Keywords
Bioavailability, Liquid suppository, Mucoadhesive, Nefopam HCl, Poloxamer, Themosenstive.
Nefopam hydrochloride (NPH) is a non-narcotic analgesic that is severely affected by its extensive hepatic metabolism resulting in low oral bioavailability. This study aimed to develop NPH thermosensitive in situ gel to enhance its bioavailability by avoidance of first pass effect through rectal administration.
A cold method was employed to develop NPH thermosensitive rectal in situ gel utilizing various concentrations of poloxamer 407 (P 407) and poloxamer 188 (P188) alone or in mixture as thermosensitive polymers and hydroxypropyl methylcellulose K4M (HPMCK4M) as well as carboxymethyl cellulose (CMC) as mucoadhesive polymers.
The achieved formulas were assessed for various in vitro constraints including: solution-gel temperature, gelation time, appearance, pH, gel strength, viscosity, in vitro drug release study. Furthermore, the optimized formula was evaluated for in vivo localization and permeability.
The obtained outcomes demonstrated a direct correlation between solution-gelation temperatures and poloxamer 188 concentration as well as an inverse correlation with the concentration of both P407 and HPMCK4M. A direct correlation was perceived between the mucoadhesive forces and viscosity with HPMCK4M concentration. Additionally, an inverse correlation was observed between NPH released with HPMCK4M concentration.
The optimal NPH gel formula (F8) (18% P407/2% P188 and 0.6%) presented a compatible pH value (7.2±0.35), an acceptable sol-gel T (35.4 °C), gel strength (39.54 ± 0.803), a mucoadhesion force of 6340.6 dyne/cm2 and sustained drug release of 85% at 8 hrs. Additionally it showed sufficient localization and a permeation flux of 0.0398 mg/cm2/h, and apparent permeability (Papp) of 1.99*10−3.
It was concluded that this drug delivery system may serve as a promising alternative to other dosage forms containing NPH, owing to avoidance of first-pass metabolism, enhanced bioavailability, non-invasiveness, and reduced adverse effects associated with other forms.
Bioavailability, Liquid suppository, Mucoadhesive, Nefopam HCl, Poloxamer, Themosenstive.
Pain mainly postoperative pain is a common condition that is the most frequent complaints of hospitalized patients. Even though the most effective analgesic in this situation is opioids, many serious side effects are related to them such as respiratory depression and sedation.1,2
Nefopam hydrochloride (NPH) is a non-narcoticanalgesic that is frequently used to relieve moderate to severe postoperative pain. It acts by increasing neurotransmitter activity (serotonin, norepinephrine, and dopamine) which influences pain signaling in the brain and spinal cord. Additionally, it prevents glutamate release, which is known as a main neurotransmitter related to the pain signaling progression.3 NPH is categorized as a class I drug in the biopharmaceutics classification system (BCS) with a molecular mass of 289.8, log P 3.4, and pKa 8.98.4
NPH is administered orally, intramuscularly, and intravenously to avoid the multitude of side effects produced by opioid drugs and reduce their consumption by 30–40%.5,6
After oral administration, NPH reaches peak plasma concentration by one to three hours with an elimination half-life of approximately four hours. NPH has a low bioavailability (about 36%) due to extensive hepatic metabolism and first pass effect which results in conversion into inactive metabolites that are primarily eliminated by urine.7,8
Rectal administration could be an effective alternative to oral administration to overcome bioavailability issues related to first-pass degradation. Additionally, among the various noninvasive methods available, the rectal route is a safe substitute for oral and intravenous drug administration, especially for patients with swallowing difficulties, nausea, or vomiting, as well as for infants and elderly patients.9
Upon insertion into the anus, a traditional solid suppository melts, dissolves, or softens in the rectal region to exert its effect. It has several drawbacks among them an alien feeling, pain sensation, anal leakage, low patient compliance, and patient discomfort which could result in patient rejection.10
One of the novelties in rectal dosage forms is in situ liquid suppositories, which are fluid-liquid preparations that are transformed into gel inside the body upon insertion due to the effect of external stimuli for instance body temperature.11
Poloxamer is the pillar of a thermosensitive in situ gelling system, a type of water-soluble, nonionic triblock copolymer.12 Its thermosensitive properties and micellar formation are mainly related to its triblock structure, which combines hydrophilic and hydrophobic segments. In appropriate concentration poloxamer forms a gel at physiological body temperature, accordingly leakage prevention and extreme distribution in the rectum.13
Additionally, mucoadhesive polymers are usually combined with thermosensitive polymers to exhibit an elevated level of retention at the site of application as well as provide a more sustained release pattern.14 Consequently, an in situ thermoresponsive mucoadhesive gel has been considered to be more appropriate than a conventional solid suppository due to its simple administration to the rectum, safe to the rectal mucosal layers, adherence to the mucosal rectal tissue deprived of outflow and a foreign body feeling reduction.9
The present work aimed to develop an in-situ thermos-gelling, mucoadhesive liquid suppository of NPH to improve the bioavailability and decrease the side effects associated with oral administration as the absorbed drug avoids the liver’s first-pass metabolism as well as avoid the drawbacks associated with solid suppositories.
This research was done at the Department of Pharmaceutics, College of Pharmacy, University of Mustansiriyah, Baghdad, Iraq (1st of December 2023).
NPH was purchased from Targetmol Chemicals Inc., USA. Poloxamer 407 (P 407), poloxamer 188 (P188), and hydroxypropyl methylcellulose K4M (HPMC K4M) were purchased from Macklin Biochemical Co, Shanghai, China. Carboxymethylcellulose (CMC) was purchased from Shanghai Honest Chemicals, China. All chemical ingredients a reagent utilized were of analytical grade. Distilled water was purchased from SDI, Iraq.
The investigations were performed after approval by the ethical committee at Mustansiriyah University, College of Pharmacy (Approval No. 40 on 3/11/2024). We conducted the experiments following the guidelines established by the Committee for Control and Supervision of Experiments on Animals (CPCSEA). We applied methylated spirit as an antiseptic to prevent infection and used careful, gentle handling to minimize animal distress. Two animals were divided into two groups and housed in separated cages under optimal conditions of 25 ± 2°C, 50 ± 1% relative humidity (RH), and a 12-hour light/dark cycle, with regular access to water.
Method of euthanasia
According to AVMA guidelines for the euthanasia of animals 2020 edition, IV barbiturate was used as following steps:
1. Pre-Euthanasia Preparation:
Ensure a calm and quiet environment to minimize stress for the rabbit and Gather necessary equipment and medications, including anesthetics and euthanasia solutions.
2. Sedation/Anesthesia:
Administer a sedative or anesthetic, common combinations (Ketamine (20-40 mg/kg) and Xylazine (2-5 mg/kg) ‘given intramuscularly’). Then monitor the rabbit until it is adequately sedated and unconscious. This may take a few minutes, and sedation may be necessary to gain venous access for the administration of the euthanasia solution.
3. Euthanasia Solution:
Once the rabbit is fully sedated, administer the euthanasia solution, typically an injectable solution containing pentobarbital sodium (barbiturate).
4. Confirmation of Death:
Lack of breathing, palpable heartbeat, and a fixed dilated pupil are some of the easiest recognized indicators of death.
Method of disposal of euthanized animals: Regardless of the euthanasia method chosen, animal remains must be handled appropriately and following state and local law.
