Keywords
Biodegradation, Dumpsite soil, Eco-friendly, Microbial consortia, Pot condition
Plastic waste is becoming the most significant environmental pollutant because it is challenging to degrade naturally due to its composition of long hydrocarbon polymer chains. The present study aims to find out the biodegradation of low-density polyethylene (LDPE) using both pure microbial strains TA1 (Aspergillus tubignesis), TA2 (Aspergillus fumigatus), TA3 (Aspergillus niger), TA6 (Pseudomonas aeruginosa), TA7, and TA8 (Proteus mirabilis) and a consortium of these strains under pot conditions.
The experiment used sterilized dumpsite soil surface-sterilized with 5% sodium hypochlorite for 3 minutes, rinsed with distilled water, and dried pots. A completely randomized design (CRD) was used with 32 treatments submerged in low-density polyethylene (LDPE) plastic of two thicknesses: 25 microns and 40 microns. Each treatment was replicated three times and conducted over two intervals: 6 months and 9 months.
The results showed that the maximum biodegradation of 25-micron-thick LDPE films was achieved using the pure fungal isolate Aspergillus niger (TA3) 13.69%, followed by Aspergillus fumigatus (TA2) 11.9% and Aspergillus tubignesis (TA1) 10.54% after 9 months. For 40-micron-thick LDPE films, the degradation rates were 7.8%, 5.8%, and 4.6% respectively. Using a consortium of three fungal species resulted in a maximum weight loss of 19.17% for 25-micron-thick LDPE bags and 9.8% for 40-micron-thick LDPE bags over the same period. FTIR analysis revealed changes in the functional groups on the LDPE surface, indicating hydrocarbon degradation.
The study found that pure microorganisms resulted in less weight loss compared to microbial consortia, and fungi were more effective than bacteria in degrading the LDPE plastic. Despite the differences, the current study suggested that microbial consortia provide an eco-friendly solution to address LDPE-related environmental issues with minimal adverse effects.
Biodegradation, Dumpsite soil, Eco-friendly, Microbial consortia, Pot condition
Plastics play an important role in several aspects of our lives due to their numerous applications. Among plastics, polyethylene has been widely used for packaging, accounting for around 30% of all plastic applications worldwide.1 Among polyethylene types, low-density polyethylene (LDPE) has emerged as a pressing global issue due to its substantial environmental impact. Originating in the twentieth-century petrochemical industry, LDPE is recognized as a significant anthropogenic discovery.2 Its widespread adoption stems from its strength, cost efficiency, abundant production capability, and ability to withstand various weather conditions, rendering it indispensable in modern life.3
Low-density polyethylene (LDPE) is resistant to microbial degradation. Irresponsible dumping and poor waste management regulations have unintentionally led increased in waste accumulation worldwide, disrupting the natural balance.4 In response to these negative consequences, numerous countries have recently banned single-use LDPE products from their marketplaces, addressing the concerns of their national leaders.5 Consequently, plastic bags end up in landfills, roadside litter, and water bodies.6 This situation creates serious environmental threats and poses health risks to society.7
To date, numerous solutions have been explored to handle non-biodegradable plastic waste. These include landfill disposal, incineration, and recycling. However, each strategy has limitations in actual use.8 Additionally, all are expensive and emit carcinogenic substances (Bisphenol A and phthalates), harmful byproducts (dioxins, heavy metals, and furans), and greenhouse gases (CO2, CO, and CH4).9 Promising research on a sustainable, eco-friendly, and cost-effective alternative to plastic materials is still ongoing.10 Microbial degradation is an environmentally friendly and economically viable method for reducing plastic waste.11
In the other way, the degradation rate was significantly increased, reaching up to 10% when Pseudomonas PL01 was supported by 1 g/L monosodium glutamate (MSG) using a soil burial method.12 As well as fungal species of Aspergillus, Penicillium, and Trichoderma are efficient PE-degrading microorganisms.13
Research indicates that a consortium of microbial species outperforms pure organisms in various areas, including the degradation of low-density polyethylene.14 This observation is consistent in polyethylene biodegradation, a consortium of microorganisms outperformed pure microorganisms in vitro conditions.15 While a few studies have reported plastic degradation by pure microbial isolates under field conditions without achieving significant environmental relevance, others have demonstrated more promising results with microbial consortia breaking down plastics in natural waste under pot conditions. Thus, this study aims to identify the most cost-effective and environmentally friendly approach to reducing LDPE waste by evaluating the biodegradation of low-density polyethylene under controlled pot conditions, using both single microbial isolates and consortia sourced from municipal solid waste disposal sites in Gondar City, Ethiopia.
This study was conducted on the balcony of the Biology Department laboratory at the University of Gondar from 2022 to 2023.
Low-density polyethylene (LDPE) bags were obtained from the local market and were cut into sets of square strips sized 2 × 2 cm2 of 25 and 40 microns thick. Polyethylene strips from each set were weighed with a microbalance (Bio base company, Model BA2204C). LDPE bag sterilization was performed by immersing in 70 % alcohol for 45 minutes and then drying at 50 °C under sterile conditions.
