelial function and increases BBB permeability. HBP and dyslipidemia accelerate atherosclerotic changes in the cerebral vasculature, compromising neuronal and glial perfusion. Progressive atherosclerosis induces chronic cerebral hypoxia, whereas TNF-α-mediated glutamate dysregulation triggers excitotoxic neuronal injury. The resulting energy deficit leads to mitochondrial dysfunction, oxidative stress, lipid peroxidation, and ultimately neuronal apoptosis.26–28 These damaged neurons lead to the release of intracellular proteins, including Ng, into the bloodstream, thereby providing a biomarker of neuronal injury. Moreover, our analysis revealed that modifiable risk factors, such as hypertension, diabetes mellitus, smoking, alcohol consumption, obesity, and lifestyle (physical inactivity)—represent critical targets for preventive interventions. In contrast, Non-modifiable factors, such as age, sex, and previous stroke, were significant predictors of increased stroke risk. As these factors cannot be modified, their presence in patients emphasizes the critical importance of early identification and effective management of controllable risk factors to reduce stroke risk and optimize prevention strategies. These findings are consistent with those reported by Murphy et al. and Nindrea et al.5,29
Our findings indicate that serum Ng levels suggest that it can be used as a successful diagnostic tool for AIS patients during the early stages of the disease. The performance was better in the embolic group, which may be due to the larger infarct size. Moreover, Ng levels demonstrated positive correlations with several biomarker parameters that may improve the diagnostic efficiency of Ng, specifically platelets, TC, and LDL, in the embolic group. Furthermore, having had a previous stroke is a factor that can help to identify individuals at the highest risk of recurrent ischemic stroke.
Figshare Repository: Serum Neurogranin as a Diagnostic Biomarker for Acute Ischemic Stroke: Performance Comparison Between Thrombotic and Embolic subtypes, https://doi.org/10.6084/m9.figshare.30664889.v1.17
This dataset contains the following underlying data:
Figshare Repository: Serum Neurogranin as a Diagnostic Biomarker for Acute Ischemic Stroke: Performance Comparison Between Thrombotic and Embolic subtypes, https://doi.org/10.6084/m9.figshare.30664889.v1.17
This dataset contains the following underlying data:
Figshare Repository: Serum Neurogranin as a Diagnostic Biomarker for Acute Ischemic Stroke: Performance Comparison Between Thrombotic and Embolic subtypes, https://doi.org/10.6084/m9.figshare.30664889.v1.17
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
For their invaluable help in patient recruitment clinical assessment and sample collection, the authors would like to thank the neurology departments at all participating centers, physicians, nurses, and laboratory staff for their invaluable assistance in patient recruitment. Finally, we would like to sincerely thank all of the patients who voluntarily participated in this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)