Keywords
Nigella sativa, Atorvastatin, Interleukin-1ß, Tumor Necrosis Factor-α, High-Fat Diet, Atherosclerosis
Nigella sativa L., known as black cumin, is thought to possess anti-inflammatory properties that may help alleviate related conditions. This study examined the effects of black cumin extract on levels of Interleukin-1β (IL-1β) and Tumor Necrosis Factor-α (TNF-α), both inflammatory markers.
The experimental design included a control group used solely for post-testing. Five groups of Wistar rats were studied: a negative control group (N), a dyslipidemia group as a positive control (P), a dyslipidemia group given black cumin (P1), a dyslipidemia group treated with atorvastatin (P2), and a dyslipidemia group receiving both atorvastatin and black cumin (P3).IL-1β and TNF-α levels were measured using ELISA, and statistical analysis was conducted using ANOVA followed by the Duncan test.
After treatment, the average IL-1β levels were 38.26 pg/mL (N), 102.16 pg/mL (P), 57.05 pg/mL (P1), 29.16 pg/mL (P2), and 54.06 pg/mL (P3). The Duncan test indicated no significant differences in IL-1β levels among groups N, P2, and P3 (p>0.05), while group P exhibited the highest IL-1β levels, significantly different from the others. For TNF-α, average levels post-treatment were 30.42 pg/mL (N), 22.02 pg/mL (P), 27.25 pg/mL (P1), 16.33 pg/mL (P2), and 13.29 pg/mL (P3). The Duncan test showed that group P3 had the lowest TNF-α levels, which were not significantly different from P2 (p>0.05) but significantly different from groups P, P1, and N (p<0.05).
In conclusion, black cumin extract effectively reduces IL-1β levels in high-fat diet rat models, while the combination of atorvastatin and black cumin extract yields the most significant reduction in TNF-α levels.
Nigella sativa, Atorvastatin, Interleukin-1ß, Tumor Necrosis Factor-α, High-Fat Diet, Atherosclerosis
Obesity is characterized by an abnormal or excessive accumulation of fat that can hinder the maintenance of optimal health. Overweight and obesity have increased substantially in recent decades, reaching epidemic proportions. Globally, at least 2.8 million deaths are linked to obesity or being overweight. While obesity was once primarily associated with high-income countries, it has now become common in low- and middle-income nations as well. According to 2022 statistics, prevalence rates vary significantly, from 12% in the South East Asian Region to 82.8% in the Western Pacific Region.1 Nearly 40% of the world’s population is currently classified as overweight or obese, with projections indicating a steady increase by 2030.2 This trend is also reflected in rising healthcare costs associated with obesity, which vary between countries, accounting for 3% to 21% of medical expenditures. Additionally, obesity is linked to noncommunicable diseases such as diabetes, hypertension, and cardiovascular conditions.1
Excess macronutrients in adipose tissues can increase inflammatory mediators including interleukin-1b and tumor necrosis factor-α, and decrease adiponectin levels, leading to oxidative stress and pro-inflammatory state.3 The finding that pro-inflammatory cytokines are expressed in obesity was made about thirty years ago. This preliminary research served as a trigger for the currently recognised paradigm, which holds that inflammation can be harmful and that food overload increases inflammation by connecting the immune and metabolic systems.4
Inflammation is primarily mediated by the NF-κB and mitogen-activated protein kinase (MAPK) pathways. When these pathways are activated, proinflammatory cytokines including IL-6, IL-8, IL-1, and TNF-α are released, potentially leading to an inflammatory response.5,6 Tumor necrosis factor (TNF) is one of the traditional, multifunctional pro-inflammatory cytokines; it was the first identified “adipokine” produced by adipose tissue, regulated by obesity, and is believed to contribute to metabolic diseases associated with obesity.7 Conversely, the IL-1 family of ligands and receptors has been extensively linked to the pathophysiology of both acute and chronic inflammatory conditions.4 Given the significant role of inflammation, recent studies have focused on identifying effective therapeutic strategies that leverage the anti-inflammatory properties of various compounds.8
Black cumin (Nigella sativa L.) is recognized for its ability to inhibit inflammation.9 Its anti-inflammatory properties, particularly those of Thymoquinone (TQ), are significant pharmacological benefits.10 Daily supplementation with 2000 mg of black cumin oil over two 8-week periods led to notable reductions in the blood mRNA expression levels of obesity-related pro-inflammatory genes, such as IL-6, IL-1β, and leptin, in overweight or obese women.11 Additionally, long-term use of black cumin for 6 to 12 weeks can significantly decrease body weight and other anthropometric measures.12 This study aimed to explore these effects by measuring Interleukin-1β (IL-1β) and Tumor Necrosis Factor-alpha (TNF-α) in rat models fed a high-fat diet after administering black cumin. The results were then compared with those from statins, which have shown a beneficial effect on reducing inflammatory markers in patients with metabolic syndrome and related conditions.13 The inflammatory markers chosen for this research were intended to evaluate both inflammatory pathways: TNF-α indicates the MAPK pathway, while IL-1β corresponds to the NF-κB pathway.