To identify the most suitable concentration of poloxamer that exhibited thermo-reversible properties below 37 °C (the temperature of the rectal cavity 37°C), an initial screening of different concentrations of P407 and P188 solutions was performed as shown in Table 1. A dispersion of an accurately weighed amount of poloxamer (7.5-22.5%) in cold distilled water (100 ml) was prepared by slow continuous stirring (200-300 rpm) till complete desperation took place. Next, the prepared dispersions were reserved overnight in the refrigerator to confirm thorough dissolution and air bubble eradication.15
| Formula Code | P407 % (w/v) | P188 % (w/v) | D.D.W q.s mL |
|---|---|---|---|
| FP1 | 7.5 | 100 | |
| FP2 | 10 | 100 | |
| FP3 | 12.5 | 100 | |
| FP4 | 15 | 100 | |
| FP5 | 17.5 | 100 | |
| FP6 | 20 | 100 | |
| FP7 | 22.5 | 100 | |
| FP8 | 17 | 3 | 100 |
| FP9 | 18 | 2 | 100 |
| FP10 | 19 | 1 | 100 |
Formulas having appropriate gelation temperature (32-36 °C) were utilized for incorporation of NPH and mucoadhesive polymers (HPMC K4M and CMC) in different concentrations (0.4-1%) to formulate in situ gelling rectal suppositories utilizing the cold method and as shown in Table 2.16
A precisely weighed amount of poloxamer (P407 and P188) for each formula was dissolved in a proper volume of distilled water at room temperature (25 °C). Consequently, the dispersion was sited inside an ice bath (4°) with continuous stirring for 30 min till a homogenous dispersion was acquired. The prepared dispersion was then kept overnight in a refrigerator to acquire a clear preparation. The accurate weight of mucoadhesive polymer (HPMC K4M or CMC) was dispersed in 30% of DW required for the formula and constantly stirred through a magnetic stirrer. Subsequently, both poloxamer and mucoadhesive polymer dispersions were blended constantly on an ice bath at 4° located on a magnetic stirrer.
Determination of Sol-Gel transition temperature
Ten milliliters of the liquid suppository were placed in a 20 mL transparent vial containing a magnetic bar. The vial then was placed in a thermostat water bath set at 25 °C and the liquid suppository was heated at a fixed rate (2 °C/min) with constant stirring (50 rpm). When the magnetic bar stopped moving due to gelation, the temperature was documented as the gelation temperature.17,18
Gelation time (Gt)
The reported approach was followed in determining the gelation time. In summary, a 30 mL universal tube containing 10 mL of the formulation was magnetically stirred at 100 rpm while the temperature was gradually raised (1°C/min). The gelation time was defined as the moment when the magnetic bead stopped rotating.19
Appearance and pH determination
The general appearance of formulated in situ gel was assessed by employing visual inspection for clarity and homogeneity under a white and black background.20
The pH of the formulated in situ gel was evaluated utilizing a calibrated glass prob pH meter (Hanna Instruments, Italy). The pH meter probe was immersed in each formula and the average of three determinations for each formula was taken.21,22
Measurement of gel strength
Gel strength was determined in triplicate by weighing 5 g of each formula, which was placed in a 10 mL measuring cylinder using a temperature-controlled water bath; after the formula congealed at the gelation point, the gel was pressured using a 3.5 g weight via a circular cup. The time taken by weight of 3.5 g to penetrate 0.5 cm through the gel was recorded in seconds.23
Drug content
One mL of prepared formula equivalent to 2 mg of NPH was accurately taken and diluted to 10 mL by phosphate buffer (pH 7.4), the solution was filtered through a 0.45 μm pore size Millipore filter, The ultraviolet/visible spectrophotometer (Shimadzu®, Japan) was used to analyze the resultant solution spectrophotometrically at 266 nm against phosphate buffer (pH 7.4) as a baseline. The concentration of the ingredient in the in-situ gelling liquid suppository formula was determined using a calibration curve that had been previously established. Drug content tests were conducted in triplicate.24
Determination of the Mucoadhesive Force
To measure the detachment force required to separate the developed liquid suppositories from fresh specimen of rectal sheep mucosal tissue, a modified mucoadhesive measuring apparatus was implemented. A tissue specimen from the mucosal side of the sheep rectum cavity was obtained from a nearby slaughterhouse and kept in normal saline. A piece of the rectum (surface area 3 cm* 3 cm) was cut and glued with cyanoacrylate adhesive on the ground surface of a tissue holder made of wood attached to the right arm of the balance. The measured amount (30 mL) of gel to be examined was added to the modified container located below the tissue holder. The tissue holders with rectal tissues and gel were subjected to uniform and constant pressure for 5 minutes (preload time) to promote adhesion bonding. Following a preload period, water was introduced into a pre-weighed container positioned on the left arm of the balance via an intravenous infusion set at a rate of one drop per second until the two adhered surfaces separated. The water in the container was subsequently measured and weighed.25
The measured weight was transformed into the force required for detachment using the equation:
A Brookfield-DVE-USA viscometer was used to determine the viscosity of the formulated in situ gels. Initially, 20 mL of the prepared formula was placed in a small vial at room temperature (25 °C). The temperature of the prepared formula was then elevated to body temperature (37 °C) employing a water bath. Measurements were performed in triplicate for each prepared formula and recorded for 25 °C and 37 °C using spindle number 64.27
A modified method was implemented to simulate an in vivo drug release from prepared in situ gel formulations. The study was conducted by using a USP type II dissolution apparatus (Vanguard Pharmaceutical Machinery, INC. USA).
An accurately measured volume (10 mL) of in situ gel (containing 20 mg of NPH) was placed in a dialysis bag prepared from a previously soaked dialysis membrane (molecular weight of 8000-14000 Dalton) in PBS pH 7.4 for 24 hrs. The dialysis membrane was secured from one side using rubber bands prior to the placement of the gel. The other side of the bag was also secured post-gel settlement by the same method and then attached to the paddle firmly and immersed in a 350 mL phosphate buffer (PBS) pH 7.4, which was maintained at 37 ± 0.5°C and rotated at 50 rpm. Samples from the dissolution medium were withdrawn at scheduled times and replaced with fresh buffer medium to provide the sink condition and absorbance was measured with UV spectrophotometry at 266 nm.28,29
The selection of the NPH-loaded in situ gel formula was based on parameters such as gelation temperature, Gt, pH, gel strength, viscosity, mucoadhesive force, and in vitro drug release studies. The selected formula was then subject to further evaluation tests.
A modified method was employed to study the extent of localization of NPH thermosensitive in situ gel. The inclusion and exclusion criteria for animals encompassed parameters such as health status, age, weight, and sex. We divided two albino rabbits weighing 2–2.5 kg into two groups: Group I (control, formula 8 without mucoadhesive polymer) and Group II (formula 8). We caged them separately according to their groups, providing them with water and proper environmental conditions. Since the study did not identify a single primary outcome measure for hypothesis testing, we did not perform an a priori sample size calculation. Instead, we established the sample size based on existing literature. Prior to the experiment two male albino rabbits were fasted for 24 hours with free access to water to reduce the rectum fecal content. We used an electric clipper to remove the hair from the male albino rabbits’ abdominal surface (5*5 cm) without compromising the skin’s integrity. Then we applied methylated spirit as an antiseptic with cotton wool to the shaved area to prevent bacterial infection. Formula 8 (NPH-18% P407/2% P188/0.6 HPMCK4M) and control formula (NPH-18% P407 2% P188) (gelation temperature = 35.4 °C) were injected into the rectum 4 cm above the anus through a catheter which was affixed to a disposable syringe afterward incorporation of 1% methylene blue to each formula. At 30 minutes and 6 hours following administration, the rectum was sectioned, and the localization of the liquid suppository formulas in the rectum was identified by the blue color.30
Animal handling and care during the experimental procedure adhered to the “Experimentation on Animals in the Course of Medical Research and Education” (CPCSEA) criteria and the AVMA 2020 guidelines.31 The investigations were performed after approval by the ethical committee at Mustansiriyah University, College of Pharmacy (Approval No. 40 on 3/11/2024). In our animal studies, we employ several strategies to alleviate animal suffering. This involves ensuring a calm and quiet environment to minimize stress, modifying experimental procedures to minimize pain and distress by using less invasive techniques, improving housing conditions, and providing better care. Anesthesia during procedures helps alleviate suffering.
