Keywords
Organophosphate poisoning; Prevalence; Nepal; Systematic review; Meta-analysis
This article is included in the Meta-research and Peer Review collection.
To estimate the prevalence of organophosphate (OP) poisoning in Nepal.
PubMed, Web of Science Core Collection (WoSCC), Cochrane Library, Ovid, and Springer databases were searched. The search strategies were used in different databases until December 13, 2024. The Joanna Briggs Institute (JBI) critical appraisal checklist was used to assess the quality of the included studies. The R project was used to estimate the pooled prevalence rate and 95% confidence interval (CI) based on the results of the heterogeneity test.
Eleven studies, involving 4809 participants. Heterogeneity indicated a P value of <0.100; therefore, we applied the random effects model for data synthesis. The overall prevalence of OP poisoning in Nepal was 36.7% (95% CI: 24.99-49.34) in Nepal. Funnel plot dots were not distributed symmetrically on either side of the central line, suggesting a potential publication bias. The results of the heterogeneity analysis revealed that hospital-based studies showed a higher prevalence rate of 36.9% (95% CI: 24.2-50.6) than community-based studies. Additionally, studies conducted in Other cities-based demonstrated a pooled prevalence rate of 43.38% (95% CI: 28.95-58.39), which was higher than that in Kathmandu-based studies.
The overall prevalence of OP poisoning was 36.7% in Nepal. The hospital-based studies indicated a higher pooled prevalence than community-based studies and other cities-based studies reported a higher prevalence than Kathmandu-based studies. Future research is warranted to provide more accurate and comprehensive evidence regarding the prevalence of OP poisoning in Nepal.
Organophosphate poisoning; Prevalence; Nepal; Systematic review; Meta-analysis
(1) The pooled prevalence rate of organophosphate (OP) poisoning was 36.7% in Nepal.
(2) The hospital-based studies had a higher pooled prevalence than community-based studies.
(3) Most studies were performed in Kathmandu rather than in other cities. However, other studies have shown a higher prevalence of OP poisoning compared to those based in Kathmandu.
Organophosphate (OP) [broader term organophosphorus] poisoning predominantly occurs in agriculture-based countries, where these substances are easily accessible. Exposure to OP can result from occupational or accidental contact with pesticides, intentional self-harm, or chemical warfare and terrorist attacks.1,2 OP is responsible for approximately two-thirds of the estimated 60% of pesticide poisoning-related deaths from self-harm that occur annually in rural Asia.3 Suicide remains a serious public health issue, affecting approximately 79% of cases occurring in low- and middle-income nations, frequently due to pesticide self-poisoning.4,5 The fatality rate of OP poisoning is as high as 25%, surpassing that of other types of poisoning.6
OP poisoning is commonly observed in Nepal, China,7 Taiwan,8 India,9 Bangladesh,10 and other parts of the world.4 In this study, data from 108 countries illustrated that pesticide self-poisoning deaths comprised 13.7% of all global suicides.4 Pesticides are widely accessible in Nepal, which is located in South Asia where most Nepalese work in agriculture and farming, making them the most common cause of poisoning. Commonly used OP compounds in Nepal include malathion, metacids, dichlorphos, cypermethrin, defox, and chlorpyriphos.11 However, the Nepalese government does not have exact data regarding the prevalence of OP poisoning. The absence of strict market inspections and widespread availability of OP have further exacerbated their misuse in agriculturally dominant areas.
This study aimed to explore the prevalence of OP poisoning in Nepal by including all relevant articles available in major databases such as PubMed, Web of Science Core Collection (WoSCC), Cochrane Library, Ovid, and Springer. By analyzing a broader range of research data, we seek to provide stronger evidence on this critical public health issue in Nepal.
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. First, a search strategy was developed, and the major databases, including PubMed, Web of Science Core Collection (WoSCC), Cochrane Library, Ovid, and Springer, were searched separately until December 13, 2024. All the studies were included in the meta-analysis. A total of 423 studies were obtained from different databases. The search strategies used for different databases are presented in Table 1 (refer to extended data).
The inclusion criteria were as follows: (a) the study must be conducted in Nepal, and (b) the prevalence of OP poisoning.
The exclusion criteria were as follows: (a) case reports, meta-analyses, editorials, and systematic reviews of OP poisoning; (b) full text not available; (c) prevalence data not available in the study; and (d) academic papers written in languages other than English.
Selecting suitable studies that satisfied the inclusion and exclusion criteria required two investigators to independently assess the research using the aforementioned methodologies and to adhere closely to the inclusion and exclusion criteria. For divergent literature, we decided whether to include it through discussion or consultation with a third researcher. The original author was contacted as much as feasible to add to the literature because it lacked certain facts. The study was discarded if the original author could not be reached. To determine the possibility of bias in the included studies, the Joanna Briggs Institute (JBI) critical appraisal checklist for prevalence studies was employed.12 A score of (yes=1), (no=0), and (unclear or not applicable=0) is assigned to each of the nine items that make up the JBI appraisal checklist. Each study’s overall score was displayed as a percentage, and each study was grouped based on the degree of bias risk (high risk of bias if 20–49% of items scored yes, moderate risk of bias if 50–79% of items scored yes, and low risk of bias if 80–100% of items scored yes according to the JBI checklist) (Table 3 refer to extended data).