This study is fundamentally experimental in nature and utilized a post-test control group design. Conducted over eight weeks, from 1st August to the end of September 30, 2023. The research took place in the Experimental Animal Laboratory at the Faculty of Veterinary Medicine, Syiah Kuala University. The protocol for this preclinical investigation adhered to the Animal Research: Reporting in vivo Experiments (ARRIVE) Guidelines as set forth by the Institutional Animal Care and Use Committee.
Every rat was given a standard diet and given a week to acclimatise. 6 rats were fed a regular diet during the first stage (2nd–5th week), while 24 rats were provided a high-fat diet. In the second stage (6th–9th week), 24 rats on a high-fat diet were split into 4 groups [positive control (P); dyslipidemia received black cumin (P1); dyslipidemia received atorvastatin (P2); and dyslipidemia received black cumin and atorvastatin (P3)], whereas 6 rats on a standard diet were kept on the diet as a negative control (N). Each week, measurements of each rat’s body weight were made to track their weight gain. After collecting blood samples at the end of week 9 to measure IL-1β and TNF-α levels, the animals were put to sleep by intraperitoneal injection of ketamine at a dose of 15-20 mg/kg, followed by cervical dislocation.
Male Rattus novergicus strain Wistar white rats (n = 30) at 4 weeks of age, in good health, and weighing between 50 and 100 grammes were utilised in this investigation. The rats were procured from the Universitas Syiah Kuala Faculty of Veterinary Medicine. Every rat was given proper care, kept at a constant temperature of +24o C, and given unlimited access to food and drink.
The seed of Black cumin came from Surakarta, Central Java, Indonesia, and was recognised at Javaplant Factory by its product number, 2065J91N. The maceration method was used to carry out the extraction, using three 24-hour cycles of 95% ethanol solvent and one 24-hour cycle of water solvent. Crude oil with residual ethanol solvent NMT 5000 ppm was the final extract form. Phytochemical analysis was carried out at the Chemistry Education Laboratory, Faculty of Teacher Training and Education, Syiah Kuala University, Banda Aceh, using the DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2’-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid)) methods. The purpose of this study was to detect alkaloids, flavonoids, polyphenols, tannins, quinones, saponins, triterpenoids, and steroids.
Vitamin D3, 0.2% cholic acid, 2% egg yolk, 5% goat fat, and 92.8% conventional diet are all present in a high-fat diet. To make this feed, 1000 g of rat food in the form of corn rice is combined with 50 g of egg yolk and 100 g of goat fat. To get the yolk from cooked eggs, first heat goat fat until it melts. Next, 1000 g of corn rice is combined with egg yolk and goat fat. Every rat received 20 mg of diet every day.14
The data’s normality was determined using the Shapiro-Wilk test. The data analysis used a one-way ANOVA test (α=0.05) to compare IL-1β and TNF-α levels between groups. SPSS Statistics version 26 for Windows was applied to conduct this statistical test. To identify significant changes across groups, Duncan’s Test post hoc tests (α=0.05) were used.
During the research, several rats died due to unknown cause, and leaving 5 mice from each treatment group. The rat’s body weight was calculated at the start of the study, week 4 (after giving the high-fat diet and before treatment), weeks 5, 6, 7, and 8. At the end of week 4, there was a significant difference in body weight between the groups given normal feed (N) with groups given atherogenic feed (P, P1, P2, P3). At the end of the 8th week, the average body weight of the rats starting from the highest, was the positive control group/P1 (286.6 gr), negative control/N (264.8 gr), atorvastatin group/P2 (233.6 gr), Nigella Sativa group/P1 (229.6 gr), and the Nigella Sativa and Atorvastatin groups/P3 (188.8 gr). Table 1 shows the rats’ average body weights throughout the research.