An ex vivo permeation study for the optimum selected formulation (F8) and control containing NPH aqueous solution (drug concentration 2 mg/mL) was implemented by Franz diffusion cell using a fresh specimen of a sheep rectum received from a local slaughterhouse. After thoroughly washing with PBS pH 7.4, the mucosal tissue was cut into sections measuring (2.5 cm * 2.5 cm). The prepared sections were placed in cooled PBS pH 7.4. The diffusion surface area was 2.5 cm2. The receptor compartment was filled with 28mL of PBS pH 7.4 and the cell was set at 37 ± 0.5 °C with the aid of a thermostatic bath and magnetic stirrer.32,33 Formulation equivalent to 20 mg of drug was placed in the donor compartment which was in connection with the mucosal surface of the membrane. A portion of 1mL was withdrawn from the receptor compartment at suitable timing intervals (15,30,45,60,90,120,150,180, 210, 240, 270,300,330,360 and 480 min) and replaced with fresh buffer medium to maintain the sink condition and analyzed for drug concentration spectrophotometrically double beam UV-Vis spectrophotometer at lambda max.34
The permeability coefficient was calculated at steady-state conditions by using the following equation35:
Obtained data were analyzed via a one-way analysis of variance (ANOVA) test. Statistical dissimilarities are counted as significant when (p<0.05).36
Solution-gelation temperature (sol-gel T) is the temperature at which the liquid phase makes a transition to gel. The temperature of the rectal mucosa is reported to be 37 °C as a body temperature. Consequently, the in situ gel formula is designed to have a sol-gel T in the range of 32–36 °C to maintain its liquid state at room temperature and readily transformed into a gel when it comes into contact with the rectal mucosa inside the body upon administration.37,38
The sol-gel temperature of the prepared preliminary formulations was identified and the obtained results are revealed in Table 3.
| Formula Code | P407 % (w/v) | P188 % (w/v) | Sol-gel T °C |
|---|---|---|---|
| PF1 | 7.5 | 55 | |
| PF2 | 10 | 51 | |
| PF3 | 12.5 | 45 | |
| PF4 | 15 | 41 | |
| PF5 | 17.5 | 38 | |
| PF6 | 20 | 35 | |
| PF7 | 22.5 | 26.5 | |
| PF8 | 17 | 3 | > 40 |
| PF9 | 18 | 2 | 32.8 |
| PF10 | 19 | 1 | 32 |
The obtained outcomes show that the sol-gel T decreased significantly (p < 0.05) as the concentration of P407 increased (PF1-PF7). This could be related to the availability of P407 chains for micelle formation and subsequently increases in the lattice packing order. Therefore, an increase in P407 concentration favored temperature-induced gelation and caused a lower gelation temperature.39 A similar observation was obtained by Niyompanich J et al., who developed gentamicin sulfate in situ gel thermosensitive gel for wound treatment.40
Based on these findings P407 alone (PF1-PF7) could not provide the required sol-gel T. Consequently modulation of the sol-gel T was implemented and accomplished through the use of a combination of P407 and P188.16
Up on implementing the combination an opposite result was obtained since increasing P188 concentration in the blend resulted in a significant (p < 0.05) increase in sol-gel T (PF8 - PF10). This outcome could be explained by the fact that P188 is more hydrophilic than P407 due to a higher ratio of polyethylene oxide/polypropylene oxide (PEO/PPO) group content compared to P407. Consequently, the addition of a minor amount of P188 to P407 changed the PEO proportion in the mixed polymer solutions since both had an equal proportion of PPO, leading to an increase in sol-gel T. Similar results were previously reported by Ibrahim E S et al.41
Formulas (PF6, PF9, and PF10) that demonstrated an appropriate sol-gel T were subsequently selected for incorporation of NPH and various mucoadhesive polymers (HPMC K4M and CMC) in different concentrations (0.4-1%).
The impact of various mucoadhesive polymers (HPMC and CMC) on sol-gel T of prepared formulas (F6-F22) is shown in Table 4.
| Formula Code | Sol-gel T (°C) | Gt (min) | APP* | pH | Drug Content (%) | Gel strength (sec) |
|---|---|---|---|---|---|---|
| F1 | >40 | NA | ++ | NA | NA | NA |
| F2 | > 40 | NA | + | NA | NA | NA |
| F3 | >40 | NA | + | NA | NA | NA |
| F4 | 39.8 | NA | + | NA | NA | NA |
| F5 | 39 | NA | + ppt. | NA | NA | NA |
| F6 | 36.2 | 4:17 | ++ | 6.76±0.05 | 97.79±0.32 | 6.24±0.946 |
| F7 | 36 | 4:51 | + | 7.1±0.26 | 100.86±1.38 | 10.02 ± 0.012 |
| F8 | 35.4 | 4:08 | + | 7.2±0.35 | 102.12±0.37 | 39.54 ± 0.803 |
| F9 | 33.8 | 3:45 | + | 7.13±0.17 | 99.46±0.92 | 48.54 ± 0.515 |
| F10 | 32.9 | 3:28 | + ppt. | 7.15 ± 0.1 | 99.19±1.30 | 59.72 ± 0.350 |
| F11 | > 40 | NA | + | NA | NA | NA |
| F12 | 39.8 | NA | + | NA | NA | NA |
| F13 | 38.6 | NA | + | NA | NA | NA |
| F14 | 37.1 | NA | + | NA | NA | NA |
| F15 | 36 | 3:38 | ++ | 6.8±0.07 | 98.420±0.928 | 31.31 ±1.05 |
| F116 | 35.8 | 5:53 | + | NA | NA | NA |
| F17 | 34.5 | 5:28 | + | NA | NA | NA |
| F18 | 33.2 | 4:58 | + | 7.05 ± 0.1 | 98.26±0.98 | 1.06 min. ± 0.065 |
| F19 | 32.4 | 4:26 | + ppt | 7 ± 0.1 | 99.12±0.95 | 1.32 min.± 0.117 |
| F20 | 36.4 | 3:57 | ++ | 6.82±0.04 | 98.46±0.57 | 58.54±0.528 |
| F21 | 33.8 | 3:33 | + | 6.8 ± 0.14 | 97.72±1.36 | 1.21min.±0.157 |
| F22 | 32.6 | 2:55 | + | 6.85± 0.05 | 98.06±0.41 | 1.48min.±0.170 |
The obtained result demonstrated a significant (p < 0.05) decline in the sol-gel T as the mucoadhesive polymer concentration increased (0.4 to 1% w/v). A similar observation was obtained by Thomas LM et al. in the preparation of lornoxicam in situ gelling liquid suppository.42 This decline in sol-gel T could be explained by the polymer’s ability to attach to the polyoxyethylene chains in the molecules of poloxamer. Consequently, dehydration is facilitated, which improves the adjunct’s entanglement and significantly enhances intermolecular hydrogen bonding. As a result, gelation occurs at lower temperatures.43
The obtained results also indicated that upon incorporation of NPH (PF9 and F6), the sol-gel T increased significantly (P < 0.05). This could be explained by the modification of the micellar association process of poloxamer solutions by water-soluble molecules, consequently increasing the sol-gel T.44
Gelation time is similarly essential as sol-gel T since the mixture must transform into a gel after installation and persist in a gel state for sufficient time to allow the drug to release. Consequently, liquid suppositories with ideal gel strength and a relatively short gelation time will stay in the suitable part of the rectum and will not leak out the anus.45 The Gt of prepared in situ gel formulas is presented in Table 4.