All data were analyzed using the R Project Meta-package (version 4.1.0). The prevalence rate was also measured in this study. We chose the pooled prevalence rate in terms of the point estimate and 95% confidence interval (95% CI) from the results of the random effects model if P value was <0.100 or I2 was >50% in the test of heterogeneity. Possible sources of heterogeneity were estimated using a sensitivity analysis, which was performed using a subgroup analysis. A funnel plot was used to estimate publication bias.
Through an extensive review and strict compliance with the inclusion criteria, 11 articles met the inclusion criteria and were included in this study ( Figure 1). A total of 4809 participants were included. Basic information for each study is presented in Table 2 (refer to extended data). All included studies were published between 2016 and 2024 on the Nepalese population from different places in Nepal.
Table 3 (refer to extended data) shows the results of quality assessment of the included studies. It was found that Subedi et al.11 had a low risk of bias; however, other studies had a moderate risk of bias according to the JBI appraisal checklist.
The heterogeneity test showed that the P value was <0.100. As a result, we chose the pooled prevalence rate from the results of the random effects model. The overall prevalence of OP poisoning was 36.7% (95% CI: 24.99-49.34) in Nepal ( Figure 2).
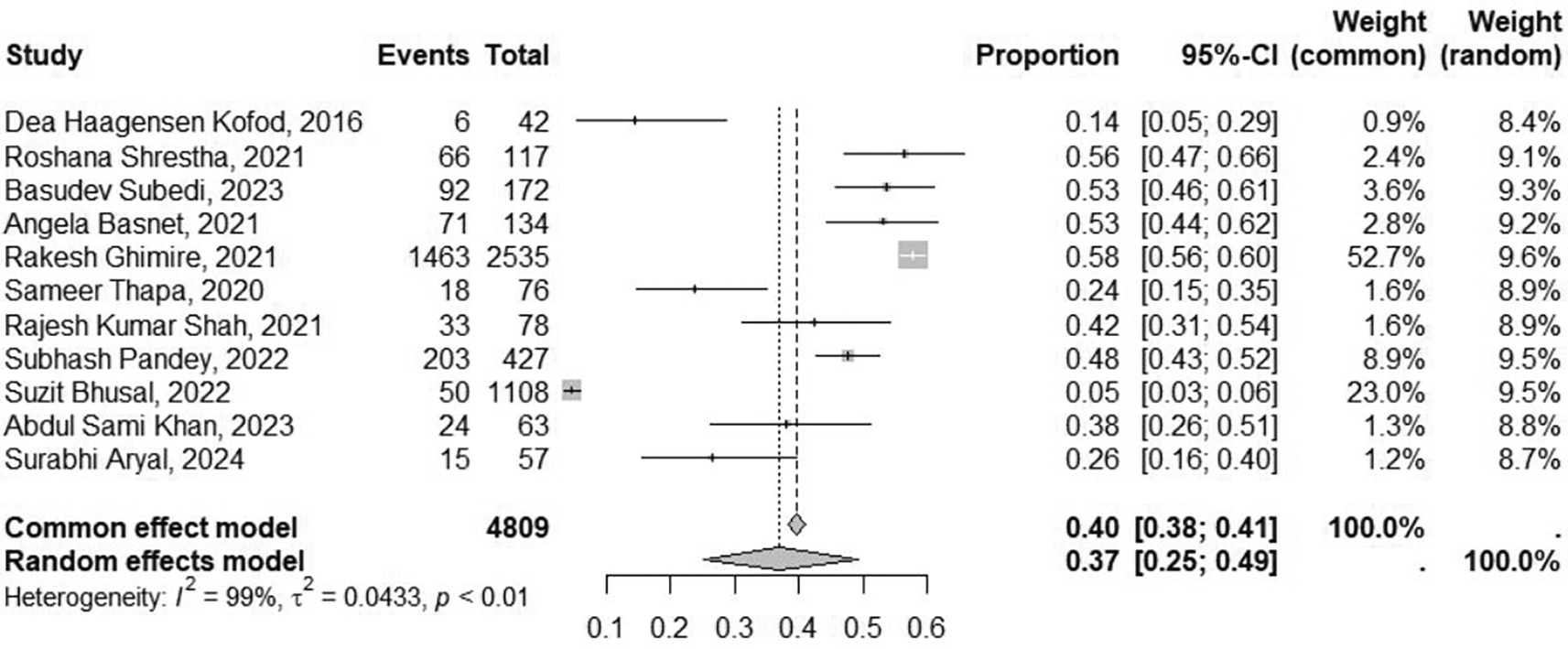
Using random effects model (I2=99.3%) 36.7% (95% CI: 24.99-49.34). CI, confidence interval.
In the sensitivity analysis, we divided the studies into hospital- and community-based studies. It was found that pooled prevalence rate of hospital-based studies was 36.9% (95% CI: 24.2-50.6), and that of the community-based studies was 35.22% (95% CI: 2.8-79.1) (Table 4 refer to extended data).