The average levels of IL-1β after treatment were 38.26 pg/mL (N), 102.16 pg/mL (P), 57.05 pg/mL (P1), 29.16 pg/mL (P2), and 54.06 pg/mL (P3). The levels of IL-1β varied significantly between the groups (p=0.000). The results of further tests (post hoc) using the Duncan Test showed that there were no significant differences in IL-1β levels between N, P2, and P3 groups (p>0.05). The P group was the group with the highest IL-1β levels, and was significantly different from the IL-1β levels in the other groups ( Table 2 and Figure 1).
| Groups | Mean | SD | 95% CI | P value |
|---|---|---|---|---|
| N ab | 38.26 | 5.47 | 32.53 – 44.00 | 0.000 |
| P c | 102.16 | 25.13 | 75.79 – 128.54 | |
| P1 b | 57.05 | 17.51 | 38.68 – 75.42 | |
| P2 a | 29.16 | 8.76 | 19.97 – 38.35 | |
| P3 ab | 54.06 | 21.43 | 21.19 – 66.17 |
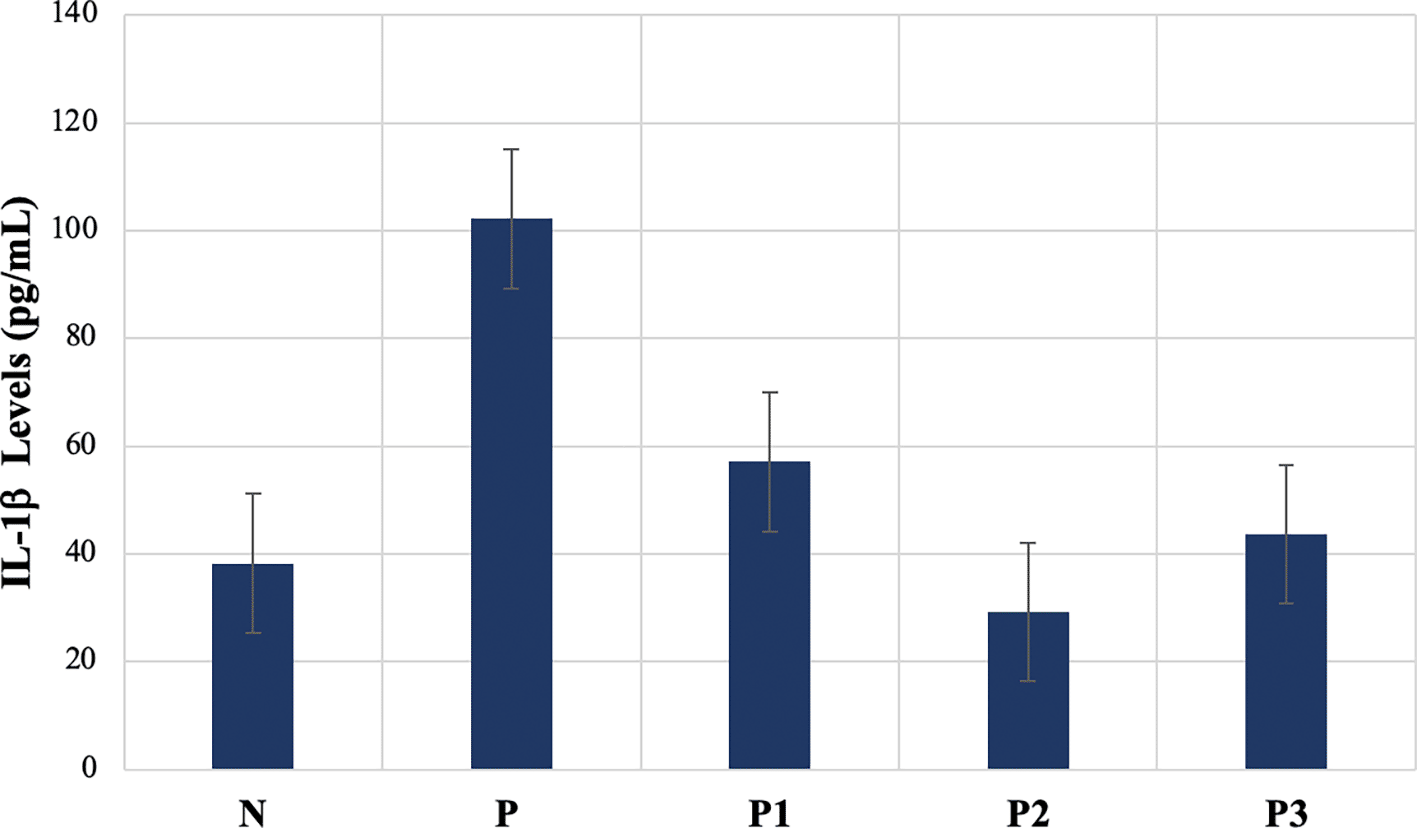
Different superscripts indicate significant differences.