The obtained outcomes demonstrated that as the concentration of mucoadhesive polymers increased as in formulas (F7-F10) and (F18-F22) a significant depletion (p < 0.05) in Gt was observed. These results could be justified by the development of hydrogen bonding concerning the poloxamer’s PEO chain and the mucoadhesive polymer and consequently a prompt growth of micellar structures.46 The obtained results were in line with the results achieved by Rençber S et al.47
A visual examination of selected formulas was done to assess for any incompatibilities resulting from an interaction between the excipients or between the excipients and the active pharmaceutical ingredient. The appearance of the prepared formulations is displayed in Table 4. Formulas (F1-F10) and (F15-F22) were clear and revealed the formation of precipitate at the bottom of the container for formulas that contain the highest concentrations of HPMC K4M (1%). On the other hand, formulas (F11-F14) were clear and revealed the absence of any precipitation which indicates the absence of any interaction.7
The pH of the rectum is normally neutral (7.2–7.4). Alterations in pH can cause discomfort or damage to the rectal mucosa, as well as affect formula stability.48 The pH of developed liquid suppositories is shown in Table 4. They were discovered to be between (6.76±0.05-7.2 ± 0.35) which is thought to be around the natural pH of the rectum and suitable for rectal administration with non-significant tissue irritation.49
In formulating a liquid suppository, the gel strength plays a crucial role in determining the ideal condition that permits the suppositories to be easily inserted and not leak out from the anus.38 A liquid suppository with a gel strength of less than 10 seconds may lose its shape and leak out of the anus, whereas a liquid suppository with a duration of more than 50 seconds might be excessively hard and painful for the rectum.50
In formulas with an optimum sol-gel T (F6-F10) and (F18 - F22), the obtained gel strength was from (6.24 ± 0.946 sec - 1.48 min.± 0.170), as shown in Table 4. The obtained findings for formulas (F6, F15, and F20) and (F7, F18, and F21) demonstrated that the gel strength of formulas was significantly (P < 0.05) increased upon the addition of mucoadhesive polymer (HPMCK4M).
Furthermore, the obtained results for formulas (F7-F10 and F20-F22) indicated that upon increasing the concentration of the mucoadhesive polymer (HPMC K4M), the gel strength of the poloxamer mixture increased significantly (p < 0.05) in a concentration-dependent manner. These results could be explained by the fact that HPMCK4M inclusion and increasing its concentration afford an increased viscosity as a result of a greater polymer chain cross-linking as well as the development of hydrogen bonds and Van der Waals between poloxamer and HPMCK4M, subsequently enhanced gel strength.51 These results were in good accordance with the results obtained by Yong et al.52
Drug content was determined to be (97.72 %-102.12 %) which is in the acceptable range according to the USP of all the formulations.53 This suggests that the technique utilized could produce gels with homogenous drug content and low variability,54 as displayed in Table 4.
The mucoadhesive force is a crucial physico-mechanical parameter for in situ gelling liquid suppositories because it prevents the formula from spreading to the upper part of the rectum, slows down rapid leakage, extends the formula’s residence duration, and prolongs drug release.14
The obtained results as shown in Table 5 and Figure 1 demonstrated that formulas (F6, F15, and F20), which contain no mucoadhesive polymer showed minor mucoadhesive properties compared to formulas that contain mucoadhesive which reflected a limited residence time as well. Consequently, the implementation of bioadhesive polymers is crucial.55
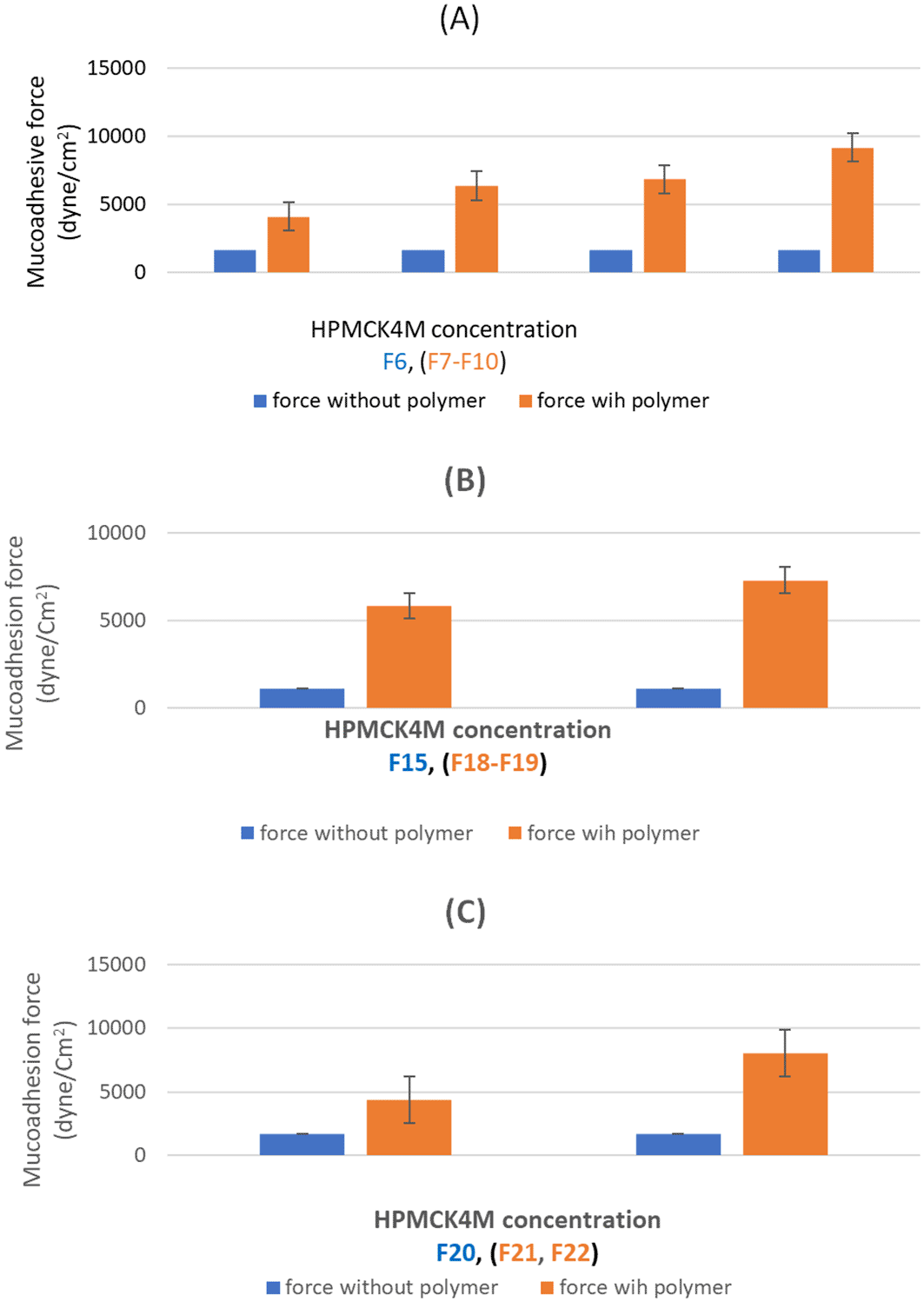
(A) mucoadhesion force of F6-F10; (B) mucoadhesion force of F15, F18 and F19; (C) mucoadhesion force of F20-F22. The results indicated that the mucoadhesive force increased significantly (p<0.05) by raising the concentration of HPMCK4M across the range of 0.4-1% in formulas (F6-F9), (F15, F18-F19) as well as (F20-F22).