The microbial isolates used for this study were obtained from our previous research, which were kept at the Microbiology laboratory, Department of Biology. The bacterial, and fungal isolates were refreshed on nutrient agar and potato dextrose agar (PDA) at 30 °C, respectively. The bacterial strains included Pseudomonas aeruginosa strain TA6 (OR823835); Proteus mirabilis strain TA7 (OR823845), and Proteus mirabilis strain TA8 (OR823836); and fungal strains were Aspergillus niger strain TA3 (OR936163); Aspergillus fumigates strain TA2 (OR801669), and Aspergillus tubingensis strain TA1 (OR810309). These strains were selected based on their in vitro potential for LDPE degradation, which was assessed through attributes such as extracellular enzyme production, weight loss, and FTIR analysis.
The experiment was carried out in 2022 up to 2023 at the University of Gondar using sterilized soil from a plastic-polluted dumpsite. The soil was placed in sterilized pots with a diameter of 20 cm. The pots were surface-sterilized for 3 minutes with 5% sodium hypochlorite, then rinsed three times with distilled water and allowed to dry before use. The soil was sterilized for 30 minutes at 121 °C. The experiment was arranged in a completely randomized design (CRD) with 32 treatments, applied to 25- and 40-micron-thick LDPE, in triplicate for each 6- and 9-month interval.
The experiment included the following treatments in both 25 and 40-micron thick LDPE for bacterial isolates.
The experiment involved fungal isolate treatments in both 25 and 40-micron-thick LDPE as follows
A 5-mm mycelial agar plug from a pure fungal culture was inoculated into 100 ml of potato dextrose broth (PDB) (200 gm potato extracts and 20 gm dextrose/L) in a 250-ml flask and incubated at 30 °C on an orbital shaker at 120 rpm for 7 days. After incubation, the spore concentration was adjusted to 4.5 × 105 spores/ml using a hemocytometer.16
A single colony from each bacterial isolate was inoculated into 100 ml of nutrient broth in 250 ml Erlenmeyer flasks, which were then incubated at 30 °C on an orbital shaker at 120 rpm until the cell concentration reached 108 cfu/ml cells/ml, as determined by a spectrophotometer at an optical density of 600 nm (OD 600 nm = 1.0).17
The dumpsite soil samples were first mixed thoroughly by hand to ensure even distribution, then sterilized for 30 minutes at 121 °C after sterilization 2.5 kg soil was placed in surface sterilized plastic pots. In the pot experiment, sterile LDPE films, measuring 2 × 2 cm2 and with thicknesses of 25 and 40 microns, were vertically buried at a depth of 5 cm in separate pots, with 3 replicates for each treatment.18 The pots, each containing 2.5 kg of sterile soil, were inoculated with the fungal isolate at an optimized spore density and the bacterial isolate at a standardized cell density. Each pot drained 50 ml of the inoculum, which was evenly distributed over the soil surface using the mulching method.19 The pots were then kept at room temperature. The soil’s water content was adjusted every seven days throughout the incubation period to maintain a consistent moisture level, supporting fungal and bacterial growth.20 The LDPE films were allowed to degrade in this artificially inoculated, pre-sterilized dumpsite soil. Control pots, which were not inoculated with fungal or bacterial isolates, were used to bury the LDPE films. The extent of LDPE film biodegradation was assessed after 6 and 9 months of incubation using the weight loss method and 9 months of incubation through Fourier transform infrared (FT-IR) analysis.
To determine weight loss, the residual LDPE film samples were collected and treated with 2% (w/v) aqueous sodium dodecyl sulfate (SDS). The mixture was incubated in a shaker at 120 rpm for 4 hours, then rinsed with distilled water and subsequently with 70% ethanol to remove any microbial film and residual medium. The residual LDPE samples were then collected on filter paper and dried overnight at 60 °C before being weighed. The extent of degradation was determined by calculating the percentage of weight loss of the LDPE films over the specified period.21
The structural changes in the functional groups of the LDPE surface were investigated using Fourier-transformed infrared (FT-IR) spectroscopy (JASCO, FT/IR 4600, UK). Spectra were recorded at 400–4000 wave numbers cm−1 with a resolution of 0.96 cm−1 for microbial-treated and untreated (control) LDPE samples. The untreated (control) plastic sample was used to create the fingerprint spectra. The absorbance mode was used to record the spectra of both the treated and untreated plastic samples. Following that, the FT-IR spectra were processed and analyzed using Origin software (Version 2019, Origin Lab Corporation). A comparison was made between the untreated and treated LDPE samples (C–O stretch, –C=C– stretch, and CH stretching) for the formation of specific peaks.22
Statistical analysis was performed using a one-way analysis of variance in SPSS version 26. A post hoc Duncan test (p < 0.05) was performed to determine the significant difference between bacterial and fungal isolates in the reduction of the weight of the LDPE film after 6 and 9 months of incubation.