In the sensitivity analysis, we divided studies into Other cities-based and Kathmandu-based studies. It was found that pooled prevalence rate of Other cities-based studies was 43.38% (95% CI: 28.95-58.39) and pooled prevalence rate of Kathmandu-based studies was 35.41% (95% CI: 19.5-53.2) (Table 4 refer to extended data).
A funnel plot was used to evaluate publication bias of the included studies. The funnel plot was asymmetric on both sides ( Figure 3), indicating that publication bias should not be ignored.
To the best of our knowledge, this is the first meta-analysis to report on the prevalence of OP poisoning in Nepal. Our findings revealed that the overall prevalence of OP poisoning in Nepal is 36.7%. The prevalence of OP poisoning varies significantly worldwide, with rates reported at 3% in Serbia,13 26.9% in China,7 and 4.6% in India.6 This implies that the prevalence of OP poisoning in Nepal may be substantially higher than in other regions. Our meta-analysis also highlighted sex differences in OP poisoning cases. Most studies showed a higher female-to-male ratio, such as 2.9:1 by Subedi et al.,11 1.4:1 by Basnet et al.,3 1.5:1 by Pandey et al.,14 2.1:1 by Bhusal et al.,15 and 1.9:1 by Aryal et al.16 In contrast, Khan et al.17 reported a higher male-to-female ratio (0.8:1). Similarly, a study conducted in China from to 2012-2016 involving 5009 patients reported a higher female ratio (1.2:1), with 56.7% of cases arising from suicide attempts.7
Significant heterogeneity was observed among the included studies, prompting subgroup analyses of hospital and community-based studies to determine the potential sources of heterogeneity. From the data of the included individual studies, it was found that the prevalence rates were higher in hospital-based studies than in community-based studies, which may be partly attributed to the fact that patients in hospital settings are more likely to undergo relevant diagnostic evaluations than those in community settings. In this meta-analysis, all authors of the included papers researched the Nepalese population and determined its prevalence. The authors of the included papers conducted research across various districts of Nepal with a primary focus on Kathmandu, ensuring that the analysis was not confined to a single district or region. Most studies were conducted in Kathmandu, where a large population resides. However, in this meta-analysis we found that Other cities-based studies shows more prevalence of OP poisoning than Kathmandu based, potentially due to larger sample size in the published papers from other cities. In terms of study design, Dea Haagensen Kofod18 performed community-based tests and Rakesh Ghimire5 performed both hospital- and community-based tests. However, the remaining studies were hospital-based.3,11,14,15,17,19–21
Our study has several limitations. First, only 11 studies were retrieved from the major literature databases. Second, significant heterogeneity and publication bias were observed, likely due to the bias of small-sample studies and study sites with different OP poisoning prevalence rates. Third, limited data were available from certain districts of Nepal, which prevented a complete understanding of national prevalence. To address these gaps, future research should include high-quality cohort and case-control studies from a broader geographical range within Nepal.
Our study provides valuable insights into the prevalence of OP poisoning in Nepal, with an overall rate of 36.7%. Hospital-based studies have reported a higher pooled prevalence rate than community-based studies, and studies conducted in other cities have shown a higher prevalence than those conducted within Kathmandu. More studies, particularly those from underrepresented regions, are necessary to obtain more accurate and comprehensive data on OP poisoning in Nepal.
Nitesh Shrestha designed the framework of the manuscript, performed the meta-analysis, and drafted and revised the manuscript. Tao Wei performed the meta-analysis and revised the manuscript accordingly. Rui Liao drafted and revised the manuscript accordingly. Qiang Xue, Jia Hu, Xia Zhao, and Qin Huang searched for relevant literature, and reviewed and revised the manuscript accordingly.
Figshare: Prevalence of organophosphate poisoning in Nepal: A Meta-Analysis. Doi: https://doi.org/10.6084/m9.figshare. 28151177.v122
This project contains the following extended data:
• Figure 1.jpg
• Figure 2.jpg
• Figure 3.jpg
• Tables.docx
• 1 The overall prevalence of OP.csv
• 1 The overall prevalence of OP Meta-analysis.txt
• 2 hospital-based studies Meta-analysis.txt
• 2 hospital-based studies.csv
• 3 community-based studies Meta-analysis.txt
• 3 community-based studies.csv
• 4 Kathmandu-based studies Meta-analysis.txt
• 4 Kathmandu-based studies.csv
• 5 Other cities-based studies Meta-analysis.txt
• 5 Other cities-based studies.csv
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Open Science Framework: PRISMA checklist for ‘Prevalence of organophosphate poisoning in Nepal: A Meta-Analysis’. Doi: 10.17605/OSF.IO/WB73R (https://osf.io/wb73r/).23
Data is available under the terms of the CC0 license
We would like to thank Dr. Tao Wei and Rui Liao, who are also authors of this paper, from the Department of Library of Kunming Medical University for their help with the R-Project (math work), paper modifications, and writing.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
No
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical Pharmacology , Clinical Toxicology
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
No
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
No
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 10 Feb 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)