Hence various mucoadhesive polymers (HPMCK4M and CMC) in different concentrations (0.4-1%) were incorporated and formulas with an optimum sol-gel T were then experimented for mucoadhesive force. The obtained outcomes revealed that none of the formulas went over the highest acceptable limit (10,000 dyne/cm2) which indicates that the prepared formulas possess ideal mucoadhesion characteristics.56
The achieved results declared a significant (p<0.05) enhancement in mucoadhesive force was obtained by the incorporation of mucoadhesive polymer (F7, F18, and F21) compared to (F6, F15, and F20) respectively. The presence of hydroxyl groups in HPMCK4M may contributed to this finding by improving the substance’s capacity to create hydrogen bonds with mucus which is considered an essential stage in the mucoadhesion process.57 A comparable result was reported by Al Hablawi et al.26
Furthermore, the results indicated that the mucoadhesive force increased significantly (p<0.05) by raising the concentration of HPMCK4M across the range of 0.4-1% in formulas (F6-F9), (F15, F18-F19) as well as (F20-F22).
The abundance of secondary bond-forming groups in the mucoadhesive polymers explains the ability of mucoadhesive polymers to bind, via hydrogen bond, to the glycoprotein chains of the rectum mucus layer and result in enhancing the mucoadhesion in a concentration-dependent manner. Consequently increasing concentration will result in increasing the binding site available for adhesion.58,59 Similar results were previously reported by Salman et al.60
The viscosity data of selected formulas (F6-F10), (F15, F18- F19), and (F21-F22) at liquid and gel phases under different shear rates (rpm) are shown in Figure 3.
All the prepared formulas showed quite low viscosity at room temperature while a significant increase (p < 0.05) in viscosity was obtained when the temperature increased to biological temperature as shown in Figure 2. This could be explained by the fact that the gelation of in situ gel is mainly related to the breakage of aqua-phobic linkage covering the poloxamer molecule at low temperatures and the separation of the solvent component as the temperature increases. Accordingly, gel formation due to hydrophobic interaction that formed due to increased chain friction and entanglement in the polymer is responsible for the gelation of P40715 Similar results were previously reported by previous literatures.61,62
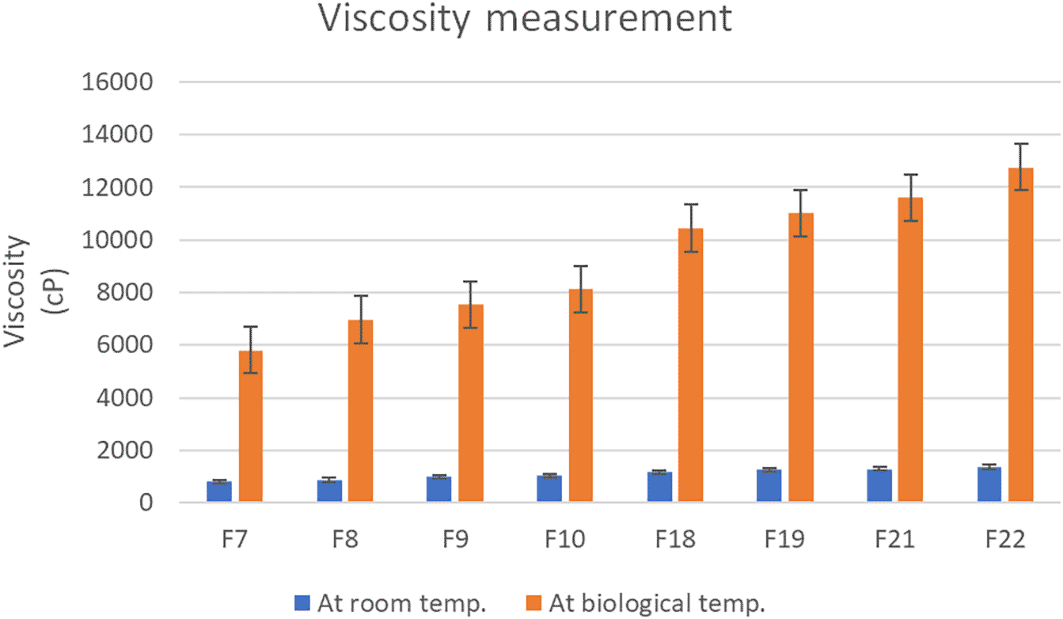
A significant increase (p < 0.05) in viscosity was obtained when the temperature increased to biological temperature.
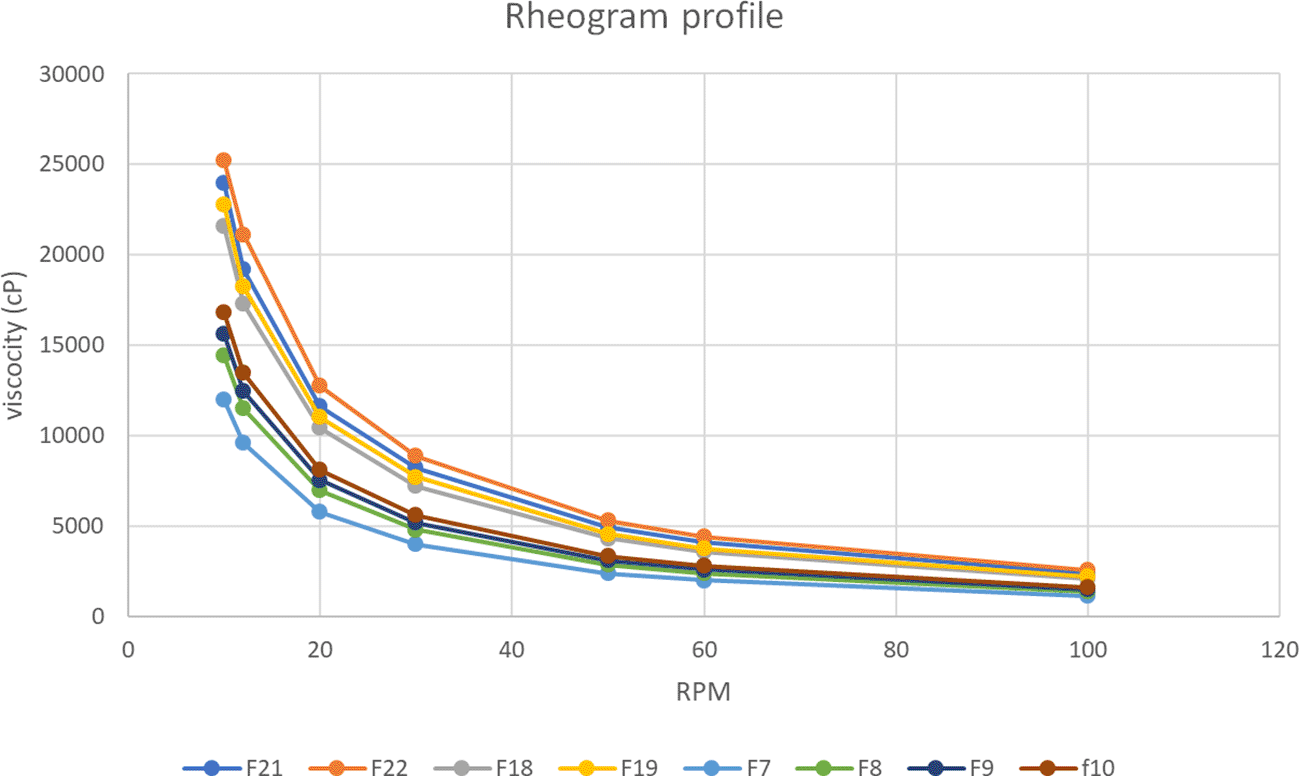
The results of this study also demonstrated that the viscosity reduced significantly (p<0.05) as the rotation speed (shear rate) increased.