After 9 months of incubation, the bacterial isolate TA6 demonstrated the maximum degradation, achieving 12.5±0.11% and 6.56±0.03% weight loss for 25- and 40-micron thick LDPE films, respectively, followed by TA8 at 11.66±0.06% and 4.6±0.05%, and TA7 at 10.69±0.06% and 4.31±0.03%. This study found significantly higher degradation rates than the previously reported 3.77% and 4.21% weight loss of LDPE film by Pseudomonas sp. after 9 months of incubation.23 It suggests that the bacterial isolates examined in the current study have a superior degrading ability, highlighting their potential for effectively addressing plastic waste management challenges.
The bacterial consortia composed of TA6+TA7, TA6+TA8, TA7+TA8, and TA6+TA7+TA8 exhibited superior biodegradation properties compared to the pure isolates use of TA6, TA7, and TA8. This study demonstrated that these formulated bacterial consortia achieved 14.24±0.05%, 15.6±0.08%, 12.32±0.08%, and 16.43±0.05% degradation of 25-micron LDPE bags, and 7.25±0.06%, 8.23±0.13%, 6.56±0.03%, and 9.31±0.06% degradation of 40-micron LDPE bags for 9 months respectively. All results were statistically significant (p < 0.05) over their individual isolates. The current study indicates that the formulated bacterial consortia degraded a greater percentage of LDPE bags than the isolates in their pure form. Another study reported that on biodegradation using microbial consortia, as opposed to pure isolates, have shown that consortia possess a higher biodegradation capacity.24
The results showed that for all bacterial isolates, the weight loss rate of the plastic films increased with longer incubation periods ( Figure 1). Another study also found that the weight loss of plastic bags and bottles increased with extended incubation times.25 This suggests that prolonged exposure to microbial systems enhances biodegradation, as evidenced by the greater percentage of weight loss over time. The longest incubation periods resulted in the most significant biodegradation, underscoring the importance of allowing sufficient time for microorganisms to effectively break down persistent plastic polymers. Additionally, optimizing incubation conditions is crucial to maximizing the potential of these microbial-mediated plastic degradation processes.
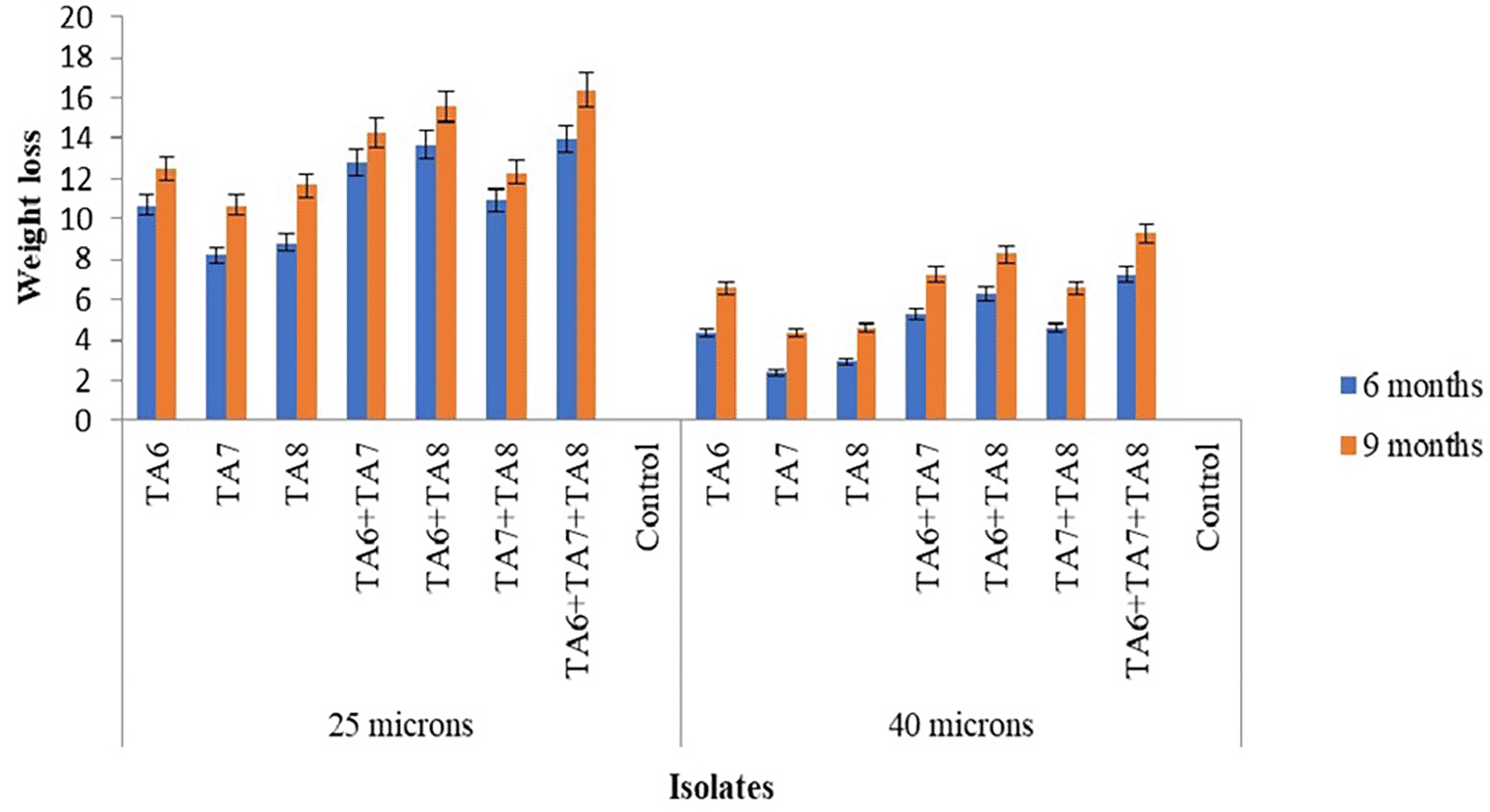