The average levels of TNF-α after treatment were 30.42 pg/mL (N), 22.02 pg/mL (P), 27.25 pg/mL (P1), 16.33 pg/mL (P2), and 13.29 pg/mL (P3). TNF-α levels vary significantly between groups. The results of further tests (post hoc) using the Duncan Test showed that the lowest levels of TNF-α was in P3 group, and was not significantly different from the P2 group (p>0.05), but were significantly different from P, P1, and N group (p<0.05). The P group did not differ significantly from the P1 group (p>0.05), the P2 group did not differ significantly from the P group (p>0.05), and the P1 group did not differ significantly from the N group (p>0.05) ( Table 3 and Figure 2).
| Groups | Mean | SD | 95% CI | P value |
|---|---|---|---|---|
| N a | 30.42 | 5.45 | 24.69 – 36.15 | 0.000 |
| P bc | 22.02 | 5.45 | 16.30 – 27.74 | |
| P1 ab | 27.25 | 5.21 | 21.78 – 32.73 | |
| P2 cd | 16.33 | 3.27 | 7.93 – 24.74 | |
| P3 d | 13.29 | 5.23 | 7.80 – 18.78 |
Nigella sativa L. is rich in bioactive compounds such as thymoquinone (TQ), dithymoquinone, thymol, and thymohydroquinone. It is widely recognized in traditional medical systems, including Unani, Tibb, Ayurveda, and Siddha. The oil and seeds have long been used in a variety of culinary and medical uses. Nigella sativa L. seeds have been widely used to treat a variety of diseases and disorders, and in Islamic literature, they are recognized as one of the most efficient therapeutic agents. Regular use of these seeds is recommended in Tibb-e-Nabwi (Prophetic Medicine).15 Among the identified compounds, TQ is the most prominent, and its therapeutic benefits are largely attributed to this component.16 Previous research has demonstrated that TQ possesses several pharmacological properties, including antioxidant, lipid-lowering, and anti-inflammatory effects.17–20
Severe obesity is defined not just by an excessive buildup of body fat but also by the infiltration of immune cells into adipose tissue and associated inflammation.1,2 Numerous clinical consequences of obesity, especially type 2 diabetes and atherosclerotic cardiovascular disease, are associated with this low-grade systemic inflammation.3,4 Substantial evidence highlights the significant role of IL-1β in atherosclerosis.21 In patients with atherosclerosis, both IL-1β protein and mRNA levels are markedly elevated compared to those in healthy individuals, and these levels are positively correlated with disease severity.22 Additionally, genetic variations in IL-1 have been associated with coronary heart disease (CHD).23 Furthermore, increased susceptibility to atherosclerosis, related to clonal hematopoiesis in peripheral blood cells, is partially regulated by the NLRP3/IL-1β pathway.24,25
The findings of this study are consistent with previous research. TQ treatment decreased the expression of NLRP3, caspase-1, IL-1β, and IL-18 genes, which were increased by a high-cholesterol diet. Following a high-cholesterol diet, TQ supplementation in LDL-R-/- mice lowered NF-κB protein expression.26 Pyroptosis, a form of programmed cell death driven by NLRP3 activation, is linked to the exacerbation of hyperlipidemia. When NLRP3 is activated, it triggers caspase-1, which modifies IL-1 and cleaves the precursors of the inflammatory cytokines IL-1β and IL-18. This mechanism stimulates the production of pro-inflammatory cytokines, which promotes pyroptosis.27
In this study, the administration of Nigella sativa extract was found to effectively reduce TNF-α levels. While significant differences were observed between the P1 and P2 groups, the combination of Nigella sativa and atorvastatin in the P3 group resulted in the lowest TNF-α levels among all samples. The P3 group also showed significant differences from the other treatment groups. The reduced TNF-α levels in the P group may be linked to the obesity present in the treatment samples, as this group had the highest body weight among all treatment groups. Several studies indicate that, in cases of obesity, lower TNF-α levels may arise due to the increased production of TNF-α receptors in adipose tissue. This phenomenon is one of the foundational concepts of the Obesity Paradox.28,29