The viscosity-temperature relationship deviates from the Arrhenius equation, which states that viscosity is inversely related to temperature.63 The results correspondingly showed that the viscosity of in situ gelling liquid suppositories increased significantly (p<0.05) as the concentration of HPMC K4M increased (F7-F10). This could be justified by the hydrophilic ability of HPMC K4M to absorb water, which led to increased viscosity.60 Additionally, the results of this study also demonstrated that the viscosity reduced significantly (p<0.05) as the rotation speed (shear rate) increased demonstrating the pseudoplastic (shear-thinning liquid) flow of the preparation.64 This outcome is in agreement with what was reported previously.65
The in vitro release profile of NPH from in situ rectal suppository formulations is shown in Figure 4.
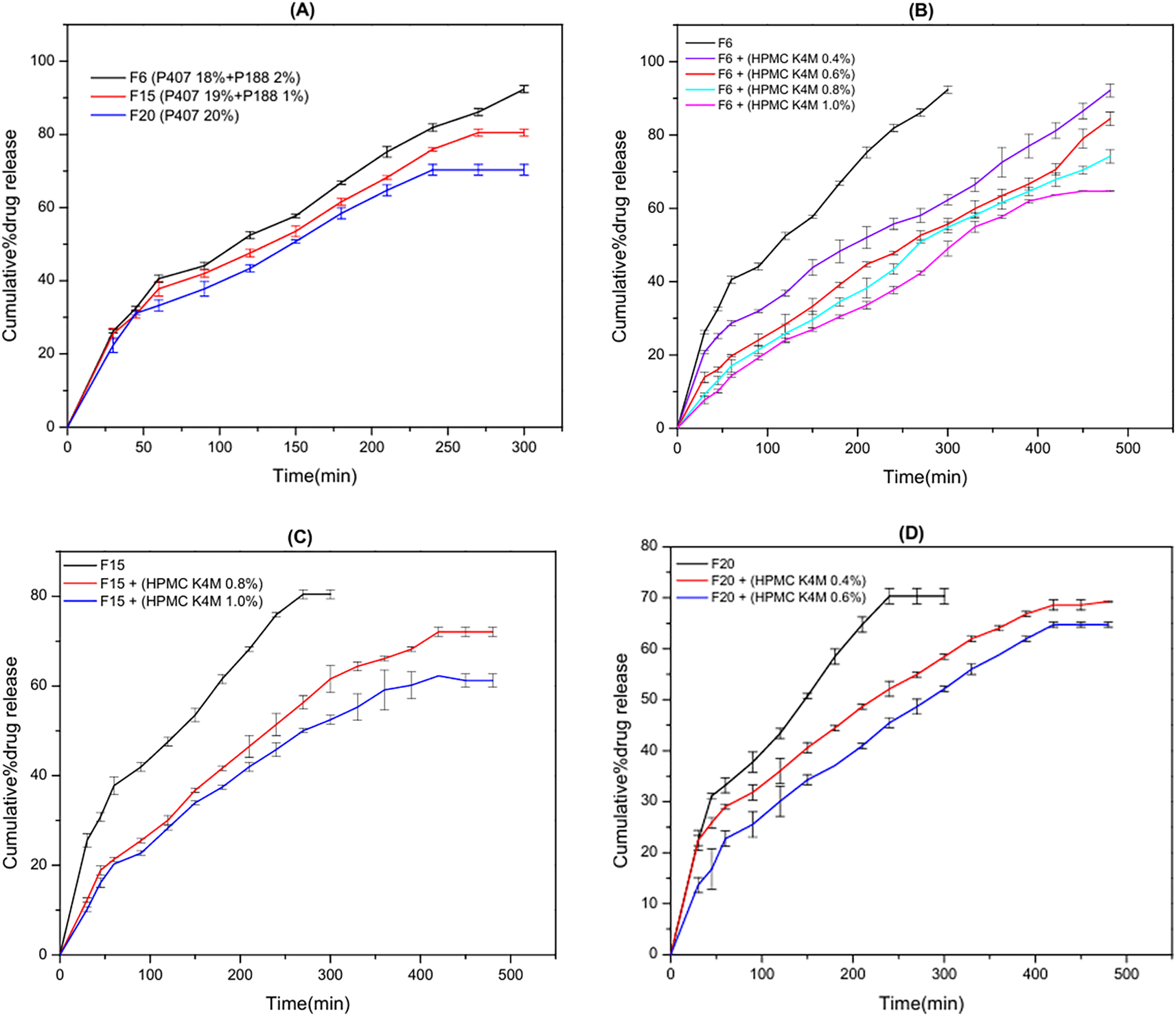
(A) NPH release profile for F6(HPMC K4M 0.0%), F15(HPMC K4M 0.0%) and F20(HPMC K4M 0.0%); (B) NPH release profile for F6, F7(HPMC K4M 0.4%), F8(HPMC K4M 0.6%), F9(HPMC K4M 0.8%) and F10(HPMC K4M 1.0%); (C) NPH release profile for F15, F18(HPMC K4M 0.8%) and F19(HPMC K4M 1.0%); (D) NPH release profile for F20, F21(HPMC K4M 0.4%) and F22(HPMC K4M 0.6%). A significant (P < 0.05) retardation in drug release was observed as the concentration of mucoadhesive polymer increased (F6-F10). The effect of the combination of mucoadhesive polymer (HPMCK4M) and its concentration is illustrated in B, C, and D.
The obtained outcomes established that the maximum drug release was obtained by F6, (blend of 2%P188 and 18% P 407) where 92 % of NPH was released within 300 min, while a minimum drug was obtained by F19, (blend) of 1.0% HPMCK4M and 19% P 407 and 1% P188 where 60% of NPH was released within 480 min.