In the present study, almost all bacterial isolates showed greater efficacy in degrading 25-micron LDPE bags compared to 40-micron LDPE bags ( Figure 1). Another study also reported that the biodegradation of polyethylene is considerably faster than that of other plastic types, which could be because polyethylene is five times thinner than other plastics.23 The higher degradation rate of 25-micron LDPE bags compared to 40-micron bags may be attributed to the greater surface area exposed to bacteria in this study. The current study found that combining TA6 (Pseudomonas aeruginosa) with TA7 and TA8 (Proteus mirabilis) strains resulted in the most effective biodegradation activity. Further investigation is needed to explore the mutual interactions and survival capabilities of these strains in nutrient broth. Additional research can identify optimal combinations of bacterial strains, allowing for a deeper investigation into the biodegradation capacity of the most effective combinations.
Figure 2 shows the biodegradation potential of fungal isolates after 9 months of incubation at room temperature under pot conditions. According to the findings of this study, the potential of the three fungal strains, both pure isolate and in consortium, showed varying degrees of degradation of LDPE film materials. The highest degradation was achieved by isolate TA3, followed by TA2 and TA1, with rates of 13.69±0.12%, 11.9±0.05%, and 10.54±0.10% for 25-micron LDPE films, and 7.8±0.06%, 5.8±0.03%, and 4.6±0.10% for 40-micron LDPE films after 9 months of incubation respectively. This indicates a better degrading ability than a previous study, which reported LDPE biodegradation percentages of 3.601% in 68 days in a humus-type soil matrix with microbial interactions by A. brasiliensis and humus-specific microbiota.26 However, the current study shows lower degrading ability compared to another study that documented a maximum of 15% weight loss of polyethylene film and no degradation of plastics by Aspergillus niger under field conditions after 9 months of incubation.18
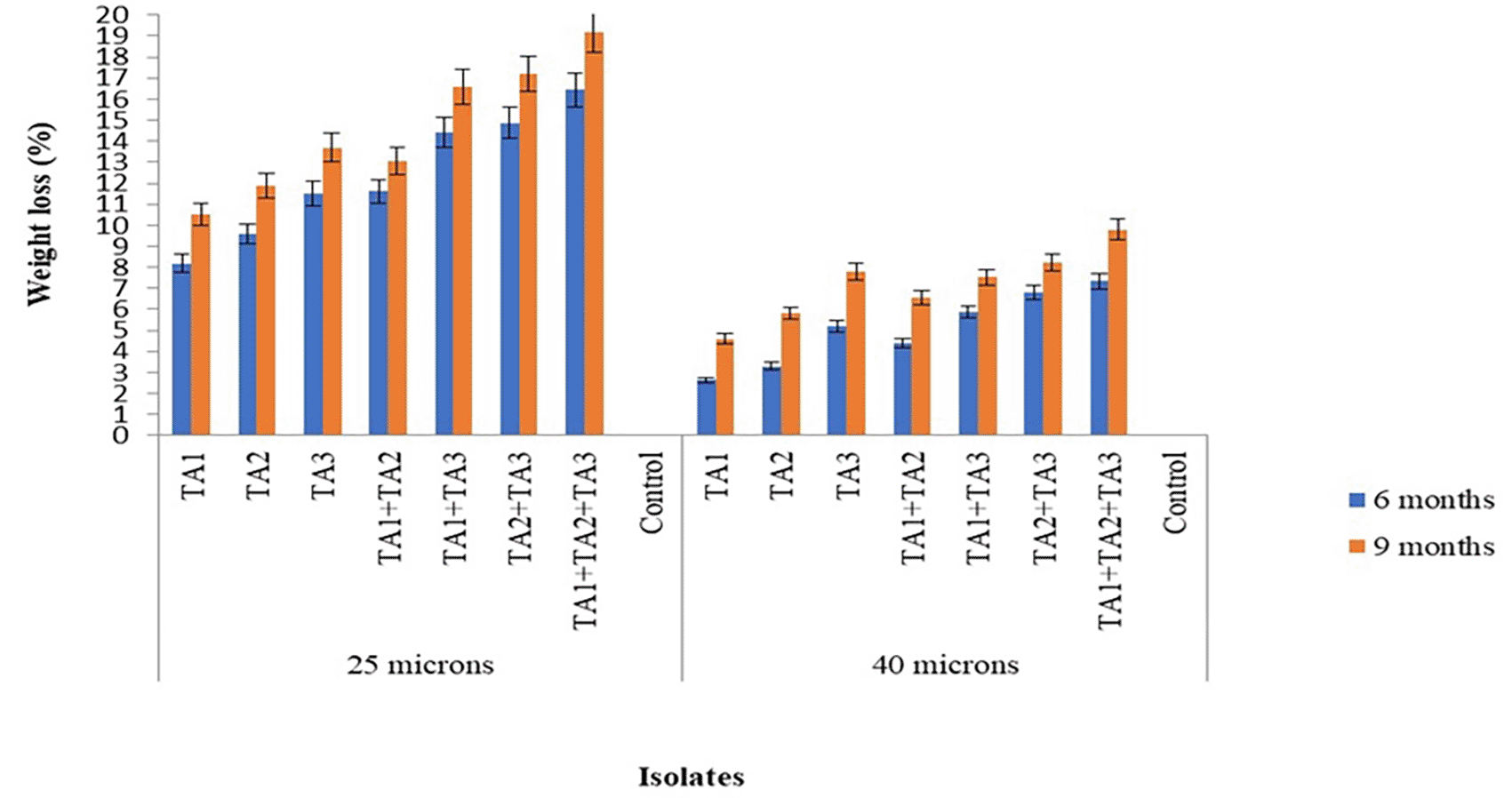