In a study involving pediatric patients, Boeck et al. reported that no detectable TNF-α was found in the serum of obese individuals.30 However, another study indicated that obese individuals produce higher amounts of TNF-α protein per mass or per unit of DNA from their fat tissue compared to lean control subjects.31 Myeloid cells release TNF-α, a powerful pro-inflammatory cytokine that stimulates MAPK and NF-κB signaling pathways. This leads to the production of additional inflammatory cytokines, including IL-1β and IL-6.32 In rodents, TNF-α is overexpressed in the adipose tissue of obese animals.33 In humans, TNF-α levels are elevated in the plasma and adipose tissue of those who are obese, with levels decreasing following weight loss.34
By suppressing adipogenic genes such CCAAT/enhancer-binding protein (C/EBP) and peroxisome proliferator-activated receptor gamma (PPARγ), TNF-α is known to prevent preadipocytes from maturing into mature adipocytes. The recruitment of more cells is made possible by this inhibition, which promotes the growth of adipose tissue mass.35 Furthermore, TNF-α inhibits genes linked to lipid absorption and storage after being activated by NF-κB.36 Adipocytes create the hormone adiponectin, which is essential for preserving the peripheral glucose and lipid balance. It also lowers the mRNA levels of this hormone. Additionally, because TNF-α promotes the synthesis of other cytokines, including IL-6, its effect on the immune response is mostly indirect.37
Several TNF-related factors are associated with atherosclerosis and elevated circulating IL-6,38 acute phase proteins like C-reactive protein (CRP),39 fibrinogen,40 and triglycerides, total cholesterol (TC), and low-density lipoprotein (LDL) are all increased in this lipid profile, but HDL and the HDL/TC ratio are low. Consequently, TNF-α is an early mediator of the acute phase response, aiding in the recruitment of leukocytes during inflammatory responses and promoting the synthesis of chemokines, IL-6, and CRP.41
In numerous clinical trials, the effects of Nigella sativa ingestion on biomarkers for oxidative stress and inflammation have been investigated, though the results remain mixed. For instance, a study found that taking 3 g/day of Nigella sativa oil for 8 weeks led to significant reductions in inflammatory markers, specifically hs-CRP and TNF-α, in obese women.42 Conversely, research by Hadi et al. indicated that a dosage of 1 g/day of Nigella sativa oil for 8 weeks did not alter TNF-α and SOD levels in patients with type 2 diabetes mellitus.43 Additionally, there is some evidence suggesting that Nigella sativa oil may be more therapeutically effective than the seed form of the plant.15
This study has been ethically assessed by Syiah Kuala University’s Faculty of Veterinary Medicine’s Medical Research Ethics Commission and it was registered on July 31, 2023, under the number 243/KEPH/VII/2023. Rats were put to sleep at the end of the ninth week by cervical dislocation after intraperitoneal injections of xylazine and ketamine. The reporting for this animal study follows the ARRIVE guidelines.
Zenodo: ARRIVE checklist for ‘Therapeutic Potential of Nigella sativa Extract against Inflammatory Markers Interleukin-1β (IL-1β) and Tumor Necrosis Factor-α (TNF-α) in Rat Models with High Fat Diet’, https://doi.org/10.5281/zenodo.13958154.44
Zenodo: Master Data, https://doi.org/10.5281/zenodo.14207758.45
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 06 Feb 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)