The results also demonstrated that both P407 and P188 reduced NPH release in a concentration-dependent way as the release rate of NPH decreased significantly (P < 0.05) with increasing P407/P188 ratio (F6, F15, and F20) as shown in Figure 3A. This could be related to the ability of P407 molecules to form constricted gel arrangements by hydrogen bond formation in the aqueous media. Additionally, the hydrophilic nature of NPH facilitates its interaction with poloxamer, consequently interrupting the drug release profile. Thus, NPH release is reduced because of the postponement in both in situ gel dissolution and NPH diffusion.66
Additionally, the achieved results revealed that the incorporation of mucoadhesive polymer (HPMCK4M) retard NPH release significantly (P < 0.05). This retardation in NPH release could be justified by the capability of mucoadhesive polymer to enhance the viscosity of the formula and subsequently affect the drug diffusion and release.67 The obtained results are consistent with the results obtained by Özgüney I. et al.68
Furthermore, a significant (P < 0.05) retardation in drug release was observed as the concentration of mucoadhesive polymer increased (F6-F10). These outcomes could be explained by an amplification of gel strength as well as a decrease in the number and size of the gel structure’s channels, consequently, limiting the drug’s release by reducing the penetration of the dissolution fluid and restricting the mobility of the drug molecule.42
The effect of the combination of mucoadhesive polymer (HPMCK4M) and its concentration is illustrated in Figure 4 (B, C, and D).
Based on results from gelation behavior, gel strength, mucoadhesion, in vitro drug release studies, and rheological evaluations, F8 containing P407/P1088/HPMC in 18%, 2%, and 0.6 %, respectively was selected for further ex vivo permeability evaluation.
The optimized NPH in situ liquid suppository (F8) and control were injected into rabbits’ rectums, and its localization was detected 30 min after administration. The blue color of the liquid suppository was noticed obviously in the rectum as shown in Figure 5A. Then 6 h post to administration, the blue tint of the liquid suppository in the rectum faded as shown in Figure 5B. Though, the formula’s position in the rectum did not change significantly over time and did not delocalize to the colon. These data revealed that the mucoadhesive force of the gel formula was sufficient to maintain it in the rectum for not less than 6 h permitting the in situ gel formula to persist in the lower part of the rectum and hence protecting NPH from extensive hepatic metabolism. These outcomes could be justified by the ability of the incorporated HPMCK4M to provide the required bonds for mucoadhesion with the chains of oligosaccharides of the mucous layer lining the rectum. Similar results were previously reported by Salem HF et al.50,69
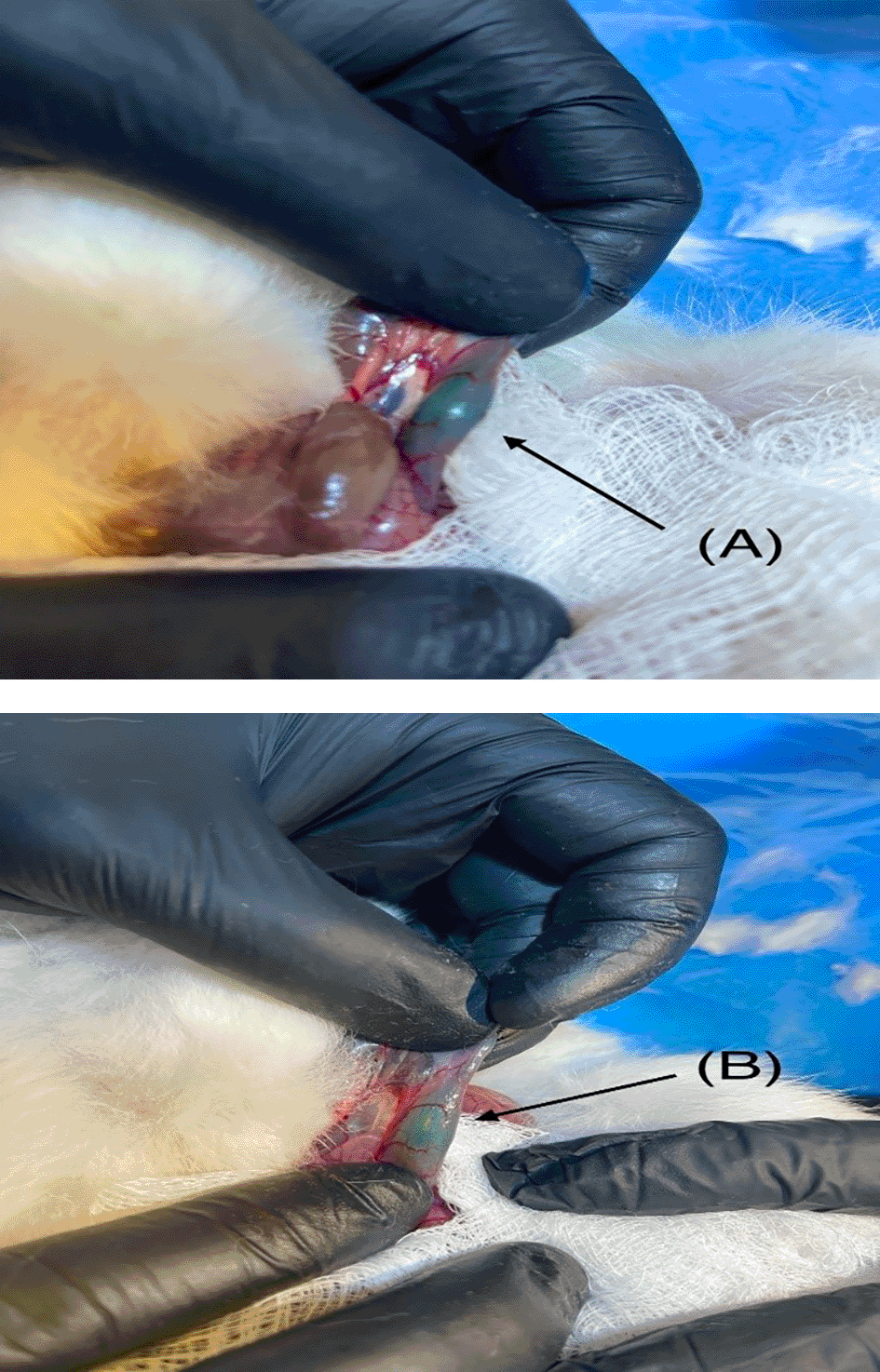
(A) NPH-LS after 30 min; (B) NPH-LS after 6 hours. The blue color of the liquid suppository was noticed obviously in the rectum as shown in A. Then 6 h post to administration, the blue tint of the liquid suppository in the rectum faded as shown in B.
The ex-vivo permeability study of the optimized in situ gel formula (F8) and the control result is shown in Figure 6. A steady state, or linear profile, was monitored for eight hours, and the slope of the linear portion of the curve was identified by the application of linear regression.