The fungal consortia composed of TA1+TA2, TA1+TA3, TA2+TA3, and TA1+TA2+TA3 demonstrated the highest biodegradation properties compared to the individual use of TA1, TA2, and TA3. Over a 9-month period, the consortia TA1+TA2, TA1+TA3, TA2+TA3, and TA1+TA2+TA3 achieved 13.05±0.00%, 16.6±0.08%, 17.2±0.08%, and 19.17±0.05% degradation of 25-micron LDPE bags, and 6.56±0.03%, 7.54±0.06%, 8.8±0.13%, and 9.8±0.06% degradation of 40-micron LDPE bags, respectively. The study found that the fungal consortia were more effective at degrading LDPE bags than the pure isolates. Other research has also shown that fungal consortia often achieve better degradation rates for polyethylene compared to individual fungi.15
In the present study, the results showed that for all fungal isolates, the weight loss rate of the plastic films increased as the incubation period extended ( Figure 2). This may be attributed to the fungi adapting to the LDPE compound and beginning to use it as a source of energy and growth, particularly after depleting all the nutrients in the medium. This adaptation encourages microbial growth and enhances enzymatic activity, which helps degrade the substrate.27
In this study almost all fungal isolates demonstrated greater efficacy in weight loss with 25-micron LDPE bags compared to 40-micron LDPE bags. Another study reported that the biodegradation of polyethylene occurs faster and earlier than that of other plastics, likely because polyethylene is five times thinner than other plastics.23
The current investigation demonstrated that combining the strains TA3 (Aspergillus niger), TA2 (Aspergillus fumigatus), and TA1 (Aspergillus tubingensis) resulted in the highest biodegradation activity. Further research is needed to explore the mutualistic interactions and survival capabilities of these microbes in potato dextrose broth. Additional fungal strain combinations can be evaluated using appropriate methods to further investigate and optimize the biodegradation capacity of the most effective combinations.
Figure 3 shows the FT-IR absorbance spectra plotted against the wavenumber for both control LDPE films and those subjected to biodegradation after 9 months of incubation with the bacterial isolates. This analysis provides valuable insights into the structural changes in the LDPE polymer resulting from microbial degradation. The FT-IR data complement the weight loss measurements from the pot experiments, offering a comprehensive view of the biodegradation mechanisms and the extent of polymer breakdown achieved by the different bacterial systems. FT-IR analysis serves as a crucial tool for validating and further elucidating the plastic biodegradation capacities of the tested bacterial cultures.
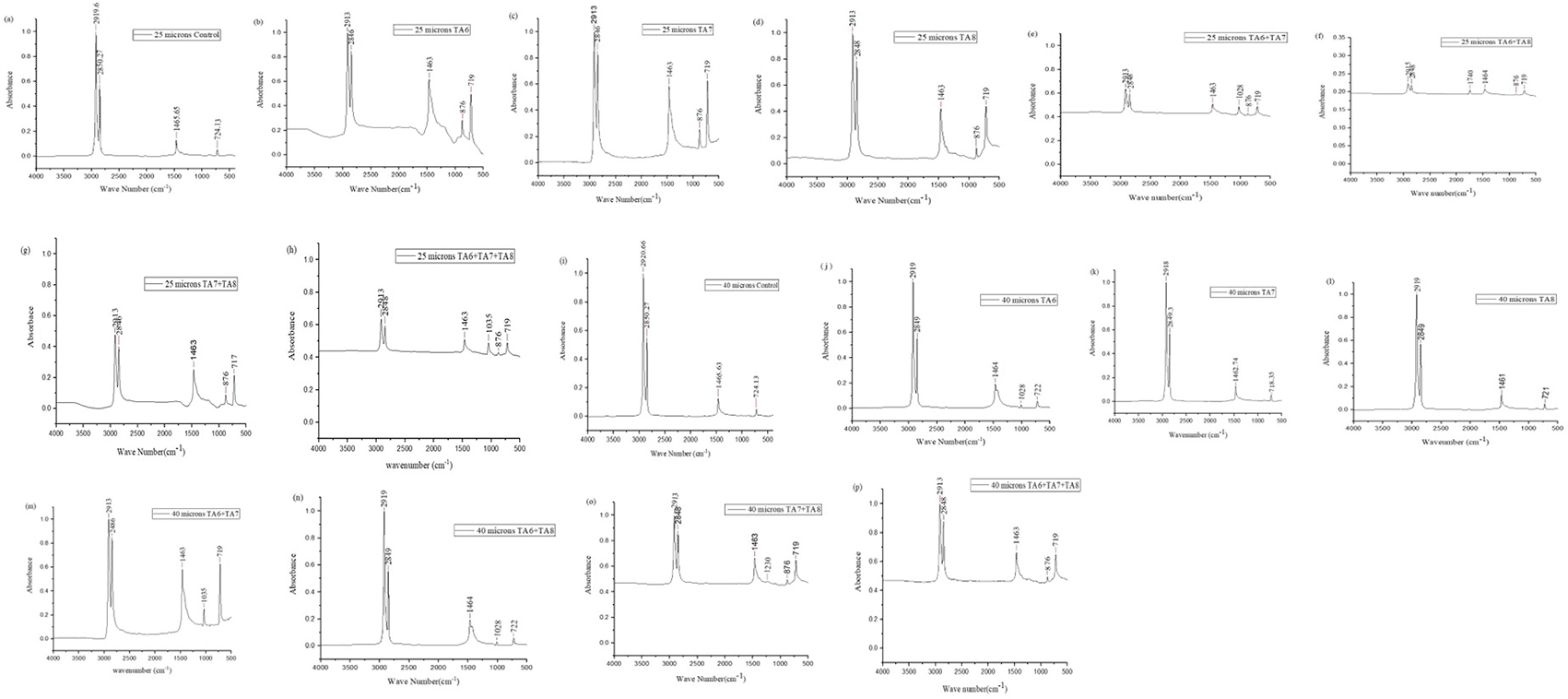
The FTIR analysis of the control LDPE sample showed characteristic peaks at 2919.69 cm−1 and 2850.27 cm−1, indicating C-H bond stretching in alkanes. A peak at 1465.65 cm−1 was attributed to C-H bending in alkane bonds, and a peak at 724.13 cm−1 was related to the =C-H bond in alkenes. These findings align with the FTIR spectrum of control polyethylene reported by Sowmya et al. (2014), which identified similar peaks: 2921.90 cm−1 and 2851.96 cm−1 for C-H stretching, 1465.47 cm−1 for C-C stretching, and 722.60 cm−1 for C-H bending. These consistent spectral features validate the characteristics of the control LDPE samples and provide a baseline for comparing the biodegraded LDPE samples.
In the present study, the analysis of polyethylene spectral data revealed new peaks at 876 cm−1 in TA6 ( Figure 3b), TA7 ( Figure 3c), TA8 ( Figure 3d), TA7+TA8 ( Figure 3e), TA6+TA8 ( Figure 3f), TA7+TA8 ( Figure 3g), TA6+TA7+TA8 ( Figure 3h), and TA7+TA8 ( Figure 3o), corresponding to the =C-H bond of alkenes. Another study also reported that weak peaks at 969 cm−1 in the =C-H structures of polyethylene films, indicating that the typical structures in PE films were disrupted.20
Additionally, new peaks appeared in the range of 1028 cm−1 in TA7+TA8 ( Figure 3e), TA6 ( Figure 3j), and TA6+TA8 ( Figure 3n), 1035 cm−1 in TA6+TA7+TA8 ( Figure 3h) and TA6+TA8 ( Figure 3m), and 1230 cm−1 in TA7+TA8 ( Figure 3o), which are associated with C–O stretching in alcohols, carboxylic acids, esters, and ethers. Previous research has also noted new C–O peaks at 1218.6 cm−1 and 1031.6 cm−1 in LDPE buried in soil, indicating structural changes.28
Furthermore, a new peak at 1740 cm−1 in TA6+TA8 ( Figure 3f) corresponds to carbonyl groups (C=O). Another study reported new absorption peaks at 1712 cm−1 in weathered polyethylene (PE), polypropylene (PP), and polystyrene (PS) pellets exposed to ultraviolet (UV) radiation in seawater for 3 months.29 The appearance, disappearance, and shift of functional group peaks are key indicators of structural changes in LDPE degradation.30
All spectra of films treated with either pure bacteria or a bacterial consortium exhibited a notable reduction in the wave numbers of peaks 2919.69 cm−1, 2850.27 cm−1, 1465.65 cm−1, and 724.13 cm−1 compared to the control samples. Park and kim, (2019)31also observed that after 20 days of incubation, the FTIR spectra of the deformed particles showed the lowest levels at most wave numbers, except for the 3447 cm−1 band (-O-H stretching), which maintained a pattern similar to that of the biologically degraded and control particles. These results suggest that microorganisms may be growing increasingly attached to the PE surface.