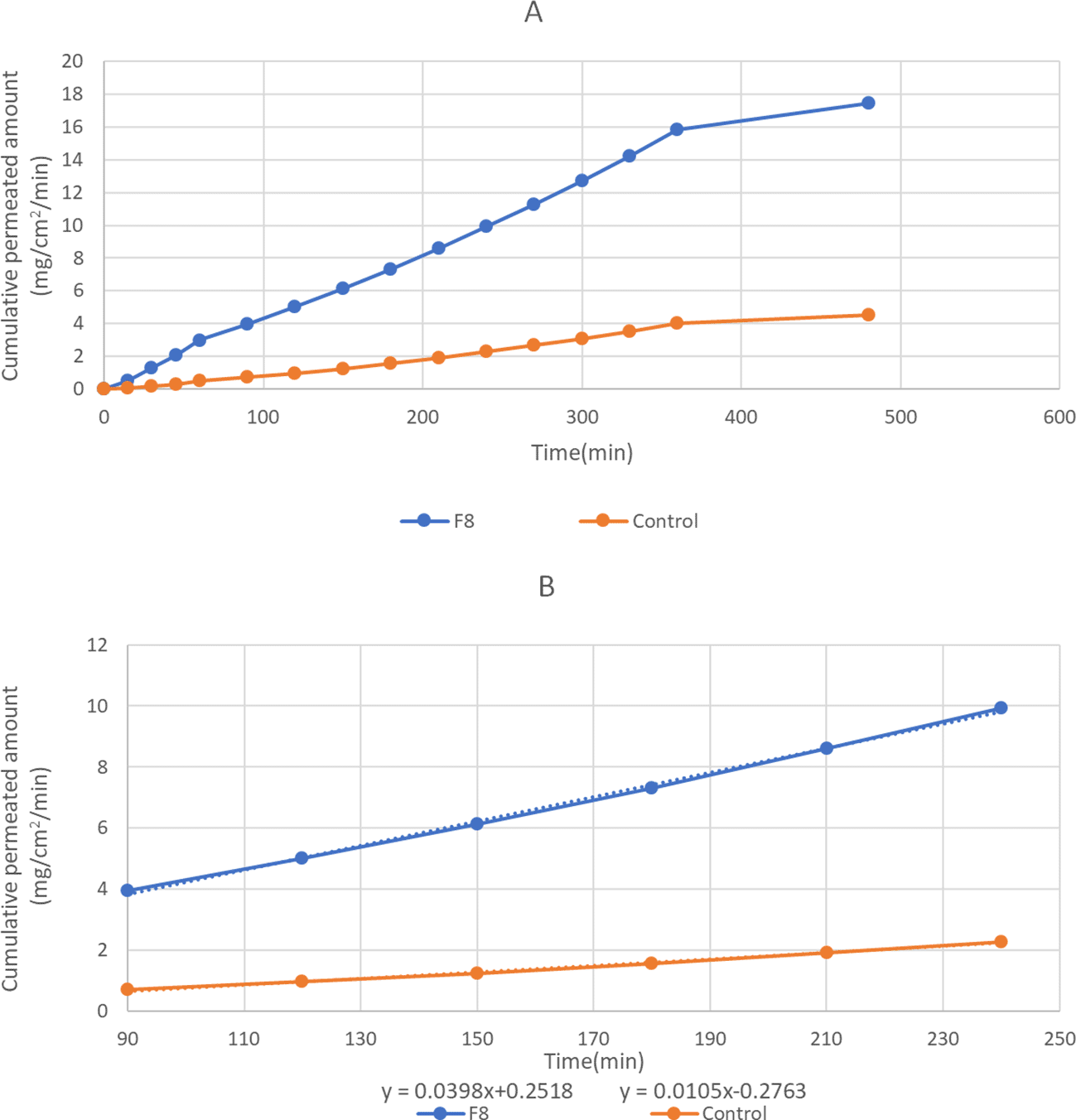
The flux values at a steady state and effective permeability were calculated and are illustrated in Table 6.
| Formula No. | Jss (mg/cm2min) | P eff. (cm/min) |
|---|---|---|
| control | 0.0105 | 5.25 * 10−4 |
| F8 | 0.0398 | 1.99 * 10−3 |
Permeation results revealed that the formula exhibited an initial rapid permeation and sustained drug permeation after 60 min. Such a delay in drug permeation may be explained by the presence of both thermosensitive polymers (P407 and P188) and mucoadhesive polymer (HPMCK4M) since the poloxamer has an improved micellar core and gel network with a mucoadhesive polymer that entraps the drug molecule and slows down the rate of drug release.24
The findings also revealed that the F8 had greater Jss and Peff compared to the control formula. This difference can be attributed to either the impact of the mucoadhesive polymer by increasing the retention period or to the permeation-enhancing effect of HPMCK4M and thermosensitive polymers (P 407 and P188).70,71
According to the attained results, it was concluded that thermosensitive in situ rectal gel of NPH could be positively formulated employing poloxamer (P407, P188) and HPMCK4M. The optimal NPH in situ gel formula (18% P407/2% P188 and 0.6%) presented compatible pH value (7.2±0.35), an acceptable sol-gel T (35.4 °C), gel strength (39.54 ± 0.803), a mucoadhesion force of 6340.6 dyne/cm2 and sustained drug release of 85% at 8 hrs. Additionally it showed sufficient localization at site of application without migration. These outcomes demonstrated that this drug delivery system could be an encouraging alternative to other dosage forms containing NPH due to avoidance of first-pass metabolism and enhanced bioavailability, non-invasiveness and reduction of side effects associated with them
Ethical approval: Animal handling and care during the experimental procedure adhered to the “Experimentation on Animals in the Course of Medical Research and Education” (CPCSEA) criteria and the AVMA 2020 guidelines. The investigations were performed after approval by the ethical committee at Mustansiriyah University, College of Pharmacy (Approval No. 40 on the 3rd of November 2024).
Conceptualization: Haydar Mahmood Ahmed, Iman Sabah Jaafar
Data Curation: Haydar Mahmood Ahmed
Formal Analysis: Haydar Mahmood Ahmed, Iman Sabah Jaafar
Funding Acquisition: Haydar Mahmood Ahmed, Iman Sabah Jaafar
Investigation: Haydar Mahmood Ahmed, Iman Sabah Jaafar
Methodology: Haydar Mahmood Ahmed, Iman Sabah Jaafar
Project Administration: Haydar Mahmood Ahmed
Resources: Haydar Mahmood Ahmed, Iman Sabah Jaafar
Software: Haydar Mahmood Ahmed, Iman Sabah Jaafar
Supervision: Iman Sabah Jaafar
Validation: Iman Sabah Jaafar
Visualization: Haydar Mahmood Ahmed, Iman Sabah Jaafar
Writing – Original Draft Preparation: Haydar Mahmood Ahmed
Writing – Review & Editing: Haydar Mahmood Ahmed, Iman Sabah Jaafar
Zenodo: Nefopam HCl in situ Mucoadhesive liquid suppository, a potential drug delivery system to enhance bioavailability, https://doi.org/10.5281/zenodo.14218213.72
This project contains the following underlying data:
Data are available under Creative Commons Zero v1.0 Universal
1. Zenodo: Materials and calberation curve ‘Nefopam HCl in situ Mucoadhesive liquid suppository, a potential drug delivery system to enhance bioavailability’, https://doi.org/10.5281/zenodo.14335091.
This project contains the following underlying data:
2. Zenodo: figures and related data to ‘Nefopam HCl in situ Mucoadhesive liquid suppository, a potential drug delivery system to enhance bioavailability’, https://zenodo.org/records/14334934
This project contains the following underlying data:
3. Zenodo: information about Anesthetic and Euthanasia used ‘Nefopam HCl in situ Mucoadhesive liquid suppository, a potential drug delivery system to enhance bioavailability’ https://doi.org/10.5281/zenodo.14335010
This project contains the following underlying data:
Creative Commons Zero v1.0 Universal
ARRIVE checklist for ‘Nefopam HCl in situ Mucoadhesive liquid suppository, a potential drug delivery system to enhance bioavailability’. https://doi.org/10.5281/zenodo.14334832.73
Creative Commons Zero v1.0 Universal
The authors are sincerely grateful for the College of Pharmacy – Mustansiriyah University (www.uomustansiriyah.edu.iq), Baghdad, Iraq for its provision.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Formulation development of Novel drug delivery system, Solubility enhancement, SNEDDS, Cubosome, NLCs
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Antimicrobials
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Drug delivery: Specializing in long acting delivery.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 04 Feb 25 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)