The control sample displayed peaks at 2919.69 cm−1 and 2850.27 cm−1, indicating C-H bond stretching in alkanes. Additionally, the peak at 1465.65 cm−1 was attributed to C-H bending in alkane bonds, and the peak at 724.13 cm−1 was related to =C-H bonds in alkenes. These observations are consistent with those reported by DSouza et al. (2021),14 who identified similar peaks at 2915 cm−1 and 2845 cm−1 for C-H bond stretching, 1464 cm−1 for CH2 bending, and 717 cm−1 for CH2 rocking vibrations.
In the present study, the analysis of polyethylene spectral data revealed new peaks at 876 cm−1 in various samples TA1 ( Figure 4b), TA2 ( Figure 4c), TA3 ( Figure 4d), TA1+TA2 ( Figure 4e), TA1+TA3 ( Figure 4f), TA2+TA3 ( Figure 4g), TA1+TA2+TA3 ( Figure 4h), which correspond to =C-H bonds in alkenes. Sowmya et al. (2015)15 also reported similar findings, noting the development of alkenes at 997.26 cm−1 in polyethylene treated with a consortium of C. lunata, A. alternata, P. simplicissimum, and Fusarium sp.
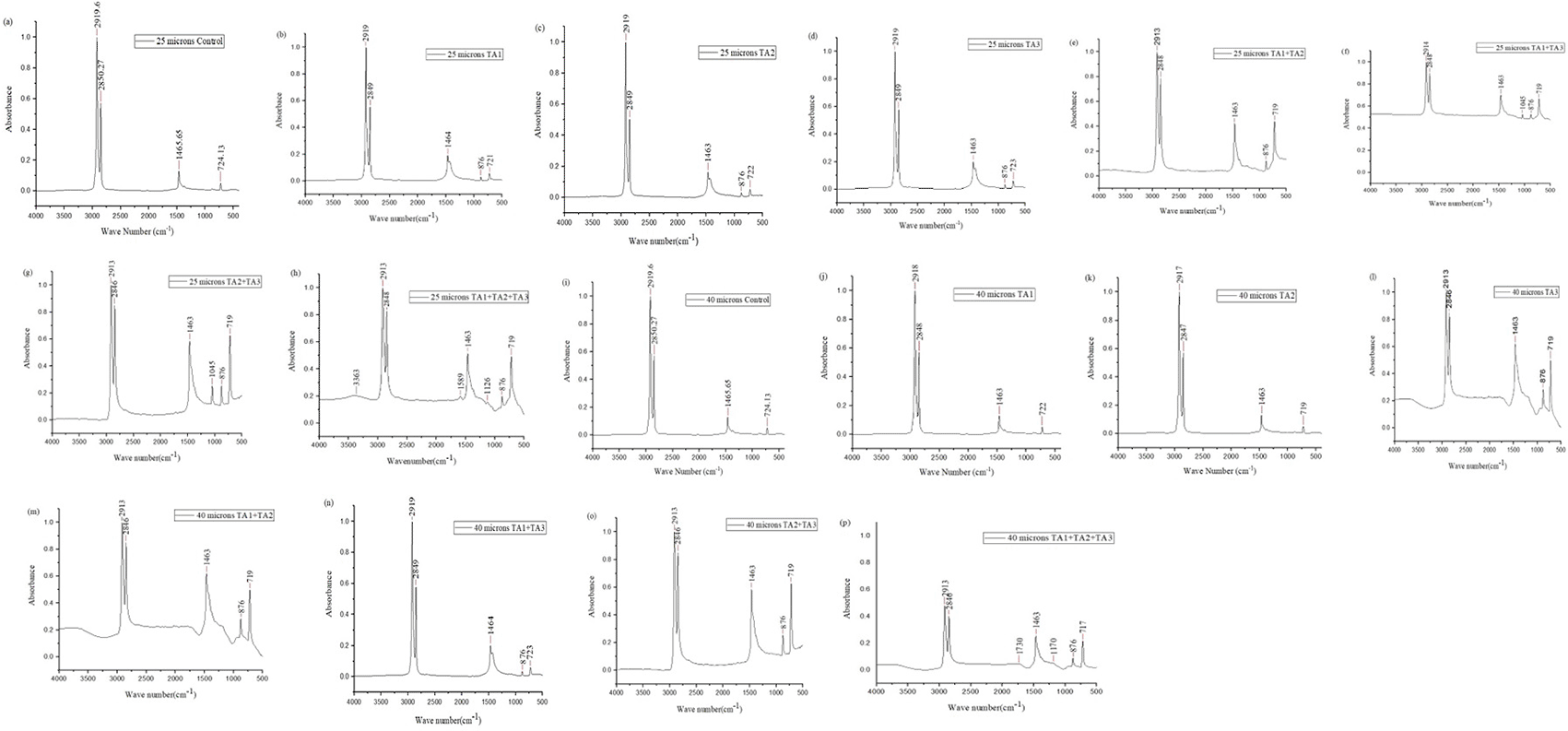
New peaks were observed at 1045 cm−1 in TA1+TA3 ( Figure 4f) and TA2+TA3 ( Figure 4g), at 1126 cm−1 in TA1+TA2+TA3 ( Figure 4h), and at 1170 cm−1 in TA1+TA2+TA3 ( Figure 4p), indicating C–O stretching vibrations linked to alcohol, carboxylic acid, ester, and ether groups. In contrast, the degradation of LDPE in the presence of consortiums showed C–O stretching frequencies between 1087.9 and 1107.5 cm−1. These findings suggest that the consortium significantly altered the carbon chain structure of LDPE, promoting bioactive hydrolysis.32
In this study, new peaks were observed at 1589 cm−1 and 1730 cm−1 in TA1+TA2+TA3 (Figure 4h and p), which correspond to ketones, aldehydes, or carbonyl groups (-C=O stretching). Similar findings were reported by Muhonja et al. (2018),11 who noted additional peaks between 1700 and 1650 cm−1, indicating the presence of aldehydes and ketones intermediate products of polyethylene biodegradation. Pinto et al. (2022)33 also observed peaks between 1500 and 1700 cm−1 in LDPE treated with community A, suggesting the presence of carbonyl (-C=O) and vinyl (-CH=CH-) groups. For community C, peaks at 1724 cm−1 and 1737-1740 cm−1 indicated the formation of carbonyl groups, carboxylic groups, and aldehydes.
Moreover, a new peak at 3363 cm−1 in TA1+TA2+TA3 (Figure 4h) corresponds to the formation of alcohols and phenols. Sowmya et al. (2015)15 also identified peaks at 3365.98 cm−1 for alcohols and phenols, 2914.58 cm−1 for alkanes, 1122.51 cm−1 for carboxylic acids, esters, and ethers, 1455.70 and 729.96 cm−1 for aromatics, and 875.39 cm−1 for alkenes, indicating polyethylene degradation.
The spectra of films treated with pure fungi and the consortium showed a significant decrease in peak intensities at 2919.69 cm−1, 2850.27 cm−1, 1465.65 cm−1, and 0724.13 cm−1 compared to control samples. Negi et al. (2011)27 observed that this reduction in peak intensities reflected weaker links in hydrocarbon chains. These spectral changes indicate modifications in the macromolecular structure of LDPE due to the consortium’s action under conditions of sunlight, moisture, and temperature.
Our research, conducted using dumpsite soil collected in pots, confirmed polyethylene degradation through weight loss and FTIR analysis.
The study revealed that naturally occurring soil microbes, including both bacteria and fungi, are highly effective in degrading low-density polyethylene (LDPE). Among these, fungi generally exhibited a higher degradation capacity than bacteria. Specifically, fungi from the Aspergillus strain, along with bacteria from the Pseudomonas aeruginosa and Proteus mirabilis strains, demonstrated the greatest potential for LDPE degradation. Based on weight loss and FTIR analyses, indicated significant structural changes and breakdown of LDPE over 9 months under pot conditions. These findings suggest that using microbial consortia with enhanced biodegradation capabilities offers a promising, eco-friendly solution for managing plastic waste.
T.D. contributed to the conceptualization, writing the original draft, methodology, investigation of the study, and formal analysis; A.M. and ZTA: conceptualization, writing, review, editing, and supervision.
Figshare: Evaluate the Efficacy of Selected Microorganism through biodegradation studies. https://doi.org/10.6084/m9.figshare.28163621.34
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors thank the Department of Biology at the University of Gondar for providing the research facilities and express their appreciation to Dr. Ebrahim Mama from the Department of Biotechnology, College of Biological and Chemical Engineering, Addis Ababa Science and Technology University, Addis Ababa, Ethiopia, for conducting the FTIR analysis.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)